1. Introduction
Oil shale is an attractive supplemental energy source for petroleum. The reserve of oil shale may be estimated at a trillion tons [
1]. The oil shale retorting technology has a long history and different technologies have been developed [
2,
3,
4,
5,
6]. Presently, in-situ mining technology is gaining more attention because of its advantages [
7]. China is rich in oil shale reserves with 4.76 × 10
10 tons that could significantly increase the petroleum supply [
8]. The in situ mining of oil shale is a potentially environmentally friendly method to recover hydrocarbons as excavation, retorting, then spoiling is not required. In the in-situ process of mining, heat is injected into the oil shale stratum and pyrolyzes the organic matter. Then, the shale oil and hydrocarbon gas can be recovered [
9,
10,
11,
12]. The advantages of such in-situ mining involves no large-scale mining, nor equipment, nor waste-rock production and allows for the control of environmental pollution.
Permeability is a key parameter which controls the potential to recover the retorted chemicals from the geological host. Many factors influence rock permeability—paramount among them are effective stresses and temperature. At room temperature (RT), the permeability of intact and fractured rocks, generally decreases with increasing effective stresses [
13,
14,
15,
16,
17]. Permeability may be enhanced by thermal fracturing when the rock is heated to a high temperature. The permeability of Beishan and Daqing granites, when heated then cooled to RT, has been shown to first increase slowly at low temperature, then increase as temperature transits from 20 °C to 900 °C. The threshold value of permeability for Beishan granite is between 300 °C and 400 °C and for Daqing granite between 200 °C and 300 °C [
18]. The permeability evolution of Luhui granite shows a threshold temperature (
Tc) for permeability change that activates permeability in the thermally cracked granite. The magnitude of permeability is 10
−7D with only a slight increase below
Tc with permeability increasing dramatically from 10
−6D as temperatures approach 300–400 °C to 10
−5D at 400 °C [
19]. This threshold temperature for permeability gain in sandstone has been identified as 400–500 °C. The initial permeability of sandstone (under certain pressure conditions) has been found to have a nonlinearity with a rising temperature [
20]. Zhang performed real-time permeability experiments on fine-grained feldspar-rich sandstone where permeability first increases at low temperature, then increases abruptly at a threshold temperature of 200 °C, then decreases at temperatures between 200 °C and 400 °C, before finally increasing from 400 °C to 600 °C [
21].
These studies mainly focus on permeability evolution with temperature of rocks composed of inorganic minerals. Few studies explore the permeability of organic-rich rocks such as coal and oil shale at incremented temperature. However, such studies are important for underground coal gasification and in-situ extraction in oil shale and low-rank coal by injecting heat. Understanding the permeability sensitivity to reservoir temperature change is crucial to defining fluid flow as an increase temperature may cause a loss in productivity in oil and gas wells. Permeability of low-rank lignite exhibits an increasing and periodic change at temperatures from RT to 650 °C [
22]. Feng et al. [
23] investigated the permeability of anthracite and coal at high temperature and under triaxial stresses and found that coal permeability could be divided into three stages by a critical temperature and peak temperature from RT to 600 °C. The critical temperature of anthracite is from 150 °C to 200 °C with a peak temperature between 450 °C and 500 °C. For coal, these range from 200 °C to 250 °C for the critical temperature of coal with no peak temperature emerging from RT to 400 °C. Kang et al. [
24,
25] reported results for heated Fushun oil shale where a small quantity of micro—fissures formed in the specimen mainly along the original bedding and at the boundaries of hard mineral particles under 300 °C. The thermal fissure surfaces are sub-parallel to the original bedding. The quantity, length and width of the fissures increased rapidly at above 300 °C due to chemical reactions within the oil shale. The corresponding permeability was measured at temperatures to 500 °C showing that permeability dramatically grew at about 350 °C. Zhao et al. [
26] also analyzed the law of pore structure and porosity of Daqing and Yan’an oil shale by computed tomography (CT) imaging and found out that organic matter thermal decomposition at high temperatures led to changes in the structure of Daqing oil shale. When the temperature reached 200 °C, many pores were developed in the original sample. At high temperature, organic matter began to decompose, and then the gas and oil was generated, and lastly many pores were formed. With the temperature rising, the number of the pores increased and became interconnected by cracks that also formed. Heterogeneous thermal dilation was a main factor leading to changes in the pore structure of Yan’an oil shale. At 200 °C, many fractures parallel to the primary bedding were developed, which expanded with the rising temperature. The internal structure of oil shale was changed by high temperature and formed a porous medium including pores and cracks [
26].
Clearly, the permeability evolution and sensitivity to temperature is a key parameter in the in-situ retorting of oil shale. Xinjiang is rich in oil shale resources and here we evaluate methods for the economic recovery at temperature up to 600 °C. We report on experiments on Jimusar oil shale from Xinjiang at in situ triaxial stresses and at temperatures to 600 °C.
3. Results
The steady flow method was used to measure permeability change in the oil shale, at different temperatures, up to 600 °C. The permeability was calculated from Darcy’s law as:
where
k,
μ,
Q,
L,
A are gas permeability in m
2, nitrogen dynamic viscosity at appropriate temperatures in
, measured flow rate through sample in m
3/s, length of sample in m, and sectional area of sample in m
2, respectively.
,
,
denote standard atmospheric pressure in MPa and nitrogen pressure of the inlet and outlet (MPa).
3.1. Permeability Evolution with Temperature
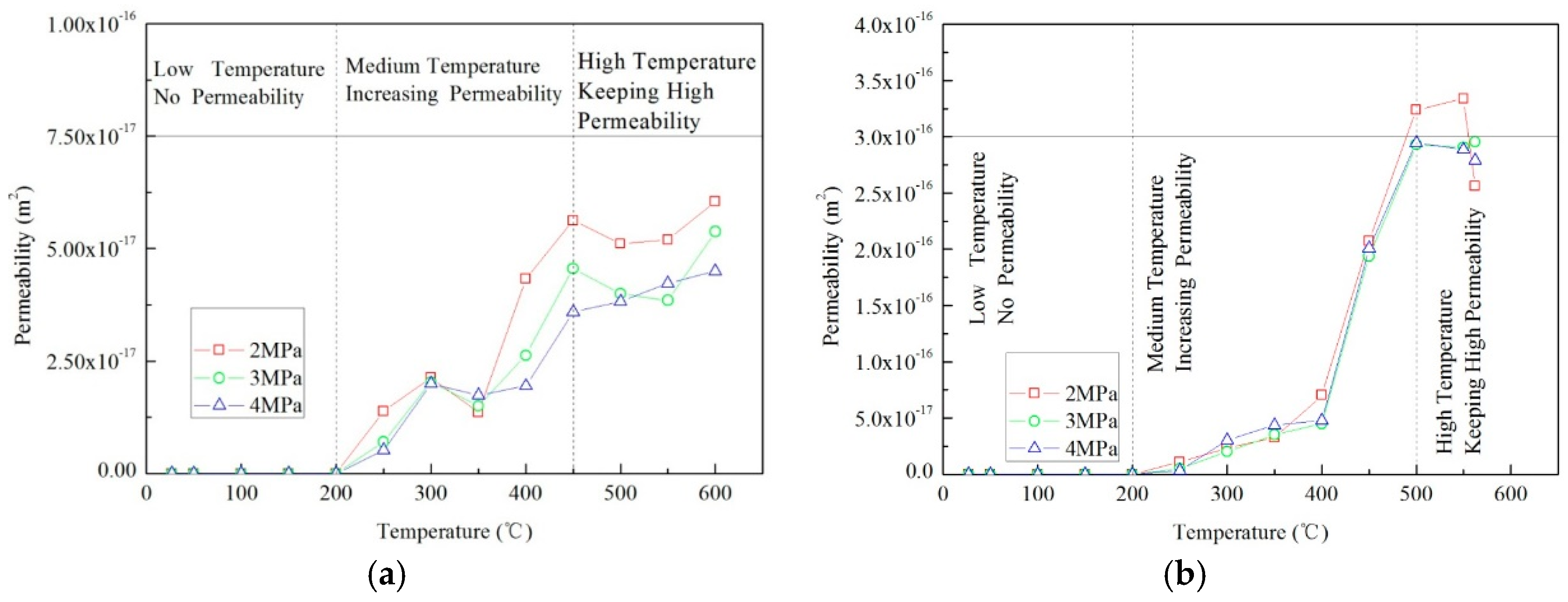
The permeability of the Jimsar oil shale evolved non-monotonically with temperature, as shown in
Figure 3. The permeability progression can be divided into three transitional states during heating.
The low temperature stage is the first stage and is present from RT to 200–250 °C. In the first stage, permeability is very low and changes little. This is closely related to the mineral composition of the oil shale. Oil shale is composed of both inorganic minerals (mass fraction >90%) and organic matter (mass fraction <10%). Fine clay minerals such as illite, smectite and kaolinite are the main components. Hence, oil shale is of very low permeability and has low porosity at RT. Thermally induced micro-cracks would appear in the rock during heating and further augment permeability. However, for oil shale, the production of thermally induced cracks is suppressed due to the presence of its fine-grained structure. In addition, thermal expansion can reduce the opening of fissures including natural fissures and thermally induced cracks. For these combined reasons, the change in permeability below 200–250 °C remains very small.
The second stage of permeability evolution is where temperature ranges from 200–250 °C to 450–500 °C. Permeability increases from zero at 200–250 °C to approximately 3.5 × 10−16 m2 at 450–500 °C. At the beginning of this stage, where temperature ranges from 200–250 °C to 350–400 °C, thermal cracking is the main mechanism for permeability increase. Permeability sharply rises at temperatures greater than 350–400 °C. The pyrolysis of organic matter at high temperatures to 500 °C is likely the main contributor to this sharp increment in permeability increase.
The establishment of a plateau of high permeability is observed above 500 °C. This is the final stage where temperatures range from 500 °C to 600 °C. In this stage, organic matter in the oil shale engages in deep pyrolysis. Gas and/or oil production induced by this pyrolysis results in the development of many pores and fissures which significantly augment oil shale permeability. However, these newly-formed pores and fissures are also compressed by triaxial stresses, which resists further increase in permeability at this stage. As a result, permeability remains significantly elevated but changes little. Pyrolysis is the key process augmenting the permeability of tight oil shales.
The permeability of tight oil shales evolves with temperature with an initial increase in permeability at a threshold temperature and a permeability peak at a peak temperature. The threshold temperature ranges from 200 °C to 250 °C and the peak temperature ranges from 450 °C to 500 °C. These two key temperatures divide permeability into three stages. These comprise: (i) a very slow evolution at low temperature ranging from RT to the threshold temperature, (ii) a considerable increment in permeability at intermediate temperatures ranging from the threshold temperature to peak temperature, and (iii) a plateau of high permeability at high temperatures ranging from this peak temperature to 600 °C.
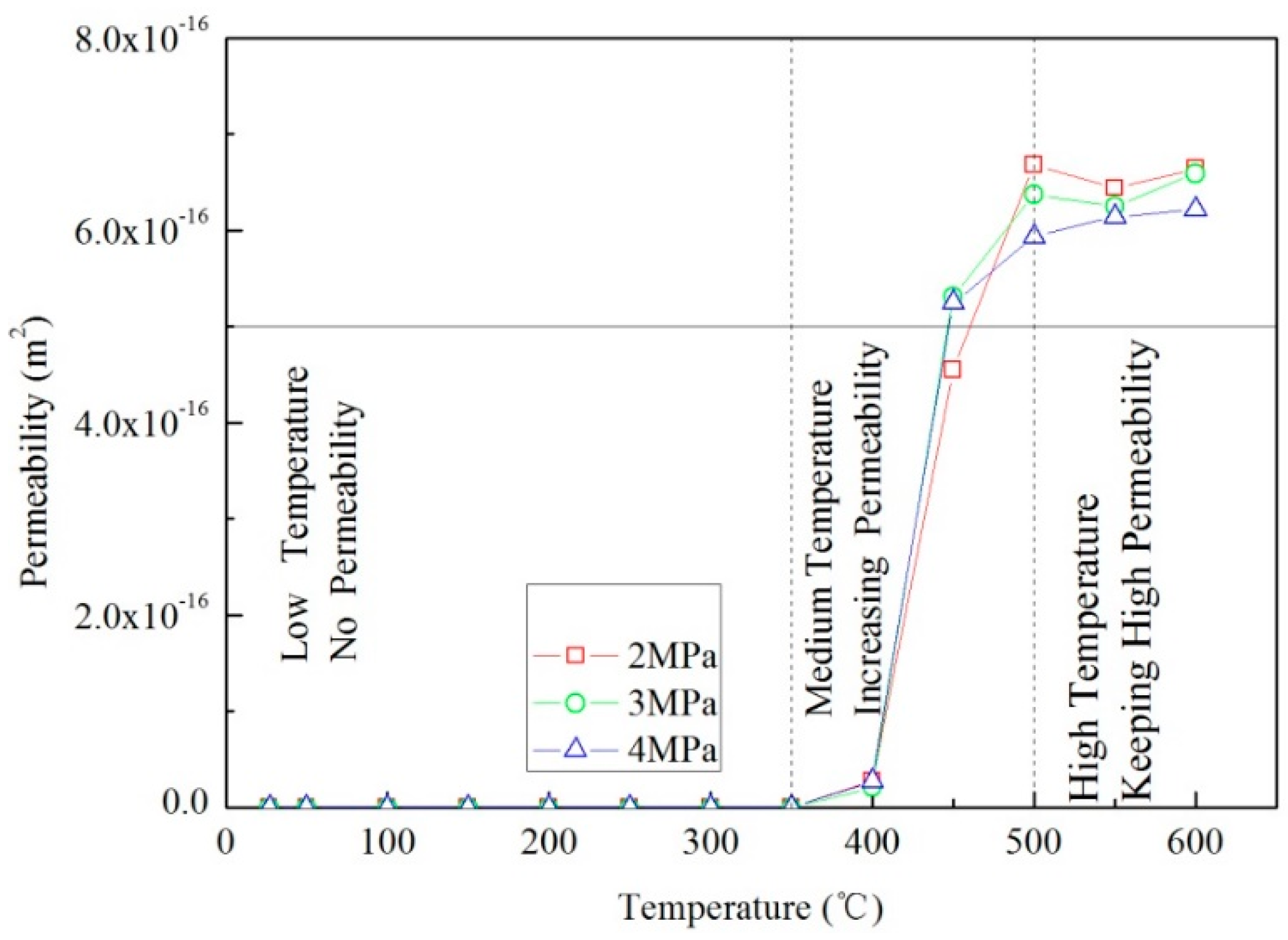
3.2. Effect of Stress on Permeability of Oil Shale at High Temperature
Generally, the incrementing of stress results in the reduction of permeability at RT. However, the few studies on oil shales reveal that this effect of stress on permeability at high temperatures is muted and countered by the positive effects of pyrolysis. We measure the permeability evolution of sample 3
# at temperatures to 600 °C under hydrostatic axial and confining stresses of 25 MPa that corresponds to a burial depth of 1000 m. Similar to samples 1
# and 2
#, the permeability of sample 3
# also experiences the three stages of permeability evolution, as shown in
Figure 4. However, the threshold temperature is considerably higher than that in samples 1
# and 2
#, ranging from 350 °C to 400 °C. The reason may be that the opening of microcracks induced by thermal cracking is more readily countered by thermal expansion at high stress and greater confinement. Once organic matter begins to pyrolyze at 400 °C, new pores and fissures will emerge and result in a significant jump in permeability. The permeability may reach 7 × 10
−16 m
2 at the peak temperature of 500 °C, which is the same as the threshold temperature in samples 1
# and 2
#. Above peak temperature, the permeability exhibits a slight drop but still remains high. Hence, pyrolysis results in high permeability of oil shale at high temperature regardless of the stress state. The stress results in permeability reduction only at low temperature when it is not countered by pyrolysis-induced permeability increase.
4. Discussion
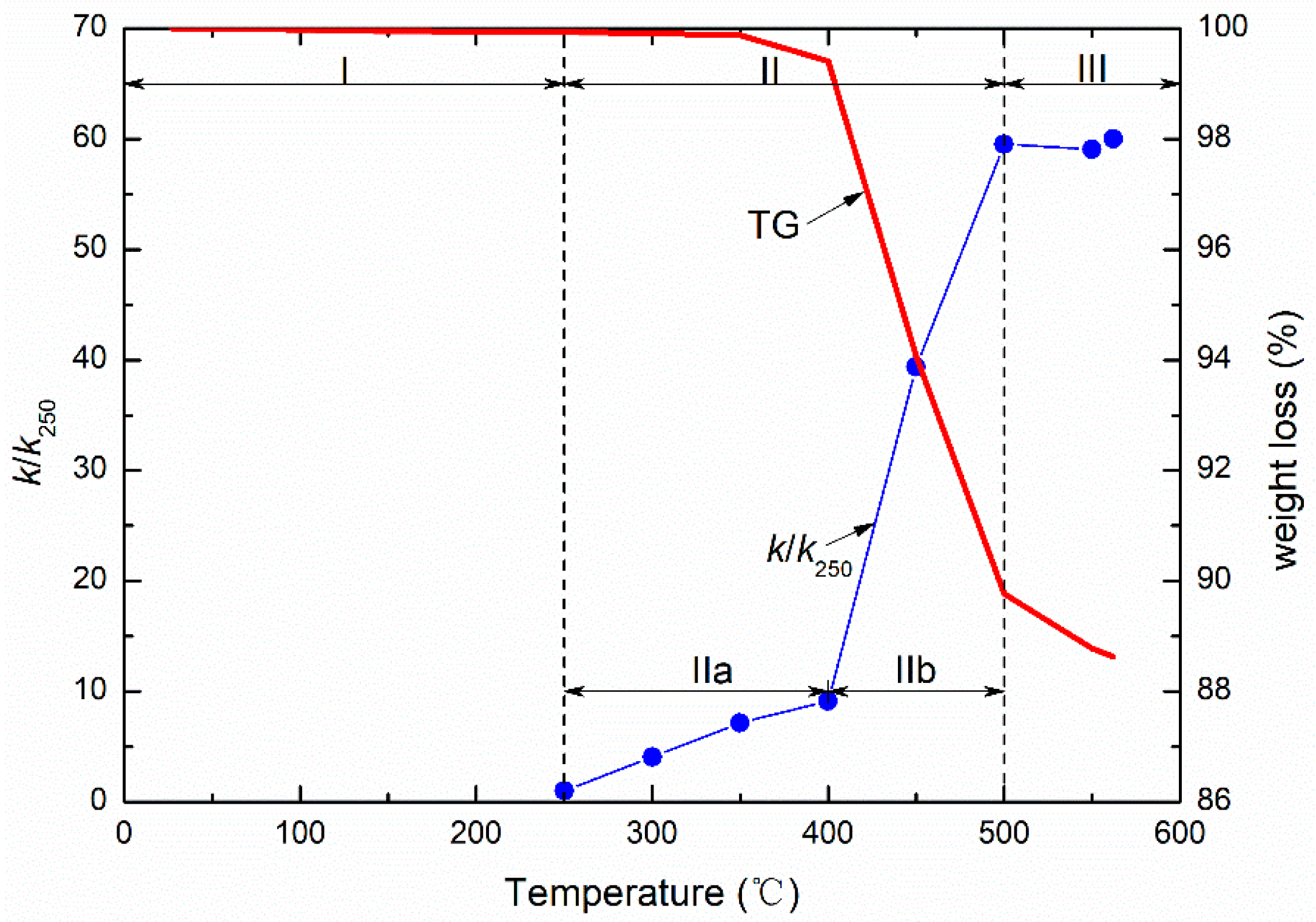
As mentioned above, permeability evolution with temperature may be closely related to the pyrolysis of organic matter. We measured weight loss of the oil shale at temperatures to 600 °C in order to analyze the effect of pyrolysis on permeability.
Figure 5 shows the variation in permeability of sample 2
# with temperature and corresponding weight loss (measured by mass thermogravimetry (TG)). The permeability increment is defined on the left axis (
Figure 5) and is the ratio of permeability at high temperature (
k) to that at 250 °C (
k250). The reason for adopting the permeability at 250 °C as a baseline is that permeability can be measured only at 250 °C and is very low below 250 °C. These three stages of permeability evolution, shown in
Figure 5, closely correspond to the three stages of pyrolysis in oil shale. At temperatures below 250 °C, effectively no permeability is measured–since pyrolysis is not yet activated in the shale. The organic matter in the oil shale begins to decompose and weight loss begins only at 250 °C. Two sub-stages IIa and IIb of permeability can be observed at 250–500 °C, shown in
Figure 5. Weight loss induced by pyrolysis can also be also divided into two sub-stages. Hence, the initial but slight pyrolysis results in a slow increment of permeability increase, corresponding to sub-stage IIa. This is followed by deep pyrolysis which results in the drastic increase in permeability, apparent in stage IIb of
Figure 5. In this sub-stage, weight loss changes from 99% to 90%, an increase of 9% (total about 12% within 600 °C). The corresponding pyrolysis-induced permeability increases from 10-fold at 400 °C to 60-fold at 500 °C. The rate of weight loss per degree centigrade slows above 500 °C, suggesting that pyrolysis has completed and changes abate at 400–500 °C. The corresponding permeability exhibits a small reduction due to stress effects driven by thermal strains but remains high. Hence, pyrolysis plays a crucial role in permeability evolution and fundamentally changes permeability tendency and magnitude.
Gas emission during heating oil shale to 600 °C can develop an oil-filled and connected fracture network. This network evolves from impermeable to permeable such that permeability can be dramatically increased. Hence, gas production during pyrolysis has a significant influence on its permeability.
Figure 6 shows the relation between gas production rate and permeability of Jimsar oil shale at different temperatures. No gas was produced at temperatures from RT to 200–250 °C, corresponding to the first stage of permeability change where the permeability is very low. Gas production rate increases with temperatures between 250 °C and 450 °C which corresponds to the main stage pyrolysis of the oil shale. In this stage, the weight loss of oil shale accounts for 90% of the total weight loss as apparent in
Figure 5. Kerogen within the oil shale is converted to hydrocarbon gas and shale oil, which further develops pores and improves the connectivity of pores. Hence, the increase in permeability is to 3.0 × 10
−16 m
2 where it reaches 89% of peak permeability. Gas production rate drops at temperatures of 450–550 °C. However, permeability continues to increase until 500 °C then decreases at 500–600 °C. Thus, permeability evolution tracks closely with the change in the gas production rate and this in turn reflects the intensity of pyrolysis. High gas production rate supports rapid mass loss in the oil shale and the rapid production of pores–and permeability increases significantly. When gas production rate decreases, the resulting pores are compressed by the tri-axial stresses and permeability slightly decreases.
5. Conclusions
The following conclusions are drawn, based on experiments on the permeability evolution of Jimusar oil shale from Xinjiang, China at both high temperature and recreated triaxial stress state:
(1) The permeability of tight oil shales evolves with temperature. The behavior corresponds small changes in permeability below a threshold temperature, then rapid pyrolysis-driven changes above this threshold temperature until a final temperature where pyrolysis is effectively complete and no significant further growth in permeability results.
(2) The threshold temperatures are dependent on triaxial stresses. For Jimusar oil shale, the threshold temperature ranges from 200 °C to 250 °C for a burial depth of 500 m and from 350 °C to 400 °C for burial depths of 1000 m. The peak temperature is insensitive to triaxial stress state and is in the range 450 °C to 500 °C for all Jimusar samples.
(3) Pyrolysis plays an important role in permeability evolution and fundamentally changes the tendency of permeability evolution and its magnitude. The first stage of permeability evolution is attributed to thermal expansion of minerals in the oil shale but this is completely overpowered by pyrolysis-driven changes (increases) in permeability at elevated temperatures.
Pyrolytically-fractured permeability in oil shale can increase the number and opening of flow channels where the production transport such as gas and oil. In addition, the enhanced flow properties can be good for heating transferring in oil shale strata. Hence, permeability enhancement is extremely conducive to oil and gas recovery from oil shale strata in practice.