Effects of Coal Deformation on Different-Phase CO2 Permeability in Sub-Bituminous Coal: An Experimental Investigation
Abstract
:1. Introduction
2. Experimental Method
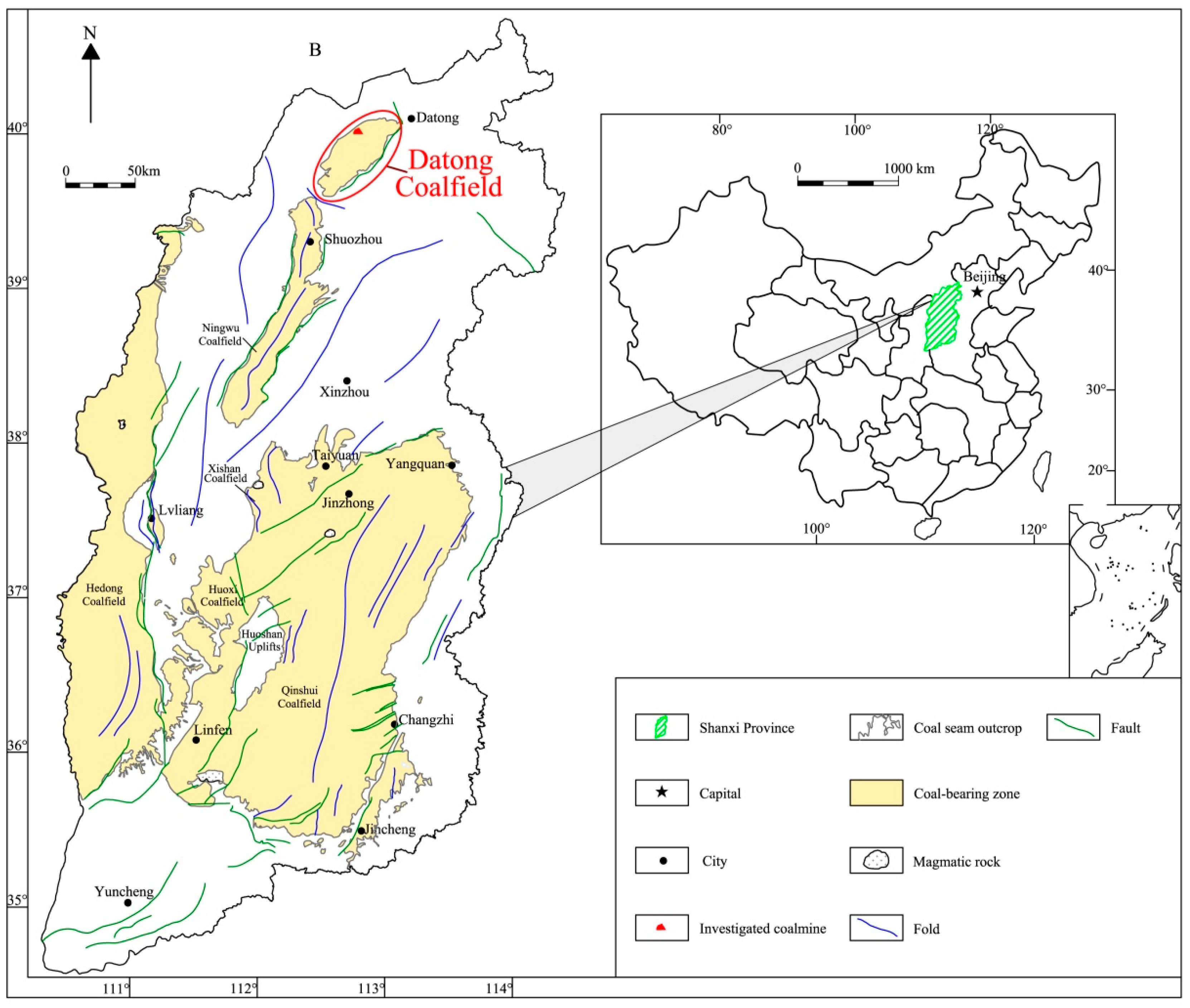
2.1. Geological Setting
2.2. Sample Description
2.3. Experimental Apparatus
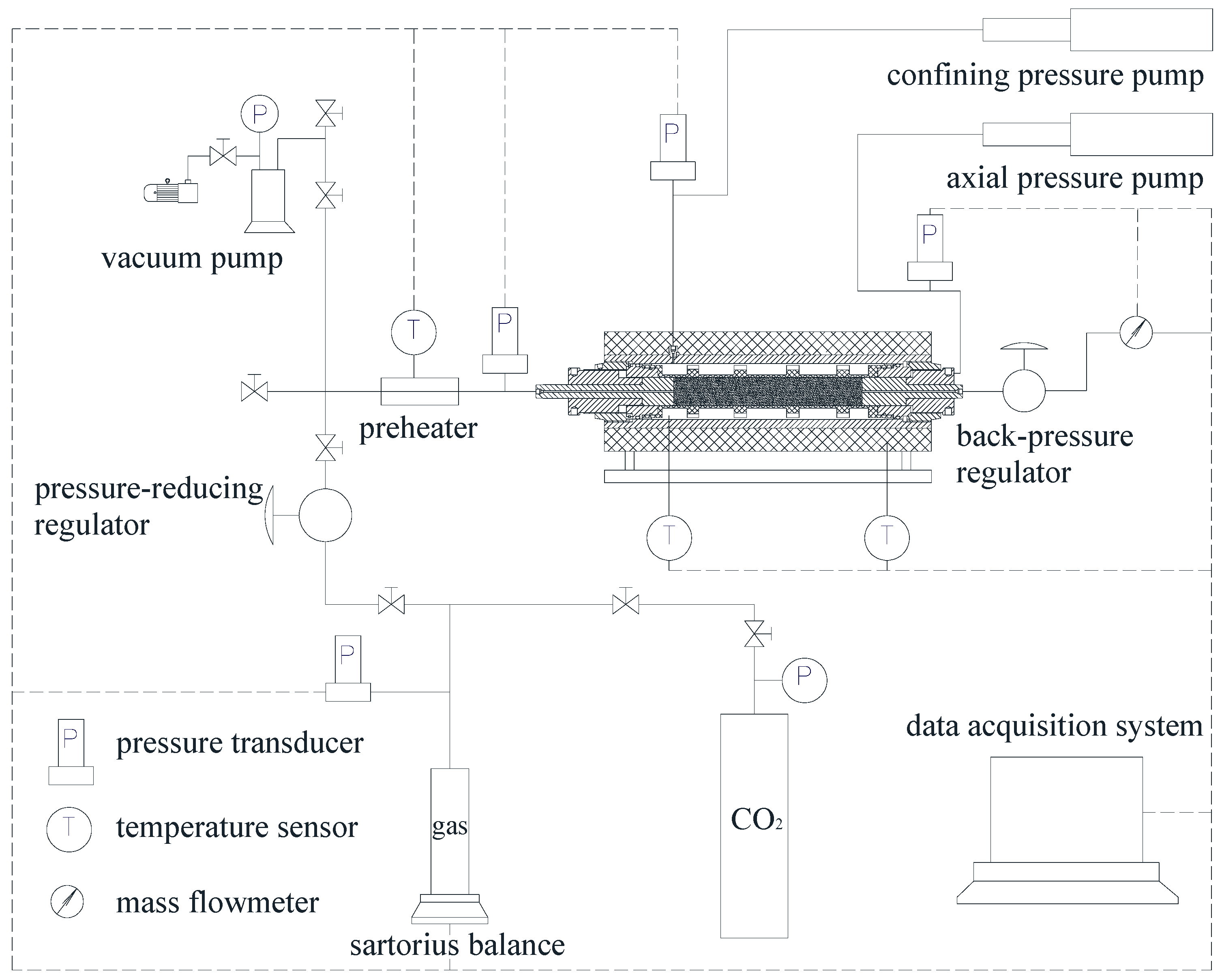
2.3.1. Permeability Testing Apparatus
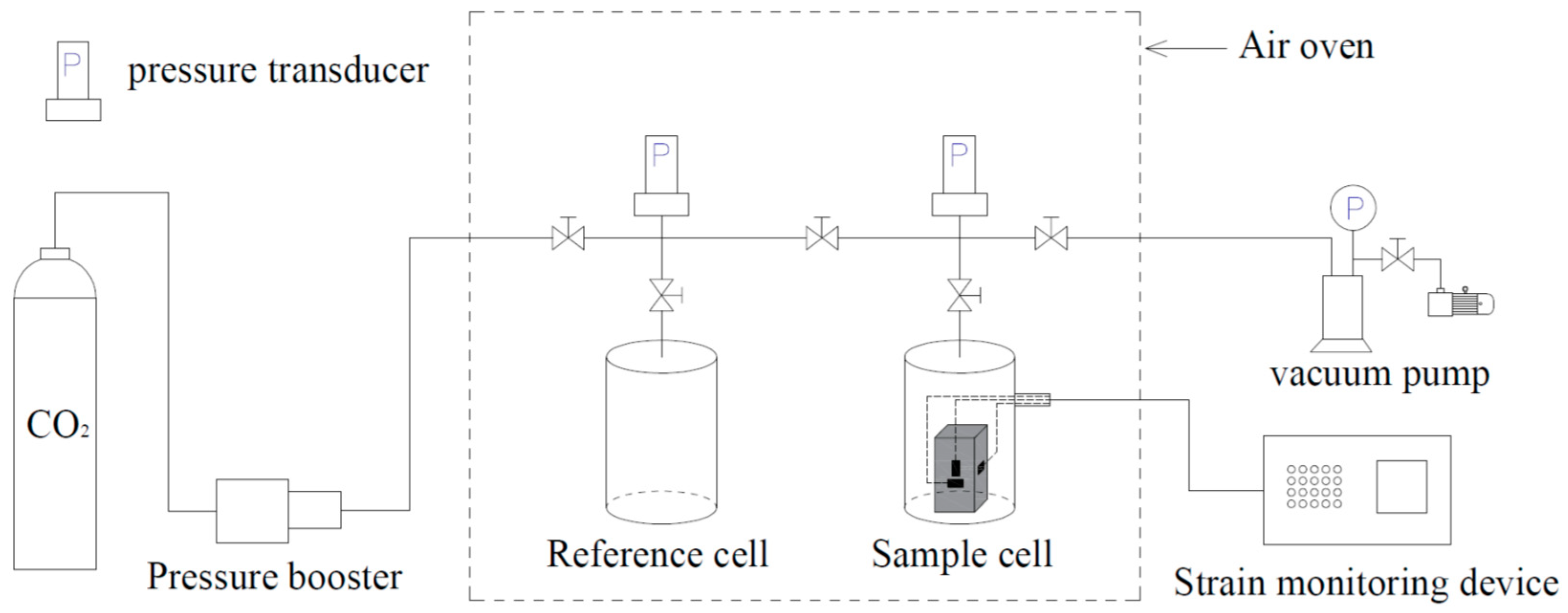
2.3.2. Adsorption Experimental Apparatus
2.4. Experimental Procedure
2.4.1. Permeability Tests
2.4.2. Adsorption and Coal Swelling Test
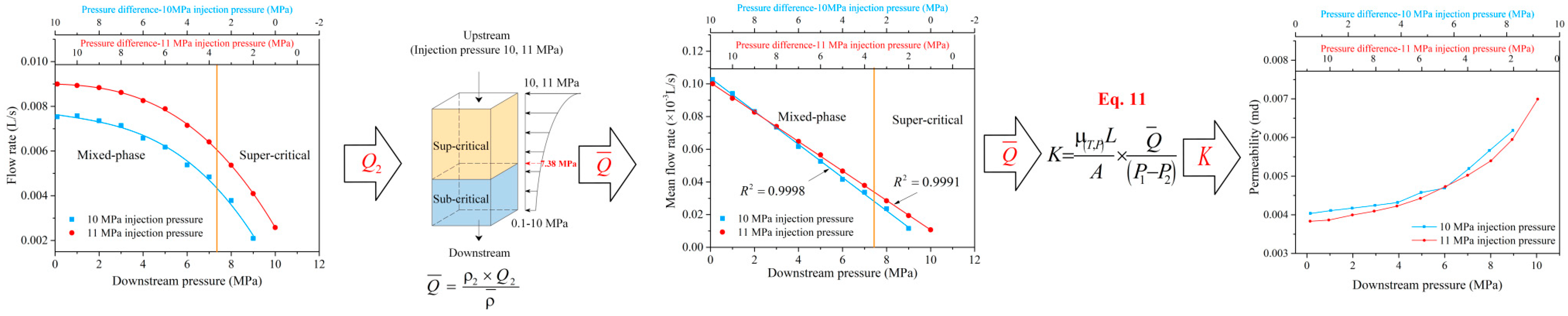
2.5. Permeability Equations for Sub-Critical and Sup-Critical CO2
3. Results and Analysis
3.1. Sub-Critical, Super-Critical, and Mixed-Phase CO2 Flow Behavior
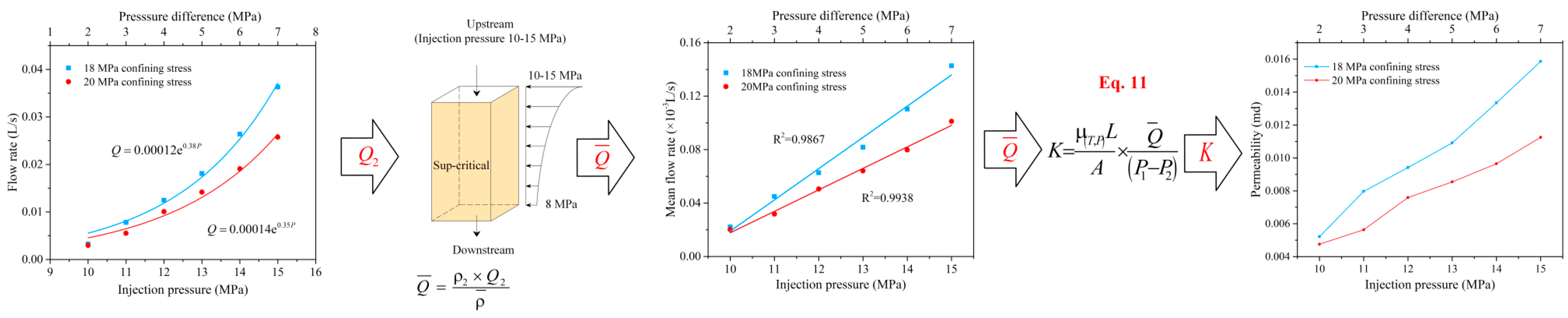
3.2. Sub-Critical, Super-Critical, and Mixed-Phase CO2 Permeability
4. Discussion
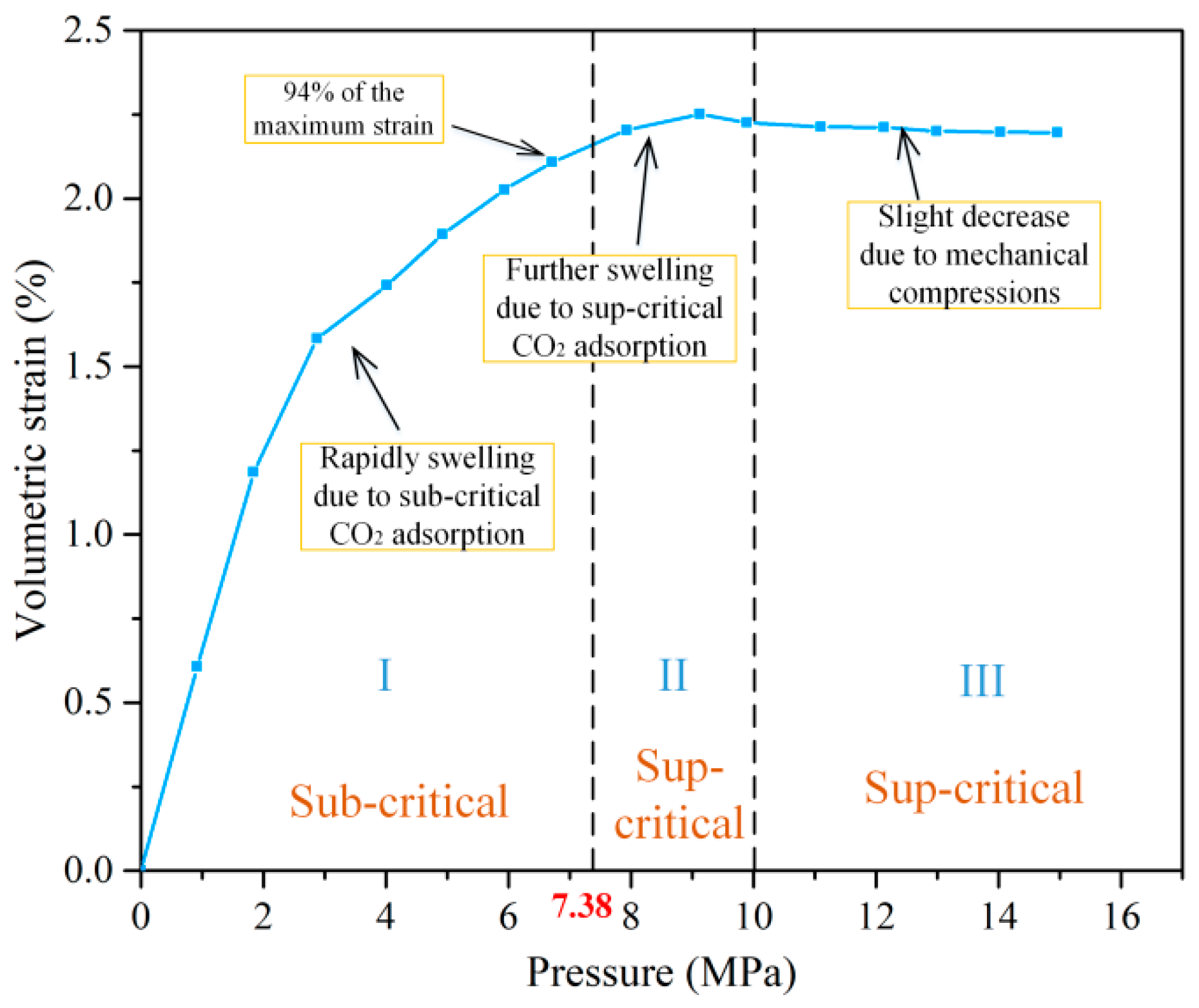
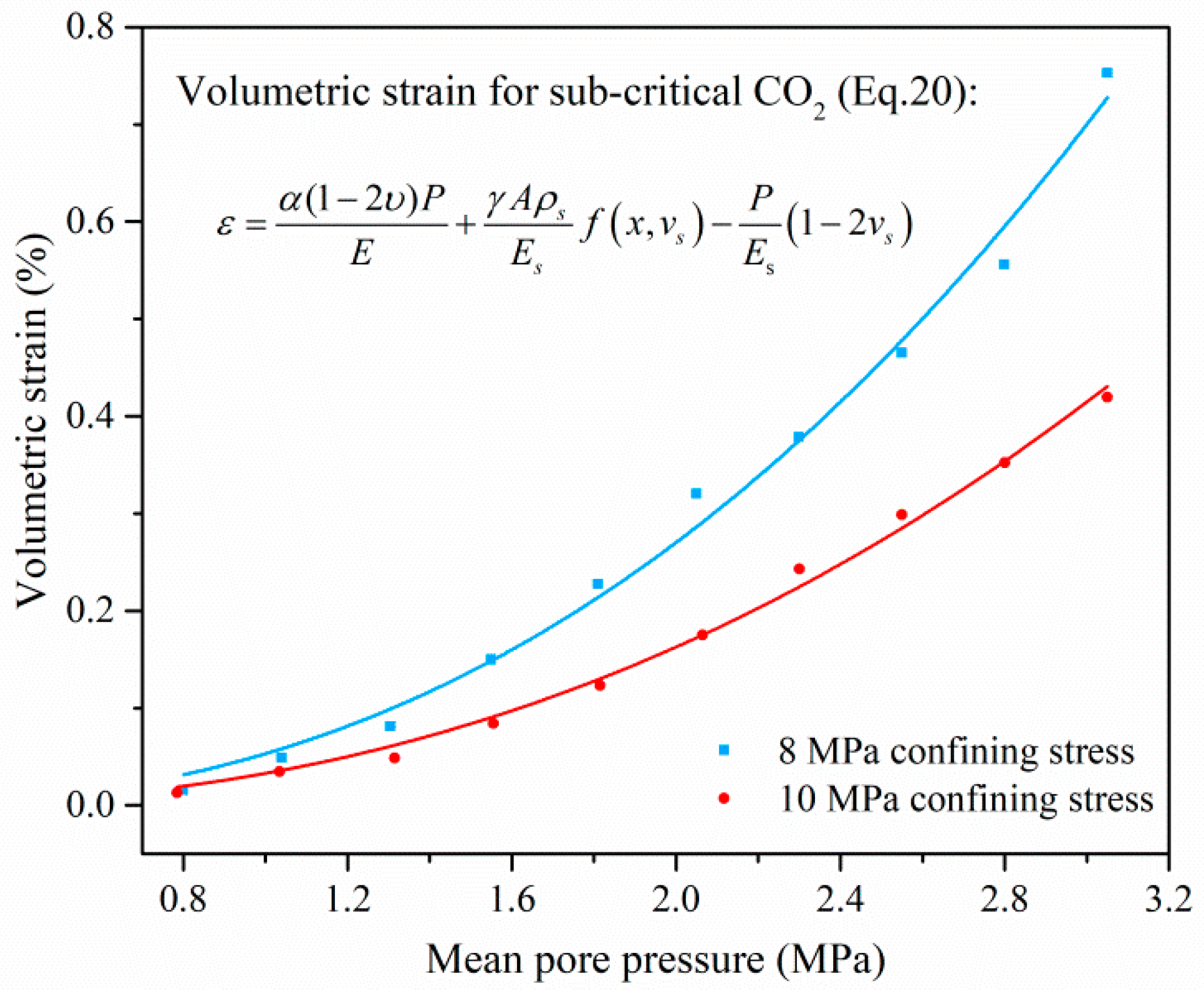
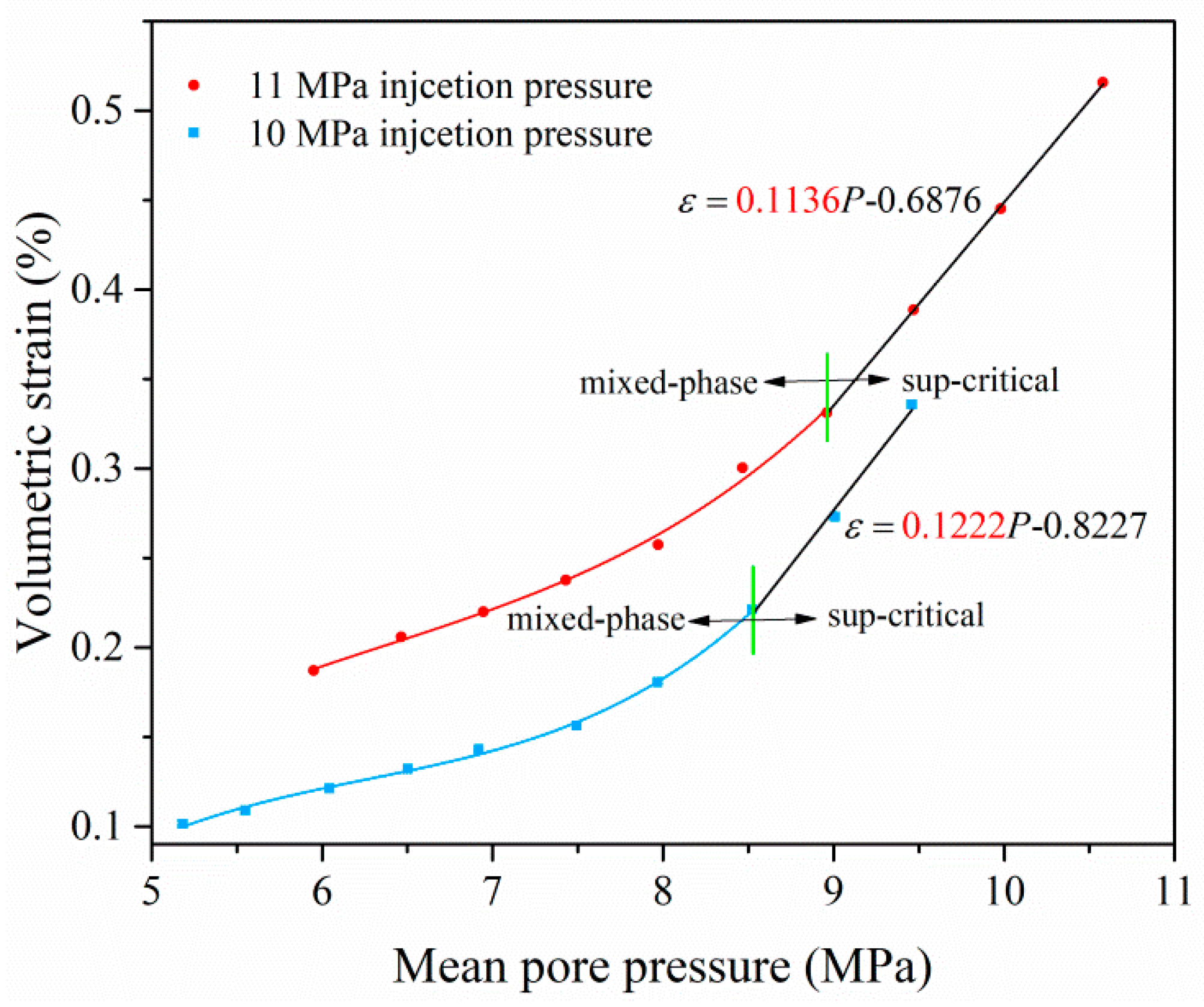
4.1. Mechanism and Behavior of Coal Swelling Due to Different-Phase CO2 Adsorption
4.1.1. Coal Swelling Mechanism Due to Different-Phase CO2 Adsorption
4.1.2. Coal Swelling Behavior Due to Different-Phase CO2 Adsorption
4.2. Mechanism and Behavior of Coal Deformation In Situ Conditions
4.2.1. Coal Deformation Mechanism In Situ Conditions
4.2.2. Coal Deformation Behavior In Situ Conditions
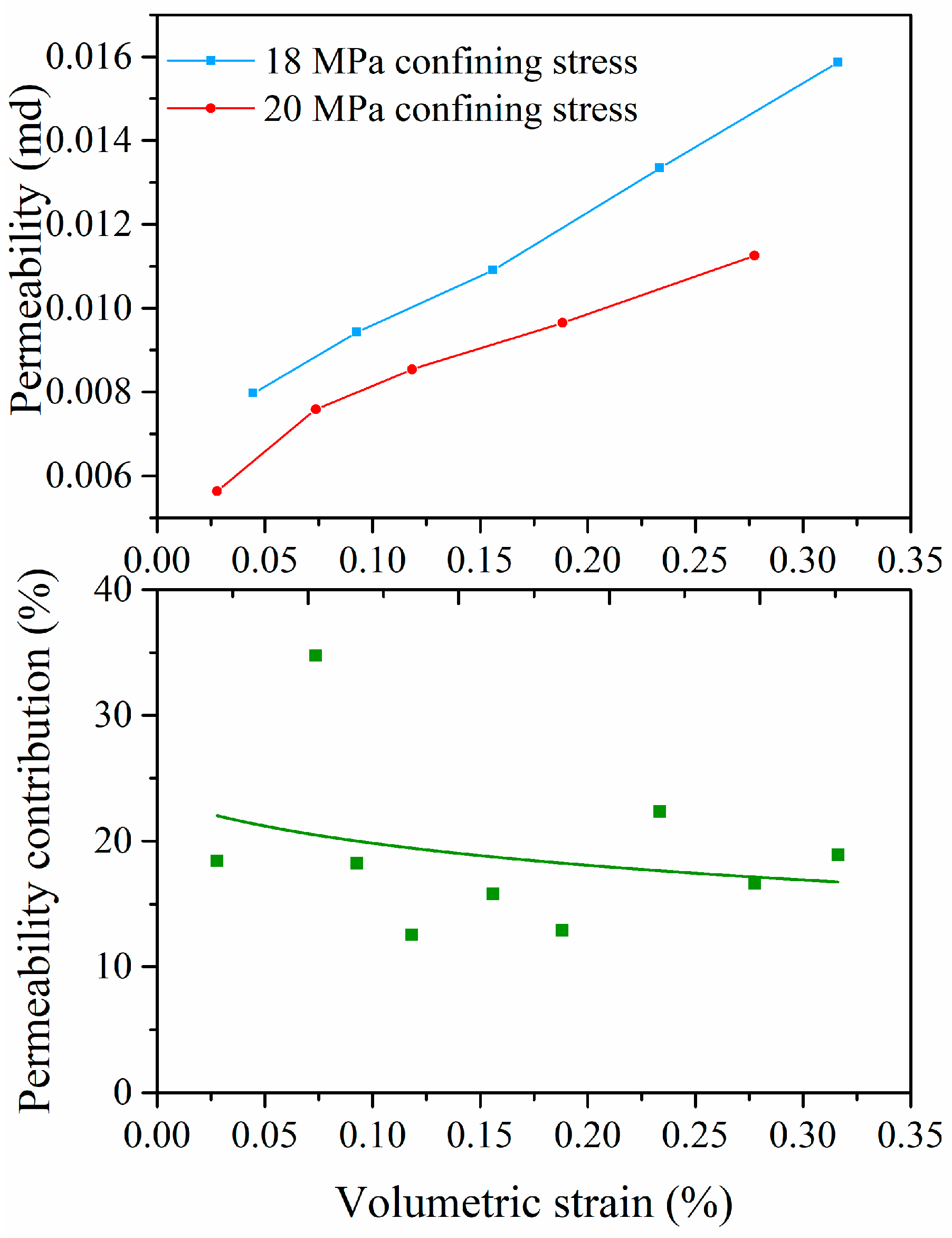
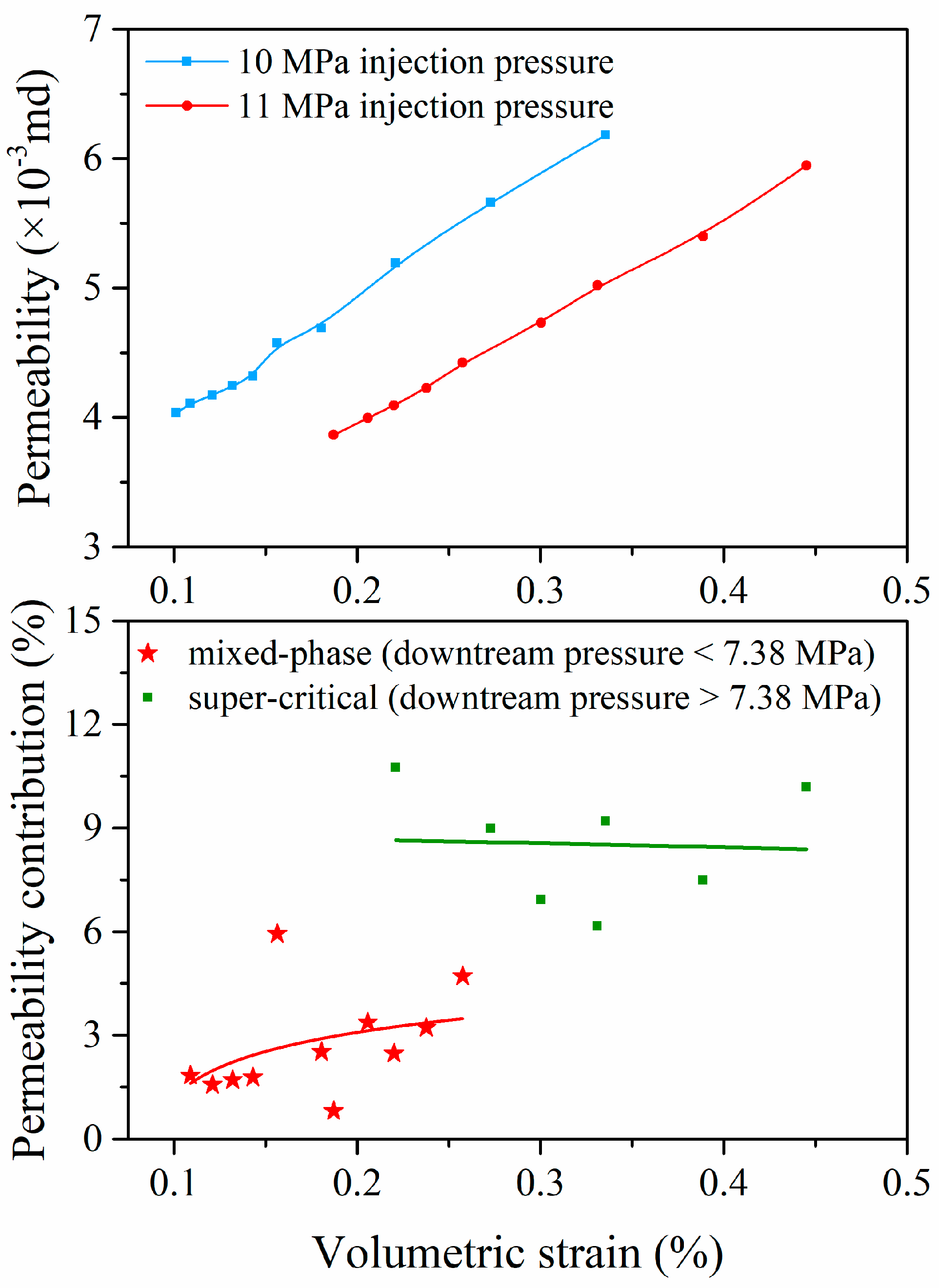
4.3. Effects of Coal Deformation on Permeability
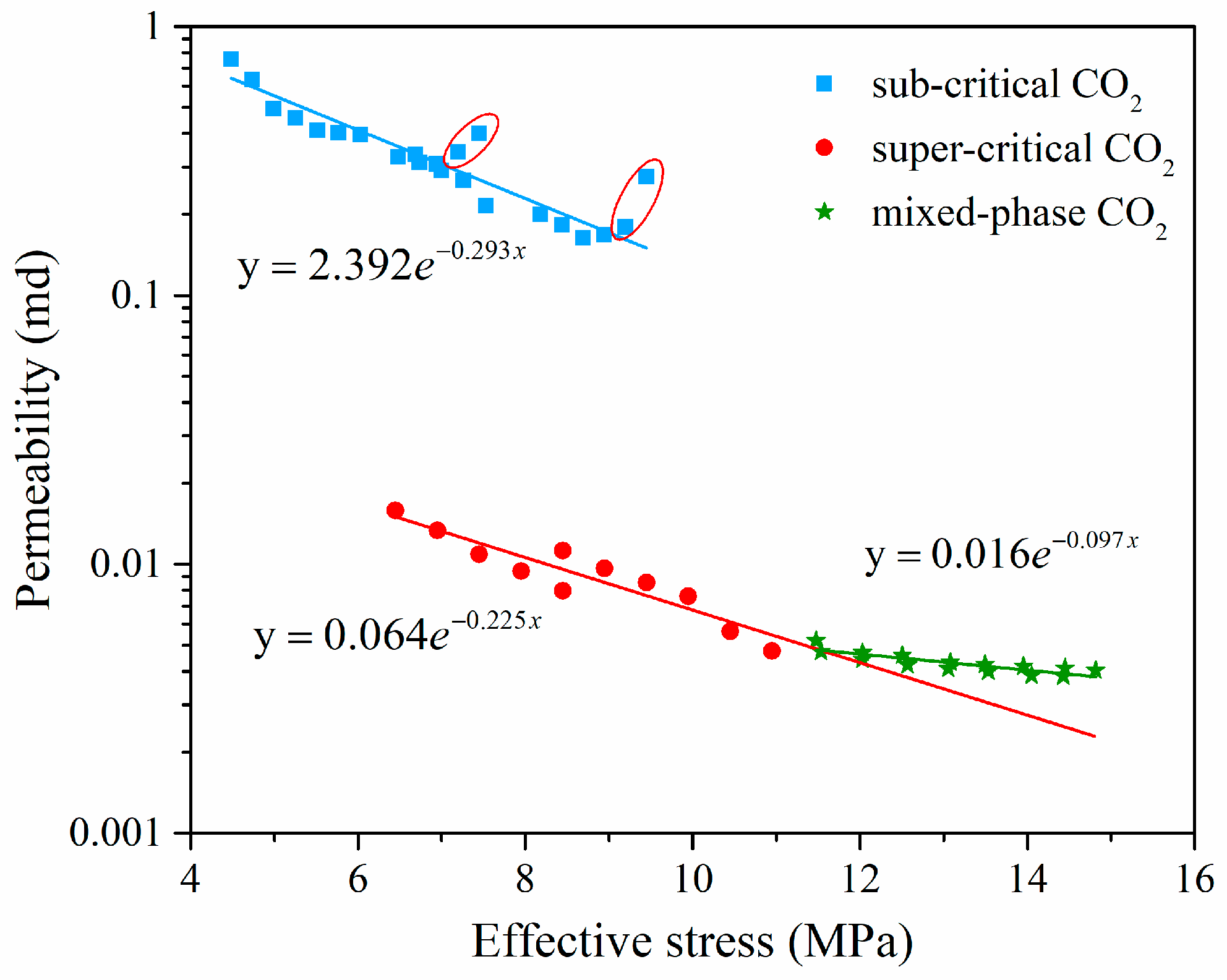
4.4. Effect of Effective Stress on Coal Permeability
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Perera, M.S.A. A Comprehensive Overview of CO2 Flow Behaviour in Deep Coal Seams. Energies 2018, 11, 906. [Google Scholar] [CrossRef]
- Razavi, S.M.R.; Marjani, A.; Shirazian, S. CO2 Capture from Gas Mixtures by Alkanol Amine Solutions in Porous Membranes. Transp. Porous Med. 2015, 106, 323–338. [Google Scholar] [CrossRef]
- Shi, J.; Mazumder, S.; Wolf, K.; Durucan, S. Competitive Methane Desorption by Supercritical CO2 Injection in Coal. Transp. Porous Med. 2008, 75, 35–54. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.; Song, X. Influence of Fluid Exposure on Surface Chemistry and Pore-Fracture Morphology of Various Rank Coals: Implications for Methane Recovery and CO2 Storage. Energy Fuels 2018, 31, 12552–12569. [Google Scholar] [CrossRef]
- Gale, J.J. Using coal seams for CO2 sequestration. Geol. Belg. 2004, 7, 99–103. [Google Scholar]
- Perera, M.S.A.; Ranjith, P.G. Carbon dioxide sequestration effects on coal’s hydro-mechanical properties: A review. Int. J. Energy Res. 2012, 36, 1015–1031. [Google Scholar] [CrossRef]
- Reeves, S.R. Enhanced CBM recovery, coalbed CO2 sequestration assessed. Oil Gas J. 2003, 101, 49–53. [Google Scholar]
- Sing, K.S.W.; Everett, D.H.; Haul, R.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.; Song, X. Multifractal analysis of Hg pore size distributions of tectonically deformed coals. Int. J. Coal Geol. 2015, 144–145, 138–152. [Google Scholar] [CrossRef]
- Wang, S.; Elsworth, D.; Liu, J. Permeability evolution in fractured coal: The roles of fracture geometry and water content. Int. J. Coal Geol. 2011, 87, 13–25. [Google Scholar] [CrossRef]
- Ranathunga, A.S.; Perera, M.S.A.; Ranjith, P.G.; Zhang, X.G.; Wu, B. Super-critical carbon dioxide flow behaviour in low rank coal: A meso-scale experimental study. J. CO₂ Util. 2017, 20, 1–13. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranjith, P.G.; Choi, S.K. Coal cleat permeability for gas movement under triaxial, non-zero lateral strain condition: A theoretical and experimental study. Fuel 2013, 109, 389–399. [Google Scholar] [CrossRef]
- Vishal, V.; Ranjith, P.G.; Singh, T.N. CO2 permeability of Indian bituminous coals: Implications for carbon sequestration. Int. J. Coal Geol. 2013, 105, 36–47. [Google Scholar] [CrossRef]
- Siriwardane, H.; Haljasmaa, I.; McLendon, R.; Irdi, G.; Soong, Y.; Bromhal, G. Influence of carbon dioxide on coal permeability determined by pressure transient methods. Int. J. Coal Geol. 2009, 77, 109–118. [Google Scholar] [CrossRef]
- Pan, Z.; Connell, L.D.; Camilleri, M. Laboratory characterisation of coal reservoir permeability for primary and enhanced coalbed methane recovery. Int. J. Coal Geol. 2010, 82, 252–261. [Google Scholar] [CrossRef]
- Durucan, S.; Ahsan, M.; Shi, J. Matrix shrinkage and swelling characteristics of European coals. Energy Procedia 2009, 1, 3055–3062. [Google Scholar] [CrossRef] [Green Version]
- Kiyama, T.; Nishimoto, S.; Fujioka, M.; Xue, Z.; Ishijima, Y.; Pan, Z.; Connell, L.D. Coal swelling strain and permeability change with injecting liquid/supercritical CO2 and N2 at stress-constrained conditions. Int. J. Coal Geol. 2011, 85, 56–64. [Google Scholar] [CrossRef]
- Wang, G.X.; Wei, X.R.; Wang, K.; Massarotto, P.; Rudolph, V. Sorption-induced swelling/shrinkage and permeability of coal under stressed adsorption/desorption conditions. Int. J. Coal Geol. 2010, 83, 46–54. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, Y.; Wu, D.; Dusseault, M.B. Experiments on methane displacement by carbon dioxide in large coal specimens. Rock Mech. Rock Eng. 2011, 5, 579–589. [Google Scholar] [CrossRef]
- Holloway, S. Underground sequestration of carbon dioxide—A viable greenhouse gas mitigation option. Energy 2005, 30, 2318–2333. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranjith, P.G.; Airey, D.W.; Choi, S.K. Sub- and super-critical carbon dioxide flow behavior in naturally fractured black coal: An experimental study. Fuel 2011, 90, 3390–3397. [Google Scholar] [CrossRef]
- Ranathunga, A.S.; Perera, M.S.A.; Ranjith, P.G.; Ju, Y.; Vishal, V.; De Silva, P.N.K. A macro-scale experimental study of sub-and super-critical CO2 flow behaviour in Victorian brown coal. Fuel 2015, 158, 864–873. [Google Scholar] [CrossRef]
- Krooss, B.M.; Van Bergen, F.; Gensterblum, Y.; Siemons, N.; Pagnier, H.J.M.; David, P. High-pressure methane and carbon dioxide adsorption on dry and moisture-equilibrated Pennsylvanian coals. Int. J. Coal Geol. 2002, 51, 69–92. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranjith, P.G.; Choi, S.K.; Bouazza, A.; Kodikara, J.; Airey, D. A review of coal properties pertinent to carbon dioxide sequestration in coal seams: With special reference to Victorian brown coals. Environ. Earth Sci. 2011, 64, 223–235. [Google Scholar] [CrossRef]
- Lyu, Q.; Long, X.; Ranjith, P.G.; Kang, Y. Unconventional gas: Experimental study of the influence of subcritical carbon dioxide on the mechanical properties of black shale. Energies 2016, 9, 516. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Elsworth, D.; Miao, X.; Mao, X. Evaluation of stress-controlled coal swelling processes. Int. J. Coal Geol. 2010, 83, 446–455. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Lebedev, M.; Sarmadivaleh, M.; Barifcani, A.; Iglauer, S. Multi-scale X-ray computed tomography analysis of coal microstructure and permeability changes as a function of effective stress. Int. J. Coal Geol. 2016, 165, 149–156. [Google Scholar] [CrossRef]
- Durucan, S.; Edwards, J.S. The effects of stress and fracture on permeability of coal. Min. Sci. Technol. 1986, 3, 205–216. [Google Scholar] [CrossRef]
- Xie, J.; Gao, M.; Yu, B.; Zhang, R.; Jin, W. Coal permeability model on the effect of gas extraction within effective influence zone. Geomech. Geophys. Geo-Energy Geo-Resour. 2015, 1, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Robertson, E.P.; Christiansen, R.L. Measurement of sorption induced strain. In Proceedings of the 2005 International Coalbed Methane Symposium, Tuscaloosa, AL, USA, 17–19 May 2005. [Google Scholar]
- Wang, S.; Elsworth, D.; Liu, J. Evolution of permeability in coal to sorbing gases: A Preliminary Study. In Proceedings of the 44th U.S. Rock Mechanics Symposium, Salt Lake City, UT, USA, 27–30 June 2010. [Google Scholar]
- Ranathunga, A.S.; Perera, M.S.A.; Ranjith, P.G.; Wei, C.H. An experimental investigation of applicability of CO2 enhanced coal bed methane recovery to low rank coal. Fuel 2017, 189, 391–399. [Google Scholar] [CrossRef]
- Goodman, A.L.; Busch, A.; Duffy, G.J.; Fitzgerald, J.E.; Gasem, K.A.M.; Gensterblum, Y.; Krooss, B.M.; Levy, J.; Ozdemir, E.; Pan, Z.; et al. An Inter-laboratory Comparison of CO2 Isotherms Measured on Argonne Premium Coal Samples. Energy Fuels 2004, 18, 1175–1182. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, Y.; Liu, B.; Li, S.; Song, W.; Lin, W. Supercritical Pure Methane and CO2 Adsorption on Various Rank Coals of China: Experiments and Modeling. Energy Fuels 2011, 25, 1891–1899. [Google Scholar] [CrossRef]
- Span, R.; Wagner, W. A New Equation of State for Carbon Dioxide Covering the Fluid Region from the Triple Point Temperature to 1100 K at Pressures up to 800 MPa. JPCRD 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Darcy, H. Les Fontaines Publiques de la Ville de Dijon; Dalmont: Paris, France, 1856. [Google Scholar]
- Cheng, L.; Ribatski, G.; Wojtan, L.; Thome, J.R. New flow boiling heat transfer model and flow pattern map for carbon dioxide evaporating inside horizontal tubes. Int. J. Heat Mass Transf. 2006, 49, 4082–4094. [Google Scholar] [CrossRef]
- Liu, H.H.; Rutqvist, J. A new coal-permeability model: Internal swelling stress and fracture–matrix interaction. Transp. Porous Med. 2010, 82, 157–171. [Google Scholar] [CrossRef]
- Zhu, W.C.; Wei, C.H.; Liu, J.; Xu, T.; Elsworth, D. Impact of Gas Adsorption Induced Coal Matrix Damage on the Evolution of Coal Permeability. Rock Mech. Rock Eng. 2013, 46, 1353–1366. [Google Scholar] [CrossRef]
- Larsen, J.W. The effects of dissolved CO2 on coal structure and properties. Int. J. Coal Geol. 2004, 57, 63–70. [Google Scholar] [CrossRef]
- Karacan, C.Ö. Heterogeneous sorption and swelling in a confined and stressed coal during CO2 injection. Energy Fuels 2003, 17, 1595–1608. [Google Scholar] [CrossRef]
- Brenner, D. The macromolecular nature of bituminous coal. Fuel 1985, 64, 167–173. [Google Scholar] [CrossRef]
- Pan, Z.; Connell, L.D. A theoretical model for gas adsorption-induced coal swelling. Int. J. Coal Geol. 2007, 69, 243–252. [Google Scholar] [CrossRef]
- Scherer, G.W. Dilation of porous glass. J. Am. Ceram. Soc. 1986, 69, 473–480. [Google Scholar] [CrossRef]
- Goodman, R.E. Introduction to Rock Mechanics; JohnWiley & Sons: New York, NY, USA, 1980; p. 173. [Google Scholar]
- Day, S.; Fry, R.; Sakurovs, R. Swelling of Australian coals in supercritical CO2. Int. J. Coal Geol. 2008, 74, 41–52. [Google Scholar] [CrossRef]
- Sircar, S. Gibbsian Surface Excess for Gas Adsorption Revisited. Ind. Eng. Chem. Res. 1999, 38, 3670–3682. [Google Scholar] [CrossRef]
- Moffat, D.H.; Weale, K.E. Sorption by coal of methane at high pressures. Fuel 1955, 34, 449–462. [Google Scholar]
- Busch, A.; Alles, S.; Gensterblum, Y.; Prinz, D.; Dewhurst, D.N.; Raven, M.D.; Stanjek, H.; Krooss, B.M. Carbon dioxide storage potential of shales. Int. J. Greenh. Gas Control 2008, 2, 297–308. [Google Scholar] [CrossRef]
- Costanzo, P.A.; Guggenheim, S. Baseline studies of the clay minerals society source clays. Clays Clay Miner. 2001, 49, 371. [Google Scholar] [CrossRef]
- Lyu, Q.; Long, X.; Ranjith, P.G.; Tan, J.; Kang, Y. Experimental investigation on the mechanical behaviours of a low-clay shale under water-based fluids. Eng. Geol. 2018, 233, 124–138. [Google Scholar] [CrossRef]
- Biot, M.A. General theory of three-dimensional consolidation. J. Appl. Phys. 1941, 12, 155–164. [Google Scholar] [CrossRef]
- Chen, Z.; Pan, Z.; Liu, J.; Connell, L.D.; Elsworth, D. Effect of the effective stress coefficient and sorption-induced strain on the evolution of coal permeability: Experimental observations. Int. J. Greenh. Gas Control 2011, 5, 1284–1293. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Wei, J.; Yang, D. The experimental approach to effective stress law of coal mass by effect of methane. Transp. Porous Med. 2003, 53, 235–244. [Google Scholar]
- Zhao, Y.; Hu, Y.; Zhao, B.; Yang, D. Nonlinear coupled mathematical model for solid deformation and gas seepage in fractured media. Transp. Porous Med. 2004, 55, 119–136. [Google Scholar] [CrossRef]
- Pan, Z.; Connell, L.D. Modelling permeability for coal reservoirs: A review of analytical models and testing data. Int. J. Coal Geol. 2012, 92, 1–44. [Google Scholar] [CrossRef]
- Izadi, G.; Wang, S.G.; Elsworth, D.; Liu, J.; Wu, Y.; Pone, D. Permeability evolution of fluid-infiltrated coal containing discrete fractures. Int. J. Coal Geol. 2011, 85, 202–211. [Google Scholar] [CrossRef]
- Klinkenberg, L.J. The permeability of porous media to liquids and gases. In Drilling and Production Practice; American Petroleum Institute: New York, NY, USA, 1 January 1941. [Google Scholar]
- Laubach, S.E.; Marrett, R.A.; Olson, J.E.; Scott, A.R. Characteristics and origins of coal cleat: A review. Int. J. Coal Geol. 1998, 35, 175–207. [Google Scholar] [CrossRef]
- Ranjith, P.G.; Perera, M.S.A. A new triaxial apparatus to study the mechanical and fluid flow aspects of carbon dioxide sequestration in geological formations. Fuel 2011, 90, 2751–2759. [Google Scholar] [CrossRef]
- Ranathunga, A.S.; Perera, M.S.A.; Ranjith, P.G.; Silva, G.P.D.D. A macro-scale view of the influence of effective stress on carbon dioxide flow behaviour in coal: An experimental study. Geomech. Geophys. Geo-Energy Geo-Resour. 2017, 3, 13–28. [Google Scholar] [CrossRef]
- Lyu, Q.; Long, X.; Ranjith, P.G.; Tan, J.; Kang, Y.; Wang, Z. Experimental investigation on the mechanical properties of a low-clay shale with different adsorption times in sub-/super-critical CO2. Energy 2018, 147, 1288–1298. [Google Scholar] [CrossRef]
- Harpalani, S.; Chen, G. Influence of gas production induced volumetric strain on permeability of coal. Geotech. Geol. Eng. 1997, 15, 303–325. [Google Scholar] [CrossRef]






| Rank | Country | Gas | Confining Pressure (MPa) | Gas Pressure (MPa) | Sample Size (mm) | Permeability (μm2) | Refs. |
|---|---|---|---|---|---|---|---|
| Anthracite | America | He, CH4, CO2 | 6–12 | 1–6 | φ25 × 46.7 | 10−10–10−6 | Wang et al. [10] |
| Brown coal | Australia | N2, CO2 | 11–17 | 6–14 | φ38 × 76 | 10−9–10−7 | Ranathunga et al. [11] |
| Bituminous | Australia | N2, CO2 | 5–20 | 2–9 | φ38 × 76 | 10−7–10−6 | Perera et al. [12] |
| Bituminous | India | CO2 | 5–13 | 1–5 | φ39 × 76 | 10−7–10−5 | Vishal et al. [13] |
| Bituminous | America | Ar, CO2 | 7–20 | 4–10 | φ37.45~50.4 × 19.15~80.1 | 10−6–10−3 | Siriwardane et al. [14] |
| Bituminous | Australia | CH4, CO2 | 20 | 1–13 | φ45 × 105.5 | 10−4–10−3 | Pan et al. [15] |
| Bituminous, semi-anthracite | France, Germany | CH4, CO2 | 7 | near 0–3.5 | φ50 | 10−6–10−4 | Sevket et al. [16] |
| Bituminous | Japan | N2, CO2 | 2–12 | 0.1–11 | φ50 × 125 | 10−6–10−3 | Kiyama et al. [17] |
| Bituminous | Australia | CH4, CO2 | 3.1–4.5 | 0.4–2 | 80 × 80 × 80 | 10−3 | Wang et al. [18] |
| Bituminous | China | CH4, CO2 | 1.0–5.5 | 0.5–2 | 100 × 100 × 200 | 10−4–10−2 | Liang et al. [19] |
| Coal Rank | Ro,max | Maceral Groups (Volume, %) | Mineral (Volume, %) | Proximate Analysis (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vitrinite | Inertinite | Liptinite | Moisture | Ash Yield | Volatile Matter | Clay | Pyrite | Carbonate | ||
| Sub-bituminous | 0.46 | 46.9 | 48.8 | 4.3 | 6.23 | 17.32 | 24.68 | 4.3 | 0.4 | 4.1 |
| CO2 Phase | Confining Stress (MPa) | Temperature (°C) | Injection Pressure (MPa) | Downstream Pressure (MPa) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub-critical condition | 8 | 50 | 1 | 1.5 | 2 | 2.5 | 3 | 3.5 | 4 | 4.5 | 5 | 5.5 | 6 | 0.1 |
| 10 | 50 | 1 | 1.5 | 2 | 2.5 | 3 | 3.5 | 4 | 4.5 | 5 | 5.5 | 6 | 0.1 | |
| Super-critical condition | 18 | 50 | 10 | 11 | 12 | 13 | 14 | 15 | 8 | |||||
| 20 | 50 | 10 | 11 | 12 | 13 | 14 | 15 | 8 | ||||||
| CO2 Phase | Confining Stress (MPa) | Temperature (°C) | Injection Pressure (MPa) | Downstream Pressure (MPa) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub-critical and super-critical | 20 | 50 | 10 | 0.1 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 20 | 50 | 11 | 0.1 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| CO2 Phase | Pressure Difference (MPa) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub-critical a | 0.9 | 1.4 | 1.9 | 2.4 | 2.9 | 3.4 | 3.9 | 4.4 | 4.9 | 5.4 | 5.9 |
| Super-critical b | 2 | 3 | 4 | 5 | 6 | 7 | |||||
| Mixed-phase c | 9.9 | 9 | 8 | 7 | 6 | 5 | 4 | 3 | 2 | 1 | |
| Mixed-phase d | 10.9 | 10 | 9 | 8 | 7 | 6 | 5 | 4 | 3 | 2 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Liang, W.; Ranjith, P.G.; He, W.; Li, Z.; Zhang, X. Effects of Coal Deformation on Different-Phase CO2 Permeability in Sub-Bituminous Coal: An Experimental Investigation. Energies 2018, 11, 2926. https://doi.org/10.3390/en11112926
Zhang B, Liang W, Ranjith PG, He W, Li Z, Zhang X. Effects of Coal Deformation on Different-Phase CO2 Permeability in Sub-Bituminous Coal: An Experimental Investigation. Energies. 2018; 11(11):2926. https://doi.org/10.3390/en11112926
Chicago/Turabian StyleZhang, Beining, Weiguo Liang, Pathegama Gamage Ranjith, Wei He, Zhigang Li, and Xiaogang Zhang. 2018. "Effects of Coal Deformation on Different-Phase CO2 Permeability in Sub-Bituminous Coal: An Experimental Investigation" Energies 11, no. 11: 2926. https://doi.org/10.3390/en11112926





