A Transient Behavior Study of Polymer Electrolyte Fuel Cells with Cyclic Current Profiles
Abstract
:1. Introduction
2. Experimental Preparation
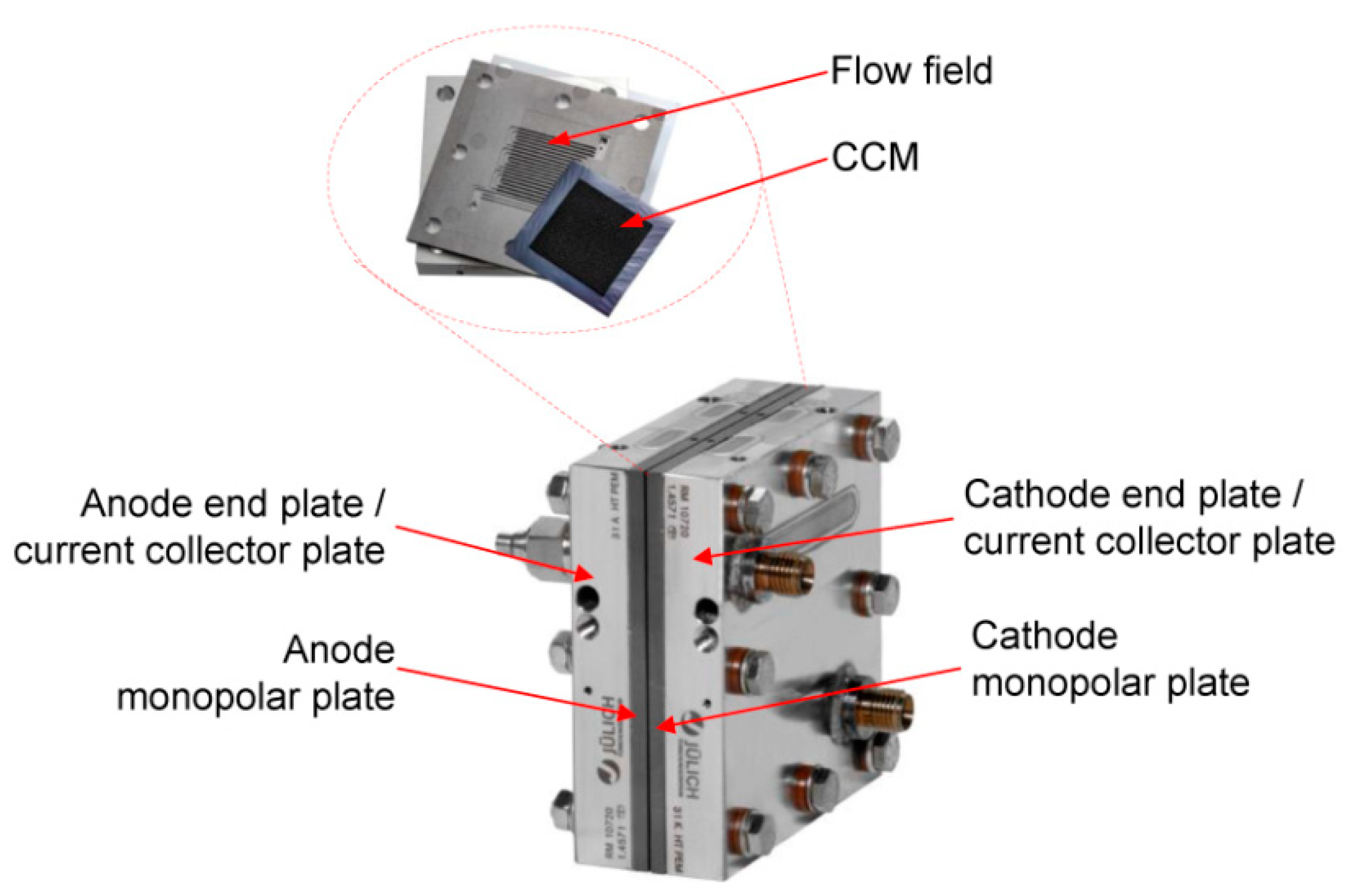
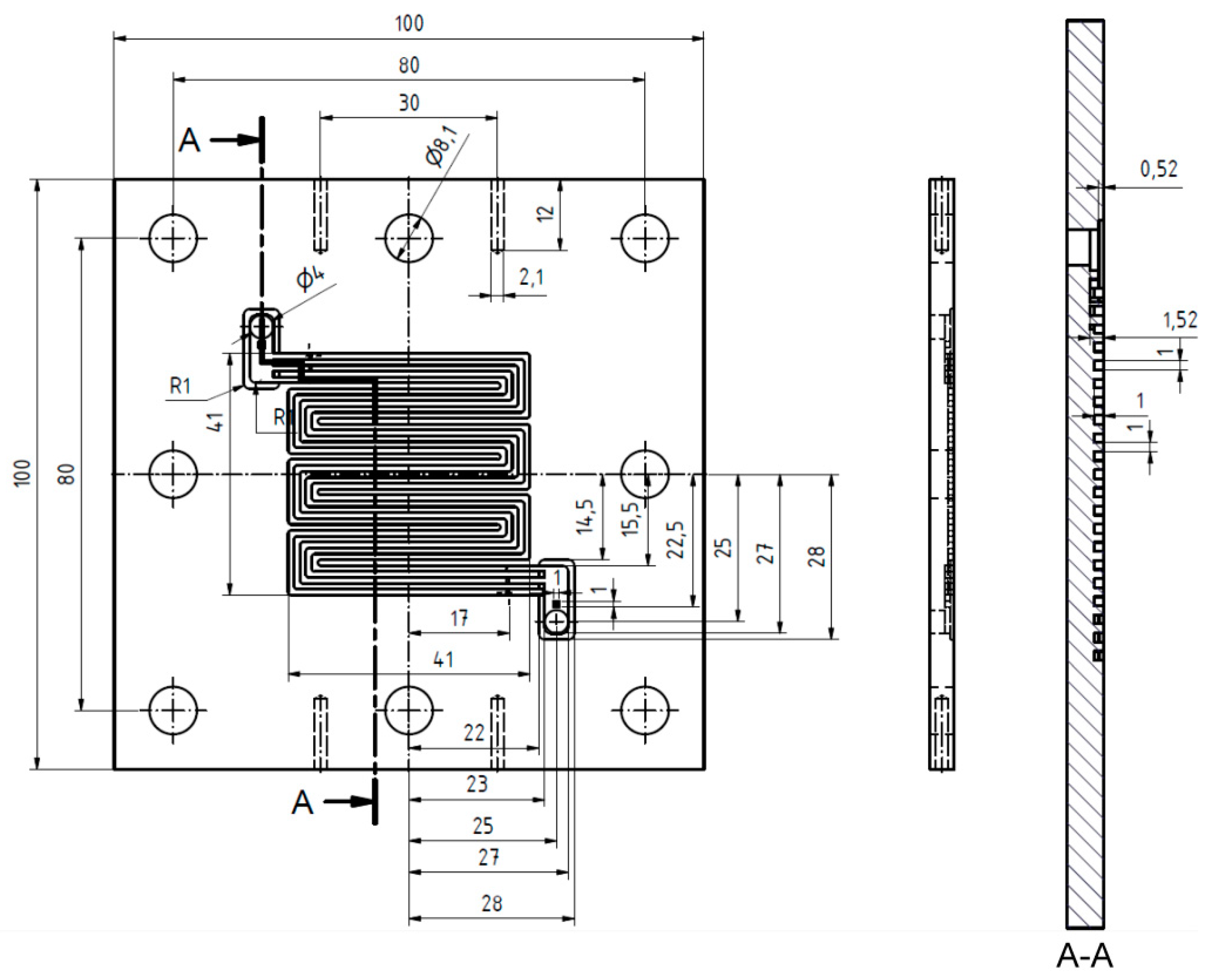
2.1. Test Cell Components
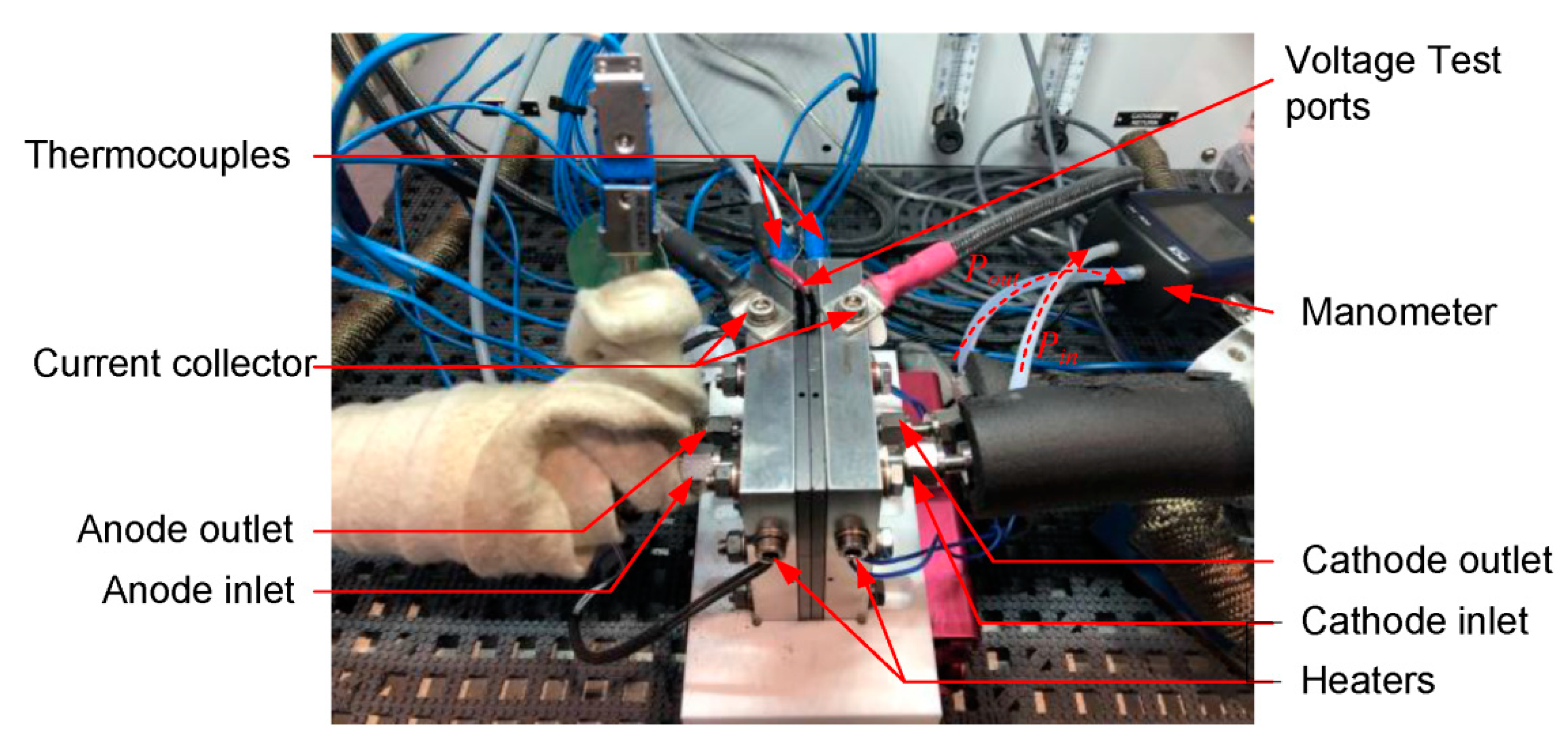
2.2. Test Equipment
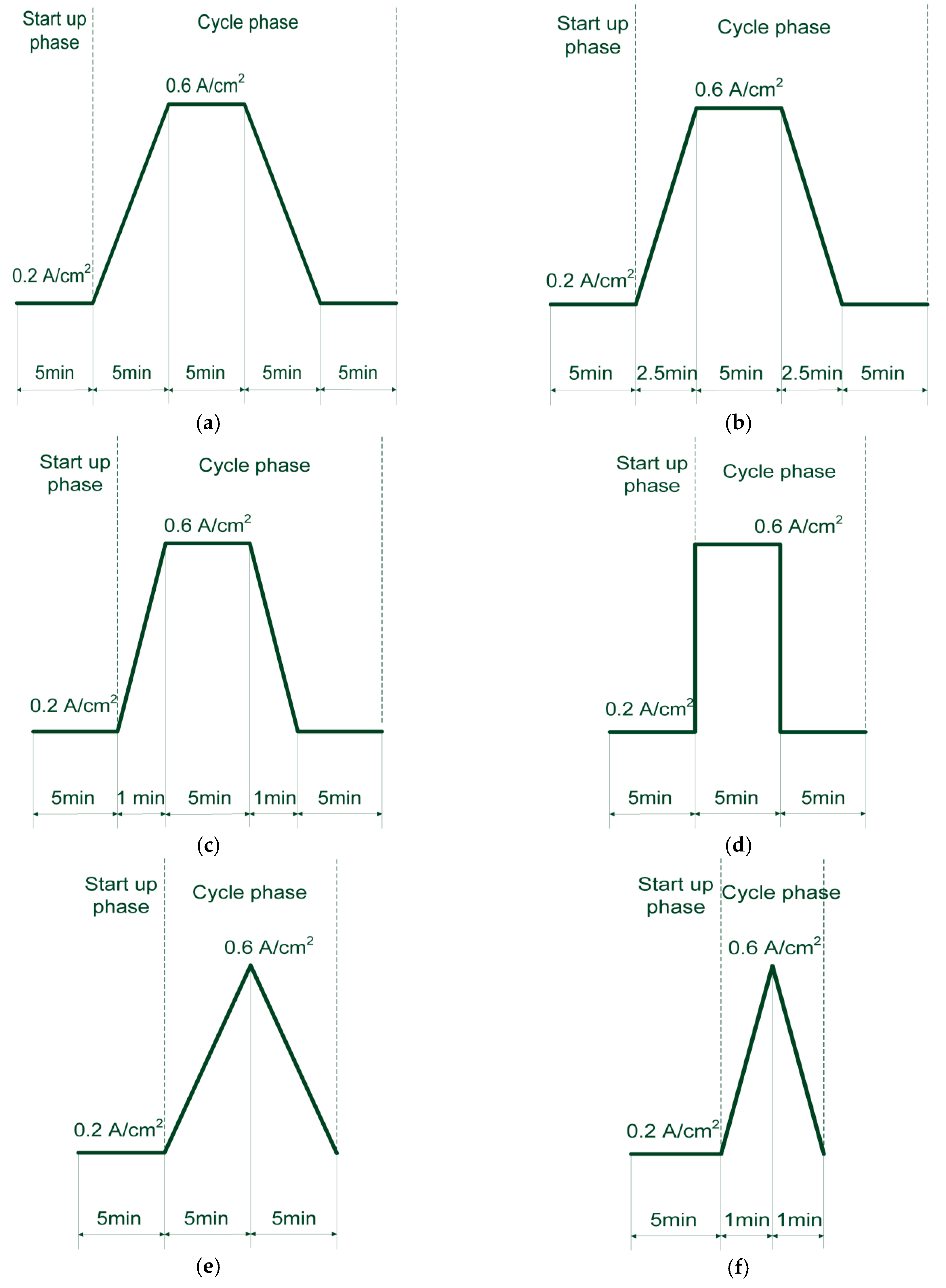
2.3. Test Procedures
2.4. Test Conditions
- Linear current increase from 0.2 A/cm2 to 0.6 A/cm2
- Constant current at 0.6 A/cm2
- Linear current decrease from 0.6 A/cm2 to 0.2 A/cm2
- Constant current at 0.2 A/cm2
3. Results and Discussion
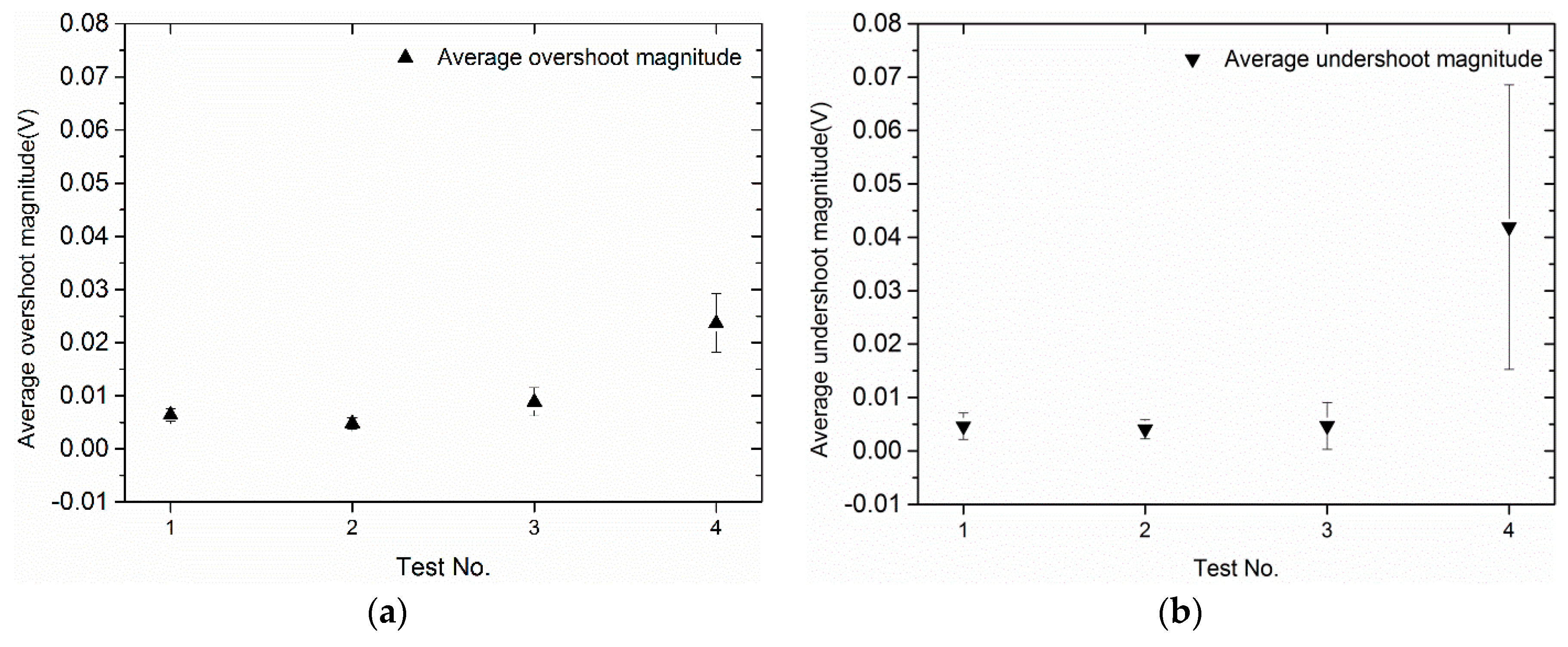
3.1. Effect of Load Changes on Voltage Overshoot and Undershoot Behavior
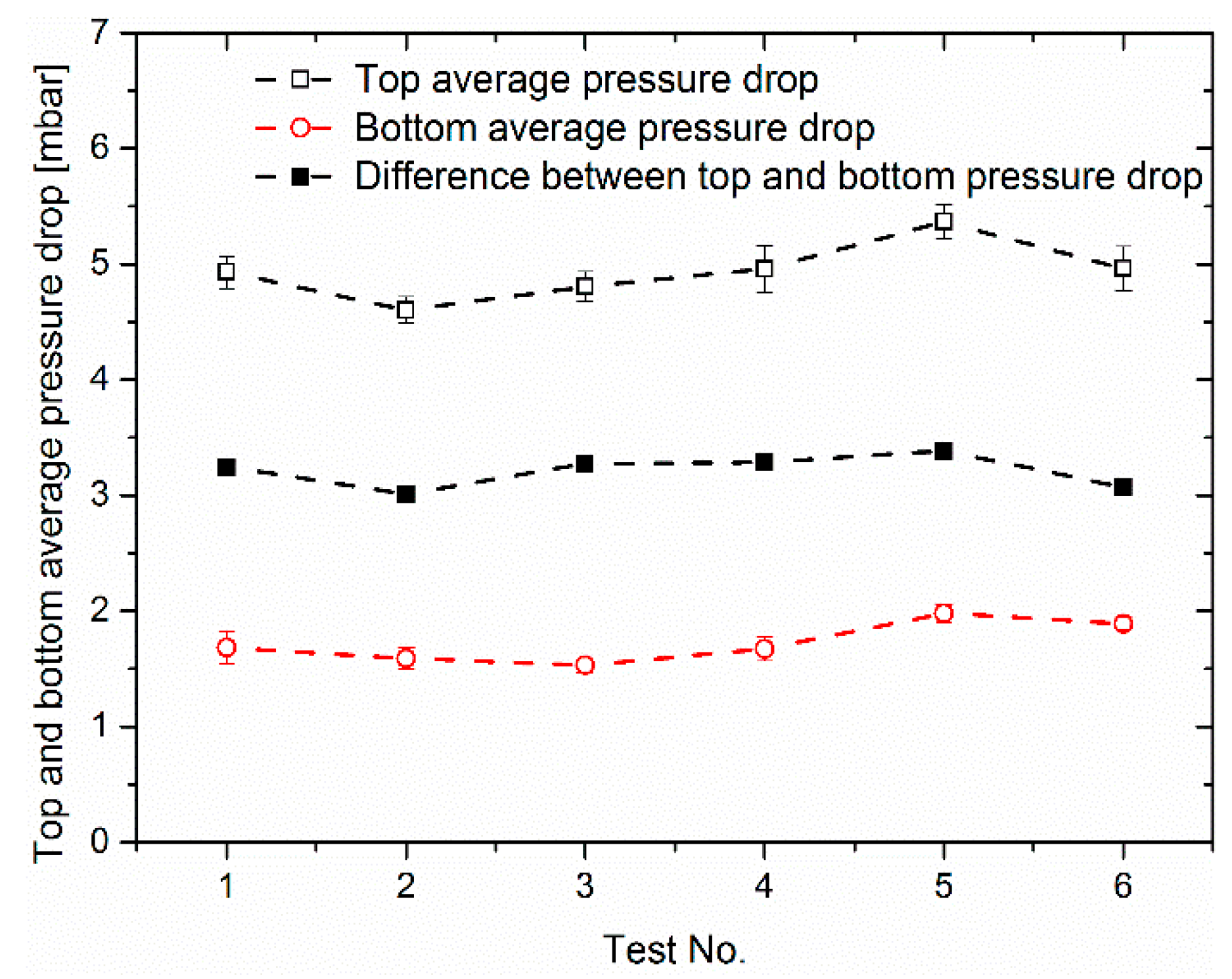
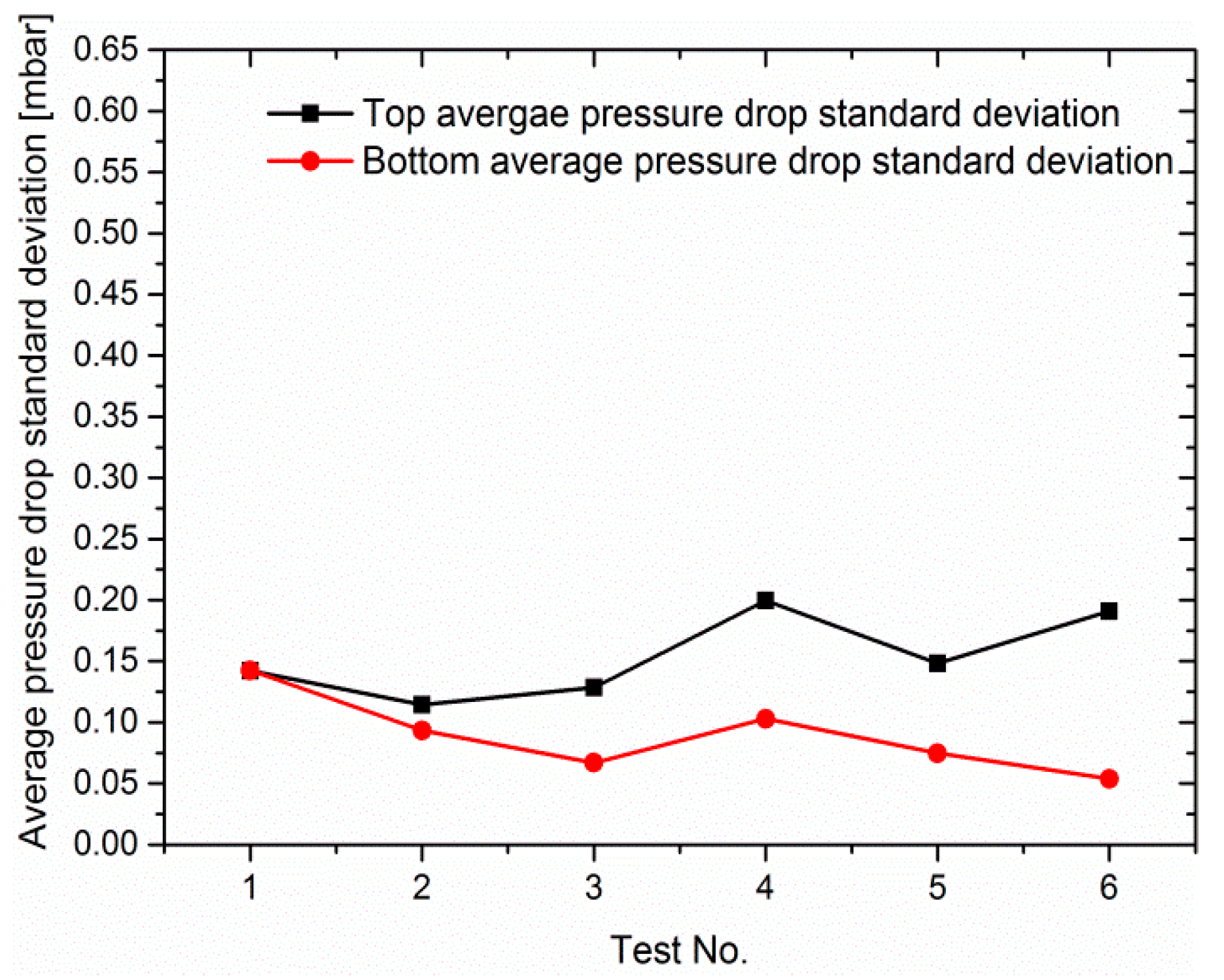
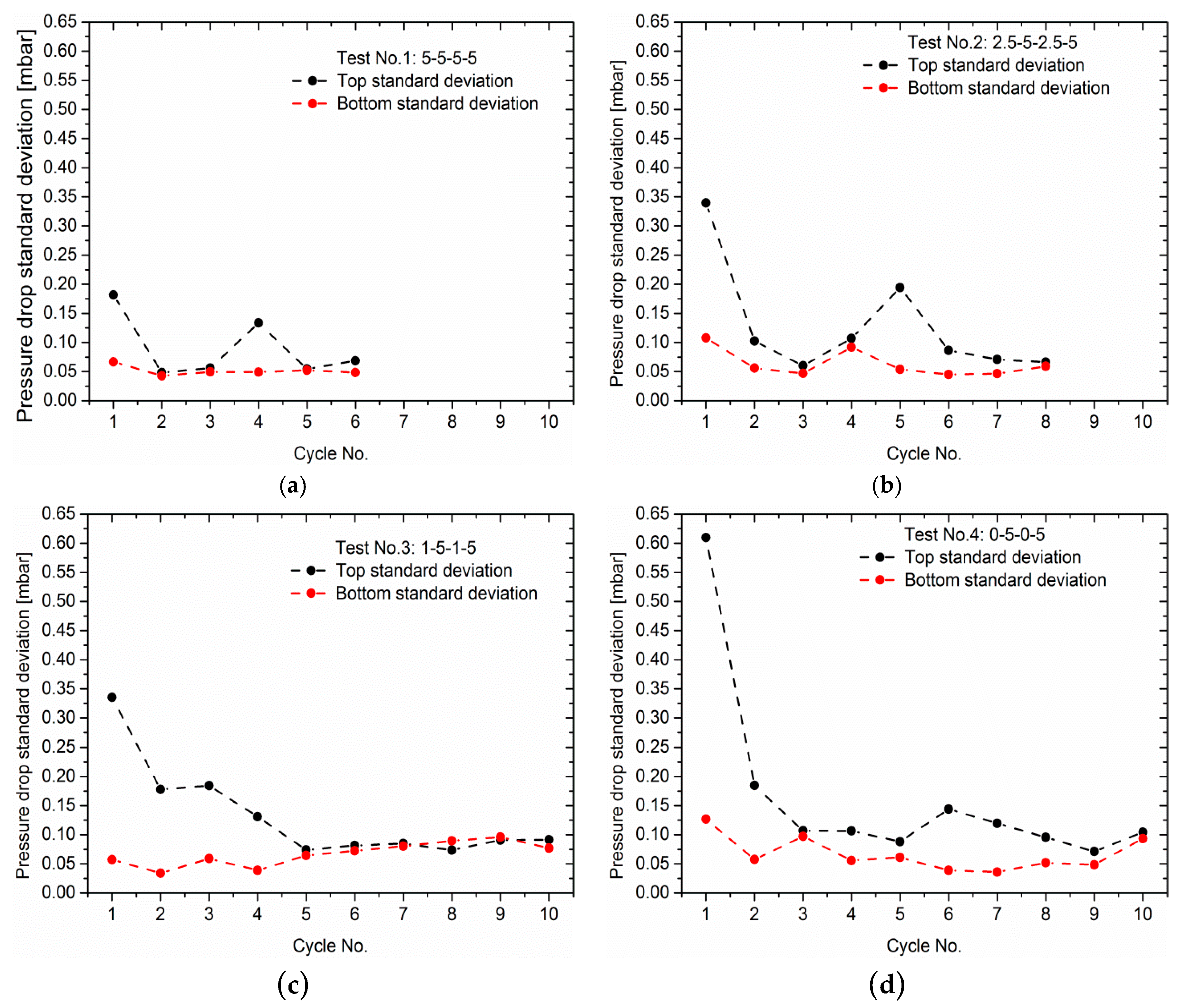
3.2. Effect of Load Ramps on Cathodic Pressure Drop Change
3.3. Effect of Load Ramps on Ohmic Resistance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Jia, X.; Pei, P.; Lu, Y.; Yi, L.; Shi, Y. Simulation and experiment for oxygen-enriched combustion engine using liquid oxygen to solidify CO2. Chin. J. Mech. Eng. 2016, 29, 188–194. [Google Scholar] [CrossRef]
- Niakolas, D.K.; Daletou, M.; Neophytides, S.G.; Vayenas, C.G. Fuel cells are a commercially viable alternative for the production of “clean” energy. J. Hum. Environ. 2016, 45, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lehnert, W.; Janßen, H.; Samsun, R.C.; Stolten, D. A review of high-temperature polymer electrolyte membrane fuel-cell (HT-PEMFC)-based auxiliary power units for diesel-powered road vehicles. J. Power Sources 2016, 311, 91–102. [Google Scholar] [CrossRef]
- Wang, F.-C.; Gao, C.-Y.; Li, S.-C. Impacts of power management on a PEMFC electric vehicle. Int. J. Hydrog. Energy 2014, 39, 17336–17346. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, W.; Zhang, L.; Chan, S.H.; Wang, Y. An experimental study on anode water management in high temperature PEM fuel cell. Int. J. Hydrog. Energy 2015, 40, 4666–4672. [Google Scholar] [CrossRef]
- Urbani, F.; Barbera, O.; Giacoppo, G.; Squadrito, G.; Passalacqua, E. Effect of operative conditions on a PEFC stack performance. Int. J. Hydrog. Energy 2008, 33, 3137–3141. [Google Scholar] [CrossRef]
- Ahn, S.-Y.; Shin, S.-J.; Ha, H.Y.; Hong, S.-A.; Lee, Y.-C.; Lim, T.W.; Oh, I.H. Performance and lifetime analysis of the kW-class PEMFC stack. J. Power Sources 2002, 106, 295–303. [Google Scholar] [CrossRef]
- Scholta, J.; Häussler, F.; Zhang, W.; Küppers, L.; Jörissen, L.; Lehnert, W. Development of a stack having an optimized flow field structure with low cross transport effects. J. Power Sources 2006, 155, 60–65. [Google Scholar] [CrossRef]
- Amphlett, J.C.; Mann, R.F.; Peppley, B.A.; Roberge, P.R.; Rodrigues, A. A model predicting transient responses of proton exchange membrane fuel cells. J. Power Sources 1996, 61, 183–188. [Google Scholar] [CrossRef]
- Um, S.; Wang, C.-Y.; Chen, K.S. Computational Fluid Dynamics Modeling of Proton Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2000, 147, 4485. [Google Scholar] [CrossRef]
- Hamelin, J.; Agbossou, K.; Laperrière, A.; Laurencelle, F.; Bose, T.K. Dynamic behavior of a PEM fuel cell stack for stationary applications. Int. J. Hydrog. Energy 2001, 26, 625–629. [Google Scholar] [CrossRef]
- Kim, S.; Shimpalee, S.; van Zee, J.W. The effect of stoichiometry on dynamic behavior of a proton exchange membrane fuel cell (PEMFC) during load change. J. Power Sources 2004, 135, 110–121. [Google Scholar] [CrossRef]
- Shen, Q.; Hou, M.; Yan, X.; Liang, D.; Zang, Z.; Hao, L.; Shao, Z.; Hou, Z.; Ming, P.; Yi, B. The voltage characteristics of proton exchange membrane fuel cell (PEMFC) under steady and transient states. J. Power Sources 2008, 179, 292–296. [Google Scholar] [CrossRef]
- Yan, W.-M.; Soong, C.-Y.; Chen, F.; Chu, H.-S. Transient analysis of reactant gas transport and performance of PEM fuel cells. J. Power Sources 2005, 143, 48–56. [Google Scholar] [CrossRef]
- Yan, Q.; Toghiani, H.; Causey, H. Steady state and dynamic performance of proton exchange membrane fuel cells (PEMFCs) under various operating conditions and load changes. J. Power Sources 2006, 161, 492–502. [Google Scholar] [CrossRef]
- Liu, D.; Case, S. Durability study of proton exchange membrane fuel cells under dynamic testing conditions with cyclic current profile. J. Power Sources 2006, 162, 521–531. [Google Scholar] [CrossRef]
- Lin, R.; Li, B.; Hou, Y.P.; Ma, J.M. Investigation of dynamic driving cycle effect on performance degradation and micro-structure change of PEM fuel cell. Int. J. Hydrog. Energy 2009, 34, 2369–2376. [Google Scholar] [CrossRef]
- Lin, R.; Xiong, F.; Tang, W.C.; Técher, L.; Zhang, J.M.; Ma, J.X. Investigation of dynamic driving cycle effect on the degradation of proton exchange membrane fuel cell by segmented cell technology. J. Power Sources 2014, 260, 150–158. [Google Scholar] [CrossRef]
- Banerjee, R.; Kandlikar, S.G. Two-phase flow and thermal transients in proton exchange membrane fuel cells–A critical review. Int. J. Hydrog. Energy 2015, 40, 3990–4010. [Google Scholar] [CrossRef]
- Sergi, J.M.; Lu, Z.; Kandlikar, S.G. In Situ Characterization of Two-Phase Flow in Cathode Channels of an Operating PEM Fuel Cell With Visual Access. In Proceedings of the ASME 2009 7th International Conference on Nanochannels, Microchannels and Minichannels (ASME), Pohang, Korea, 22–24 June 2009; pp. 303–311. [Google Scholar]
- Zhang, L.; Du, W.; Bi, H.T.; Wilkinson, D.P.; Stumper, J.; Wang, H. Gas–liquid two-phase flow distributions in parallel channels for fuel cells. J. Power Sources 2009, 189, 1023–1031. [Google Scholar] [CrossRef]
- Adroher, X.C.; Wang, Y. Ex situ and modeling study of two-phase flow in a single channel of polymer electrolyte membrane fuel cells. J. Power Sources 2011, 196, 9544–9551. [Google Scholar] [CrossRef] [Green Version]
- Pei, P.; Li, Y.; Xu, H.; Wu, Z. A review on water fault diagnosis of PEMFC associated with the pressure drop. Appl. Energy 2016, 173, 366–385. [Google Scholar] [CrossRef]
- Stumper, J.; Löhr, M.; Hamada, S. Diagnostic tools for liquid water in PEM fuel cells. J. Power Sources 2005, 143, 150–157. [Google Scholar] [CrossRef]
- Chen, J. Dominant frequency of pressure drop signal as a novel diagnostic tool for the water removal in proton exchange membrane fuel cell flow channel. J. Power Sources 2010, 195, 1177–1181. [Google Scholar] [CrossRef]
- Banerjee, R.; Kandlikar, S.G. Experimental investigation of two-phase flow pressure drop transients in polymer electrolyte membrane fuel cell reactant channels and their impact on the cell performance. J. Power Sources 2014, 268, 194–203. [Google Scholar] [CrossRef]
- Banerjee, R.; Kandlikar, S.G. Two-Phase Pressure Drop Characteristics During Low Temperature Transients in PEMFCs. In Proceedings of the ASME 2014 12th International Conference on Nanochannels, Microchannels and Minichannels (ASME), Chicago, IL, USA, 3–7 August 2014. [Google Scholar]
- Banerjee, R.; Kandlikar, S.G. Two-phase pressure drop response during load transients in a PEMFC. Int. J. Hydrog. Energy 2014, 39, 19079–19086. [Google Scholar] [CrossRef] [Green Version]
- US Fuel Cell Council. USFCC Single Cell Test Protocol # 05-014. Available online: http://www.members.fchea.org/core/import/PDFs/Technical%20Resources/MatComp%20Single%20Cell%20Test%20Protocol%2005-014RevB.2%20071306.pdf (accessed on 20 July 2018).
- Cleghorn, S. TECHNICAL DATA–PRIMEA SERIES MEAs. Available online: https://www.dropbox.com/s/lzmrz9t4vuafhi1/2000%20Gore_Primea_NT-PEM_MEA_Einfahrprozedur.pdf (accessed on 20 July 2018).
- Banerjee, R.; See, E.; Kandlikar, S.G. Pressure Drop and Voltage Response of PEMFC Operation under Transient Temperature and Loading Conditions. ECS Trans. 2013, 58, 1601–1611. [Google Scholar] [CrossRef] [Green Version]


| Anode Reactant Gas | Hydrogen |
| Rel. Humidity | 90% |
| λH2 | 2 |
| Cathode reactant gas | Air |
| Rel. Humidity air | 90% |
| λAir | 2 |
| Cell Temperature | 60 °C |
| Flow Direction | Co-flow, top to bottom |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Janßen, H.; Lehnert, W. A Transient Behavior Study of Polymer Electrolyte Fuel Cells with Cyclic Current Profiles. Energies 2019, 12, 2370. https://doi.org/10.3390/en12122370
Shi Y, Janßen H, Lehnert W. A Transient Behavior Study of Polymer Electrolyte Fuel Cells with Cyclic Current Profiles. Energies. 2019; 12(12):2370. https://doi.org/10.3390/en12122370
Chicago/Turabian StyleShi, Yan, Holger Janßen, and Werner Lehnert. 2019. "A Transient Behavior Study of Polymer Electrolyte Fuel Cells with Cyclic Current Profiles" Energies 12, no. 12: 2370. https://doi.org/10.3390/en12122370





