Chemical Characteristics of Biomass Ashes
Abstract
:1. Introduction
2. Materials and Methods
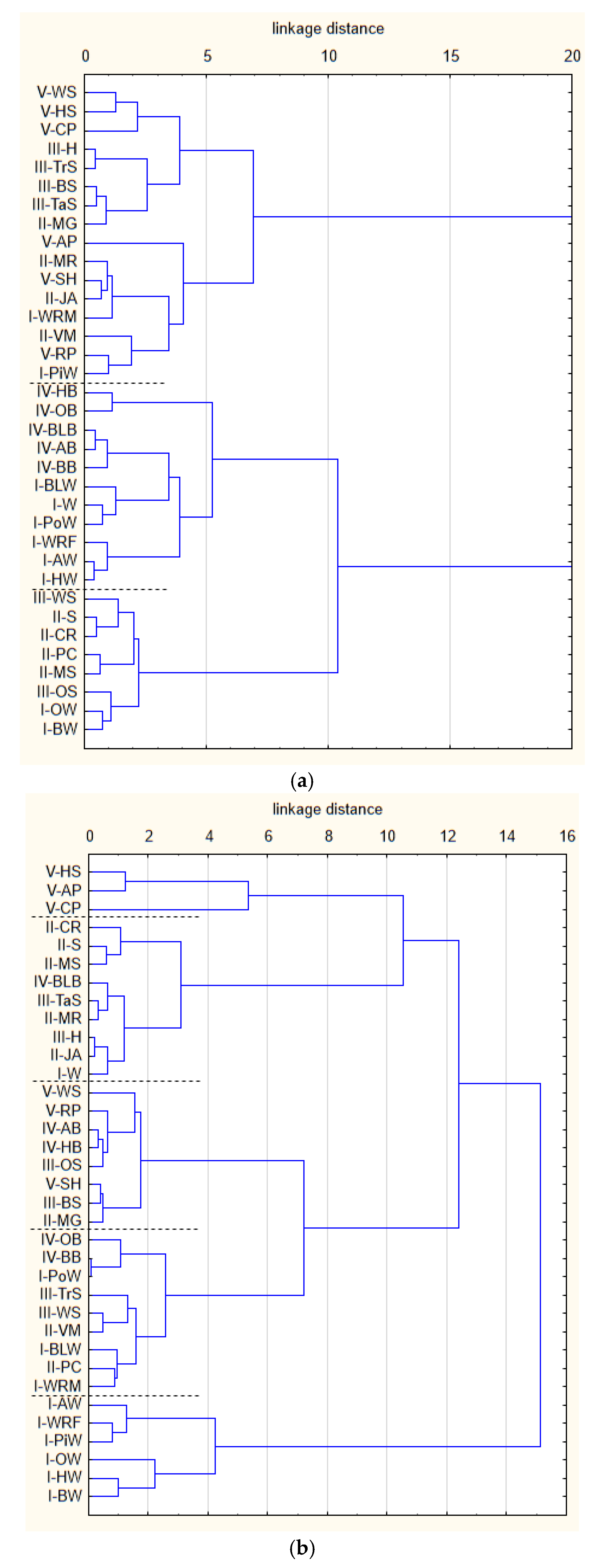
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stolarski, M.J.; Krzyżaniak, M.; Warmiński, K.; Tworkowski, J.; Szczukowski, S. Willow biomass energy generation efficiency and greenhouse gas reduction potential. Pol. J. Environ. Stud. 2015, 24, 2627–2640. [Google Scholar] [CrossRef]
- Burg, P.; Ludín, D.; Rutkowski, K.; Krakowiak-Bal, A.; Trávníček, P.; Zemánek, P.; Turan, J.; Višacki, V. Calorific evaluation and energy potential of grape pomace. Int. Agrophys. 2016, 30, 261–265. [Google Scholar] [CrossRef] [Green Version]
- Viktarovich, N.; Czechowska-Kosacka, A. Production from Biomass in a Trigeneration System. Rocz. Ochr. Sr. 2016, 18, 1007–1017. [Google Scholar]
- Mirowski, T. Utilization of biomass for energy purpose versus reduction of emission of air pollutants from municipal and households sector. Rocz. Ochr. Sr. 2016, 18, 466–477. [Google Scholar]
- Dołżyńska, M.; Obidziński, S. Effect of used cooking oil additive on sewage sludge combustion. Przem. Chem. 2017, 96, 1848–1851. [Google Scholar] [CrossRef]
- Maj, G. Emission Factors and energy properties of agro and forest biomass in aspect of sustainability of energy sector. Energies 2018, 11, 1516. [Google Scholar] [CrossRef]
- Szyszlak-Bargłowicz, J.; Zając, G.; Kuranc, A.; Słowik, T.; Dudziak, A.; Stoma, M.; Wasilewski, J. Chemical properties of selected agri-food industry waste products in the aspect of their use for energetics purposes. Przem. Chem. 2018, 97, 779–783. [Google Scholar] [CrossRef]
- Chocyk, D.; Gładyszewska, B.; Ciupak, A.; Oniszczuk, T.; Mościcki, L.; Rejak, A. Influence of water addition on mechanical properties of thermoplastic starch foils. Int. Agrophys. 2015, 29, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Oniszczuk, T.; Pilawka, R. Effect of cellulose fibers on thermal strength of thermoplastic starch. Przemysł Chemiczny 2013, 92, 265–269. [Google Scholar]
- Sornek, K.; Filipowicz, M.; Rzepka, K. Study of clean combustion of wood in a stove-fireplace with accumulation. J. Energy Inst. 2017, 90, 613–623. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Koniuszy, A.; Mikiciuk, M.; Izwikow, M.; Stawicki, T.; Sędłak, P. Analysis of ecotoxic influence of waste from the biomass gasification process. Environ. Sci. Pollut. Res. Int. 2017, 24, 15022–15030. [Google Scholar] [CrossRef] [PubMed]
- Antonkiewicz, J. Use incineration ash for binding heavy metals in soils. Environ. Protec. Nat. Resour. 2009, 41, 398–405. [Google Scholar]
- Kajda-Szcześniak, M. Characteristics of ashes from fireplace. Arch. Waste Manag. Environ. Protect. 2014, 16, 73–78. [Google Scholar]
- Ciesielczuk, T.; Kusza, G.; Nems, A. Fertilization with biomass ashes as a source of trace elements for soils. Environ. Protect. Nat. Res. 2011, 49, 219–227. [Google Scholar]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 1. Phase–mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash.: Part 2. Potential utilisation, technological and ecological advantages and challenges. Fuel 2013, 105, 19–39. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Baxter, D. Trace element concentrations and associations in some biomass ashes. Fuel 2014, 129, 292–313. [Google Scholar] [CrossRef]
- Schiemenz, K.; Eichler-Löbermann, B. Biomass ashes and their phosphorus fertilizing effect on different crops. Nutr. Cycl. Agroecosyst. 2010, 87, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Meller, E.; Bilenda, E. Effects of biomass ash on the physicochemical properties of light soil. Energy Policy J. 2012, 15, 287–292. [Google Scholar]
- Eriksson, J.-E.; Khazraie, T.; Hupa, L. Different Methods for the Characterization of Ash Compositions in Co-Firing Boilers. In Energy Technology 2018; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018; pp. 253–263. [Google Scholar]
- Demirbas, A. Heavy metal contents of fly ashes from selected biomass samples. Energy Sources 2005, 27, 1269–1276. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef] [Green Version]
- Reumerman, P.; Van den Berg, D. Reduction of Fouling, Slagging and Corrosion Characteristics of Miscanthus. (The BIOMIS Project) Report. Available online: http://cordis.europa.eu/project/rcn/48287_en.html (accessed on 24 June 2018).
- Száková, J.; Ochecová, P.; Hanzlíček, T.; Perná, I.; Tlustoš, P. Variability of total and mobile element contents in ash derived from biomass combustion. Chem. Pap. 2013, 67, 1376–1385. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion of biomass. Energy Sources 2007, 29, 549–561. [Google Scholar] [CrossRef]
- Demirbas, A. trace metal concentrations in ashes from various types of biomass species. Energy Sources 2003, 25, 743–751. [Google Scholar] [CrossRef]
- Thy, P.; Yu, C.; Jenkins, B.M.; Lesher, C.E. Inorganic composition and environmental impact of biomass feedstock. Energy Fuels 2013, 27, 3969–3987. [Google Scholar] [CrossRef]
- Umamaheswaran, K.; Batra, V.S. Physico-chemical characterisation of Indian biomass ashes. Fuel 2008, 87, 628–638. [Google Scholar] [CrossRef]
- Wang, G.; Shen, L.; Sheng, C. Characterization of biomass ashes from power plants firing agricultural residues. Energy Fuels 2012, 26, 102–111. [Google Scholar] [CrossRef]
- Vamvuka, D.; Kakaras, E. Ash properties and environmental impact of various biomass and coal fuels and their blends. Fuel Process. Technol. 2011, 92, 570–581. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.; Morgan, T.J. An overview of the organic and inorganic phase composition of biomass. Fuel 2012, 94, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, Y.; Shimogaki, H.; Abe, H.; Kagawa, A. Inorganic elements in typical Japanese trees for woody biomass fuel. J. Wood. Sci. 2010, 56, 53–63. [Google Scholar] [CrossRef]
- Monti, A.; Di Virgilio, N.; Venturi, G. Mineral composition and ash content of six major energy crops. Biomass Bioenergy 2008, 32, 216–223. [Google Scholar] [CrossRef] [Green Version]

| Group of Origin | Name | Code |
|---|---|---|
| I-WWB Wood and woody biomass | Birch wood (Betula L.) | I-BW |
| Pine wood (Pinus L.) | I-PiW | |
| Oak wood (Quercus L.) | I-OW | |
| Hornbeam wood (Carpinus L.) | I-HW | |
| Ash wood (Fraxinus L.) | I-AW | |
| Wood residue chips—Forest | I-WRF | |
| Wood residue chips—Municipal | I-WRM | |
| Poplar wood (Populus L.) | I-PoW | |
| Willow (Salix viminalis) | I-W | |
| Acacia wood (Robinia pseudoacacia L.) | I-BLW | |
| II-EC Energy Crops | Virginia mallow (Sida hermaphorodita R.) | II-VM |
| Jerusalem artichoke (Helianthus tuberosus L.) | II-JA | |
| Multiflorous rose (Rosa polyantha) | II-MR | |
| Miscanthus giganteus (Miscanthus sinensis gigantea) | II-MG | |
| Miscanthus sacchariflorus (Miscanthus sacchariflorus) | II-MS | |
| Prairie cordgrass (Spartina pectinata) | II-PC | |
| Common reed (Phragmites australis) | II-CR | |
| Switchgrass (Panicum virgatum) | II-S | |
| III-AB Agricultural Biomass | Wheat straw | III-WS |
| Triticale straw | III-TrS | |
| Oat straw | III-OS | |
| Barley Straw | III-TaS | |
| Buckwheat straw | III-BS | |
| Hay | III-H | |
| IV-FR Forest Residue | Beech bark | IV-BB |
| Oak bark | IV-OB | |
| Hornbeam bark | IV-HB | |
| Ash bark | IV-AB | |
| Acacia bark | IV-BLB | |
| V-AFIW Agri-Food Industry Wastes | Cherry pits | V-CP |
| Sunflower husk | V-SH | |
| Rape pods | V-RP | |
| Apple pomace | V-AP | |
| Hazelnut shell | V-HS | |
| Walnut shell | V-WS |
| Code | Feature | Ash | P | K | Ca | S | Cu | Fe | Mn | Zn | Ni | Cr | Pb | As |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I-BW | mean | 0.67 | 20,853 | 71,290 | 132,583 | 5631 | 97.10 | 6 518 | 17,585 | 212.67 | 34.91 | 39.07 | 40.48 | 1.01 |
| SD | 0.069 | 404.17 | 1370.92 | 3195.21 | 88.05 | 0.69 | 79.75 | 503.38 | 3.06 | 1.02 | 0.81 | 0.19 | 0.02 | |
| I-PiW | mean | 0.59 | 18,618 | 116,436 | 201,109 | 7 142 | 196.00 | 3 665 | 10,693 | 193.13 | 45.84 | 62.04 | 28.89 | 1.59 |
| SD | 0.046 | 459.43 | 1419.16 | 3407.62 | 89.46 | 1.73 | 32.65 | 244.24 | 1.12 | 1.18 | 2.99 | 0.46 | 0.32 | |
| I-OW | mean | 0.37 | 15,071 | 57,331 | 156,738 | 5107 | 190.67 | 9256 | 10,114 | 169.33 | 125.67 | 89.87 | 54.49 | 1.91 |
| SD | 0.008 | 52.85 | 5433.49 | 3508.10 | 317.16 | 2.31 | 183.01 | 376.43 | 6.11 | 4.04 | 1.70 | 3.86 | 0.08 | |
| I-HW | mean | 0.73 | 16,548 | 69,905 | 249,050 | 3956 | 140.67 | 8598 | 18,587 | 155.00 | 158.67 | 10.65 | 40.20 | 1.13 |
| SD | 0.034 | 492.33 | 1341.71 | 517.41 | 30.81 | 30.11 | 437.53 | 631.85 | 18.45 | 8.62 | 0.28 | 3.47 | 0.05 | |
| I-AW | mean | 0.43 | 17,967 | 70,442 | 279,785 | 3077 | 121.00 | 5758 | 10,545 | 183.00 | 24.84 | 30.66 | 15.31 | 0.78 |
| SD | 0.017 | 339.13 | 281.71 | 4991.02 | 32.08 | 2.00 | 56.30 | 10.00 | 2.65 | 0.47 | 0.84 | 0.17 | 0.14 | |
| I-WRF | mean | 0.96 | 17,680 | 69,104 | 203,935 | 1546 | 188.00 | 3403 | 6920 | 171.00 | 110.33 | 95.64 | 50.67 | 1.44 |
| SD | 0.065 | 198.95 | 976.94 | 3949.64 | 204.58 | 1.00 | 33.65 | 172.42 | 1.00 | 1.15 | 2.74 | 0.53 | 0.11 | |
| I-WRM | mean | 1.79 | 32,039 | 108,081 | 245,075 | 8464 | 181.00 | 4678 | 2815 | 320.33 | 176.33 | 25.00 | 12.69 | 0.13 |
| SD | 0.323 | 59.77 | 1096.39 | 5387.04 | 79.08 | 1.00 | 49.65 | 227.07 | 4.16 | 1.53 | 1.11 | 0.28 | 0.02 | |
| I-PoW | mean | 0.91 | 6419 | 64,985 | 173,872 | 5015 | 96.92 | 4612 | 549.67 | 81.41 | 26.19 | 20.57 | 9.65 | 0.18 |
| SD | 0.039 | 455.55 | 57.47 | 2696.99 | 1.00 | 3.34 | 2.08 | 10.12 | 1.05 | 0.06 | 0.18 | 0.22 | 0.01 | |
| I-W | mean | 0.38 | 3342 | 37,339 | 135,981 | 4732 | 123.50 | 2662 | 910 | 394.00 | 32.00 | 45.97 | 8.93 | 0.34 |
| SD | 0.073 | 17.58 | 280.22 | 1052.50 | 153.52 | 0.92 | 7.23 | 11.79 | 7.81 | 0.41 | 0.20 | 0.14 | 0.04 | |
| I-BLW | mean | 0.45 | 2679 | 38,799 | 227,225 | 1826 | 158.00 | 6156 | 794.33 | 244.00 | 59.72 | 36.31 | 15.83 | 0.49 |
| SD | 0.027 | 443.92 | 1088.85 | 6719.70 | 38.03 | 3.61 | 124.65 | 69.02 | 6.24 | 1.17 | 1.48 | 0.38 | 0.01 | |
| CV [%] | 58.86 | 56.99 | 34.99 | 24.25 | 45.23 | 25.59 | 38.05 | 81.76 | 40.42 | 60.60 | 61.21 | 70.57 | 67.43 | |
| II-VM | mean | 3.49 | 16,777 | 139,234 | 276,611 | 13,038 | 67.89 | 5445 | 909.67 | 157.00 | 83.89 | 47.51 | 28.19 | 0.93 |
| SD | 0.090 | 349.16 | 1766.96 | 4580.64 | 137.79 | 1.47 | 92.70 | 16.77 | 3.00 | 1.74 | 0.97 | 0.54 | 0.17 | |
| II-JA | mean | 3.77 | 31,990 | 154,359 | 193,316 | 7877 | 72.62 | 2536 | 143.33 | 463.67 | 35.83 | 50.61 | 6.43 | 0.32 |
| SD | 0.124 | 824.66 | 3157.97 | 3392.32 | 200.82 | 0.35 | 8.50 | 1.53 | 1.15 | 0.74 | 1.42 | 0.07 | 0.05 | |
| II-MR | mean | 3.21 | 26,246 | 139,384 | 217,339 | 6280 | 33.00 | 1875 | 788.00 | 413.00 | 52.58 | 23.14 | 6.11 | 0.36 |
| SD | 0.323 | 900.99 | 3791.34 | 5908.95 | 602.48 | 0.88 | 24.09 | 2.00 | 8.72 | 1.06 | 0.72 | 0.03 | 0.09 | |
| II-MG | mean | 3.63 | 24,849 | 87,462 | 46,491 | 7510 | 70.70 | 2511 | 625.33 | 222.00 | 45.65 | 60.87 | 9.23 | 0.31 |
| SD | 0.054 | 163.72 | 962.88 | 339.15 | 65.59 | 0.10 | 25.24 | 3.06 | 2.65 | 0.29 | 0.47 | 0.14 | 0.06 | |
| II-MS | mean | 3.61 | 21,323 | 49,658 | 36,409 | 2459 | 81.55 | 4464 | 529.00 | 458.00 | 51.98 | 63.77 | 7.27 | 0.39 |
| SD | 0.086 | 430.84 | 948.69 | 361.63 | 102.19 | 0.49 | 32.91 | 2.00 | 2.00 | 0.10 | 0.21 | 0.08 | 0.08 | |
| II-PC | mean | 5.38 | 22,914 | 32,556 | 78,914 | 1384 | 54.99 | 4752 | 681.33 | 319.33 | 32.73 | 50.29 | 13.03 | 0.65 |
| SD | 0.260 | 706.17 | 932.43 | 1030.57 | 51.96 | 1.44 | 167.48 | 24.44 | 9.24 | 1.39 | 2.21 | 0.37 | 0.09 | |
| II-CR | mean | 2.36 | 11,624 | 41,517 | 55,077 | 1135 | 44.27 | 3199 | 1146 | 582.33 | 36.10 | 44.48 | 14.65 | 0.13 |
| SD | 0.035 | 266.58 | 146.07 | 91.82 | 92.95 | 1.53 | 13.05 | 54.04 | 0.58 | 0.40 | 1.32 | 0.25 | 0.01 | |
| II-S | mean | 7.72 | 14,949 | 53,607 | 48,367 | 2232 | 69.11 | 5371 | 1249 | 534.67 | 32.18 | 64.57 | 28.73 | 0.73 |
| SD | 0.117 | 484.49 | 794.36 | 322.26 | 649.55 | 2.77 | 248.86 | 78.53 | 21.94 | 1.48 | 1.04 | 0.80 | 0.11 | |
| CV [%] | 40.24 | 29.81 | 54.89 | 76.04 | 76.47 | 25.28 | 35.73 | 44.55 | 36.27 | 25.53 | 62.67 | 35.59 | 55.32 | |
| III-WS | mean | 8.19 | 10,137 | 69,319 | 42,145 | 5955 | 27.84 | 4948 | 337.33 | 216.67 | 7.38 | 60.47 | 6.82 | 0.18 |
| SD | 0.212 | 157.82 | 849.32 | 611.31 | 80.55 | 0.32 | 45.83 | 4.04 | 2.08 | 0.14 | 1.92 | 0.07 | 0.07 | |
| III-TrS | mean | 8.97 | 26,225 | 187,587 | 38,350 | 7772 | 43.03 | 7723 | 460.00 | 190.33 | 12.84 | 90.85 | 7.32 | 0.14 |
| SD | 0.321 | 146.65 | 3206.92 | 785.99 | 127.08 | 0.77 | 80.25 | 11.36 | 1.53 | 0.07 | 1.11 | 0.03 | 0.03 | |
| III-OS | mean | 8.70 | 16,439 | 92,168 | 86,196 | 3415 | 22.06 | 1974 | 322.00 | 126.33 | 13.14 | 14.58 | 7.69 | 0.09 |
| SD | 0.673 | 130.31 | 939.36 | 1426.03 | 84.69 | 0.56 | 29.60 | 7.94 | 1.53 | 0.11 | 0.69 | 0.14 | 0.01 | |
| III-TaS | mean | 6.88 | 21,760 | 108,603 | 84,223 | 7877 | 64.88 | 1371 | 321.00 | 388.67 | 8.84 | 20.62 | 4.39 | 0.18 |
| SD | 0.036 | 574.04 | 2752.07 | 1288.73 | 165.08 | 0.25 | 9.42 | 4.36 | 3.21 | 0.13 | 0.85 | 0.24 | 0.02 | |
| III-BS | mean | 9.20 | 22,725 | 131,067 | 77,974 | 6885 | 38.78 | 2677 | 349.00 | 166.00 | 8.24 | 47.94 | 3.90 | 0.08 |
| SD | 0.132 | 272.29 | 742.94 | 106.57 | 17.24 | 0.73 | 30.27 | 4.58 | 1.00 | 0.51 | 1.90 | 0.12 | 0.01 | |
| III-H | mean | 8.07 | 24,799 | 175,174 | 65,546 | 8407 | 51.31 | 2676 | 675.00 | 447.00 | 37.30 | 69.56 | 33.33 | 0.07 |
| SD | 0.084 | 74.85 | 472.58 | 247.79 | 65.29 | 0.07 | 13.75 | 1.00 | 1.73 | 0.33 | 0.90 | 2.75 | 0.01 | |
| CV [%] | 10.02 | 27.84 | 34.45 | 30.11 | 25.68 | 35.48 | 62.56 | 31.95 | 47.84 | 54.29 | 100.3 | 73.05 | 42.89 | |
| IV-BB | mean | 10.18 | 11,355 | 28,763 | 353,721 | 2400 | 104.00 | 4560 | 482.67 | 68.83 | 99.07 | 20.65 | 9.08 | 0.12 |
| SD | 0.126 | 859.23 | 407.75 | 14,448.62 | 46.70 | 2.65 | 125.13 | 13.43 | 1.96 | 1.04 | 0.58 | 0.08 | 0.01 | |
| IV-OB | mean | 6.27 | 3958 | 21,102 | 322,489 | 9600 | 202.67 | 3841 | 418.67 | 145.67 | 48.07 | 31.06 | 24.18 | 0.85 |
| SD | 0.037 | 312.35 | 133.84 | 4099.19 | 104.89 | 4.51 | 59.48 | 2.52 | 2.31 | 0.49 | 3.41 | 0.42 | 0.14 | |
| IV-HB | mean | 9.17 | 3489 | 24,661 | 277,589 | 13,924 | 25.21 | 1088 | 313.33 | 160.33 | 68.57 | 12.49 | 11.23 | 0.13 |
| SD | 0.121 | 206.61 | 379.75 | 4727.50 | 935.15 | 0.89 | 36.10 | 42.92 | 5.03 | 2.43 | 0.10 | 0.30 | 0.01 | |
| IV-AB | mean | 9.14 | 5109 | 23,185 | 311,438 | 3341 | 19.83 | 936.00 | 340.33 | 108.00 | 42.95 | 13.94 | 9.71 | 0.11 |
| SD | 0.096 | 405.12 | 1094.21 | 5794.18 | 78.70 | 0.13 | 13.86 | 9.29 | 4.36 | 0.71 | 0.45 | 0.27 | 0.01 | |
| IV-BLB | mean | 10.44 | 7152 | 19,936 | 302,913 | 1724 | 61.00 | 1503 | 465.33 | 307.33 | 64.70 | 20.07 | 14.03 | 0.98 |
| SD | 0.164 | 958.96 | 127.26 | 6070.13 | 2.65 | 1.75 | 23.29 | 27.54 | 3.21 | 0.75 | 1.59 | 0.21 | 0.21 | |
| CV [%] | 32.04 | 48.49 | 13.72 | 8.50 | 79.91 | 84.30 | 65.59 | 17.88 | 53.17 | 35.37 | 42.04 | 31.62 | 94.90 | |
| V-CP | mean | 1.06 | 9450 | 193,663 | 91,717 | 14,482 | 795.33 | 9281 | 705.33 | 826.33 | 40.23 | 65.53 | 12.35 | 1.58 |
| SD | 0.050 | 118.07 | 3729.06 | 2385.99 | 63.51 | 11.15 | 145.57 | 20.26 | 11.37 | 0.40 | 1.54 | 0.07 | 0.11 | |
| V-SH | mean | 2.94 | 27,752 | 141,783 | 190,313 | 9734 | 91.72 | 3096 | 380.67 | 146.00 | 22.23 | 21.18 | 4.42 | 0.09 |
| SD | 0.14 | 428.64 | 1806.89 | 3094.12 | 117.63 | 0.75 | 31.90 | 8.14 | 1.73 | 0.82 | 0.90 | 0.10 | 0.00 | |
| V-RP | mean | 9.18 | 14,151 | 129,477 | 277,338 | 6063 | 33.18 | 1573 | 269.00 | 45.70 | 23.76 | 18.82 | 3.82 | 0.09 |
| SD | 0.324 | 175.12 | 895.40 | 2094.17 | 21.42 | 0.22 | 9.29 | 3.00 | 0.44 | 0.52 | 0.12 | 0.11 | 0.00 | |
| V-AP | mean | 3.32 | 33,208 | 143,679 | 184,430 | 19,128 | 582.00 | 1716 | 345.67 | 492.33 | 64.33 | 10.67 | 13.82 | 1.72 |
| SD | 0.266 | 176.21 | 3637.03 | 400.83 | 64.50 | 24.56 | 155.18 | 8.39 | 29.02 | 5.64 | 0.81 | 1.23 | 0.42 | |
| V-HS | mean | 5.04 | 15,674 | 184,542 | 109,089 | 9216 | 465.00 | 2144 | 118.33 | 325.33 | 61.64 | 37.62 | 7.56 | 0.13 |
| SD | 0.49 | 308.51 | 5158.78 | 4452.17 | 66.43 | 19.29 | 81.22 | 1.53 | 12.74 | 1.90 | 1.35 | 0.19 | 0.03 | |
| V-WS | mean | 3.76 | 11,981 | 185,001 | 72,773 | 4391 | 202.75 | 1108 | 123.25 | 89.21 | 42.04 | 50.93 | 6.51 | 0.12 |
| SD | 0.18 | 164.99 | 1297.41 | 405.22 | 3.79 | 0.96 | 3.00 | 0.50 | 0.13 | 0.05 | 1.25 | 0.10 | 0.02 | |
| CV [%] | 65.31 | 47.77 | 16.06 | 47.12 | 48.92 | 78.25 | 91.66 | 62.91 | 87.41 | 58.29 | 48.38 | 40.08 | 123.1 | |
| Code | Elements in Decreasing Order |
|---|---|
| I-BW | Ca > K > P > Mn > Fe > S > Zn > Cu > Pb > Cr > Ni > As |
| I-PiW | Ca > K > P > Mn > S > Fe > Cu > Zn > Cr > Ni > Pb > As |
| I-OW | Ca > K > P > Mn > Fe > S > Cu > Zn > Ni > Cr > Pb > As |
| I-HW | Ca > K > Mn > P > Fe > S > Ni > Zn > Cu > Pb > Cr > As |
| I-AW | Ca > K > P > Mn > Fe > S > Zn > Cu > Cr > Ni > Pb > As |
| I-WRF | Ca > K > P > Mn > Fe > S > Cu > Zn > Ni > Cr > Pb > As |
| I-WRM | Ca > K > P > S > Fe > Mn > Zn > Cu > Ni > Cr > Pb > As |
| I-PoW | Ca > K > P > S > Fe > Mn > Cu > Zn > Ni > Cr > Pb > As |
| I-W | Ca > K > S > P > Fe > Mn > Zn > Cu > Cr > Ni > Pb > As |
| I-BLW | Ca > K > Fe > P > S > Mn > Zn > Cu > Ni > Cr > Pb > As |
| II-VM | Ca > K > P > S > Fe > Mn > Zn > Ni > Cu > Cr > Pb > As |
| II-JA | Ca > K > P > S > Fe > Zn > Mn > Cu > Cr > Ni > Pb > As |
| II-MR | Ca > K > P > S > Fe > Mn > Zn > Ni > Cu > Cr > Pb > As |
| II-MG | K > Ca > P > S > Fe > Mn > Zn > Cu > Cr > Ni > Pb > As |
| II-MS | K > Ca > P > Fe > S > Mn > Zn > Cu > Cr > Ni > Pb > As |
| II-PC | Ca > K > P > Fe > S > Mn > Zn > Cu > Cr > Ni > Pb > As |
| II-CR | Ca > K > P > Fe > Mn > S > Zn > Cr > Cu > Ni > Pb > As |
| II-S | K > Ca > P > Fe > S > Mn > Zn > Cu > Cr > Ni > Pb > As |
| III-WS | K > Ca > P > S > Fe > Mn > Zn > Cr > Cu > Ni > Pb > As |
| III-TrS | K > Ca > P > S > Fe > Mn > Zn > Cr > Cu > Ni > Pb > As |
| III-OS | K > Ca > P > S > Fe > Mn > Zn > Cu > Cr > Ni > Pb > As |
| III-TaS | K > Ca > P > S > Fe > Zn > Mn > Cu > Cr > Ni > Pb > As |
| III-BS | K > Ca > P > S > Fe > Mn > Zn > Cr > Cu > Ni > Pb > As |
| III-H | K > Ca > P > S > Fe > Mn > Zn > Cr > Cu > Ni > Pb > As |
| IV-BB | Ca > K > P > Fe > S > Mn > Cu > Ni > Zn > Cr > Pb > As |
| IV-OB | Ca > K > S > P > Fe > Mn > Cu > Zn > Ni > Cr > Pb > As |
| IV-HB | Ca > K > S > P > Fe > Mn > Zn > Ni > Cu > Cr > Pb > As |
| IV-AB | Ca > K > P > S > Fe > Mn > Zn > Ni > Cu > Cr > Pb > As |
| IV-BLB | Ca > K > P > S > Fe > Mn > Zn > Ni > Cu > Cr > Pb > As |
| V-CP | K > Ca > S > P > Fe > Zn > Cu > Mn > Cr > Ni > Pb > As |
| V-SH | Ca > K > P > S > Fe > Mn > Zn > Cu > Ni > Cr > Pb > As |
| V-RP | Ca > K > P > S > Fe > Mn > Zn > Cu > Ni > Cr > Pb > As |
| V-AP | Ca > K > P > S > Fe > Cu > Zn > Mn > Ni > Pb > Cr > As |
| V-HS | K > Ca > P > S > Fe > Cu > Zn > Mn > Ni > Cr > Pb > As |
| V-WS | K > Ca > P > S > Fe > Cu > Mn > Zn > Cr > Ni > Pb > As |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. https://doi.org/10.3390/en11112885
Zając G, Szyszlak-Bargłowicz J, Gołębiowski W, Szczepanik M. Chemical Characteristics of Biomass Ashes. Energies. 2018; 11(11):2885. https://doi.org/10.3390/en11112885
Chicago/Turabian StyleZając, Grzegorz, Joanna Szyszlak-Bargłowicz, Wojciech Gołębiowski, and Małgorzata Szczepanik. 2018. "Chemical Characteristics of Biomass Ashes" Energies 11, no. 11: 2885. https://doi.org/10.3390/en11112885






