Gasification Performance of a Top-Lit Updraft Cook Stove
Abstract
:1. Introduction
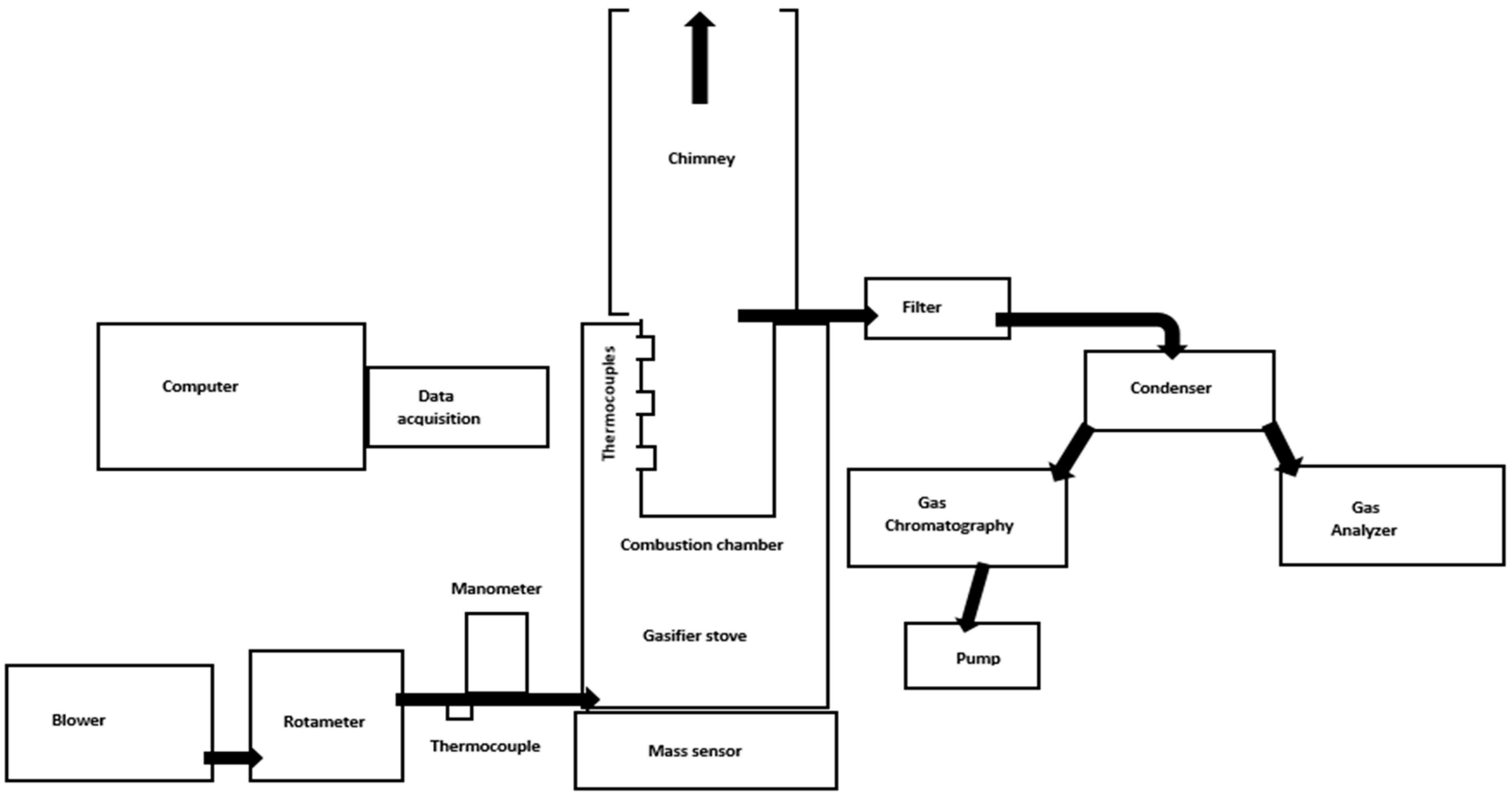
2. Materials and Methods
3. Results and Discussion
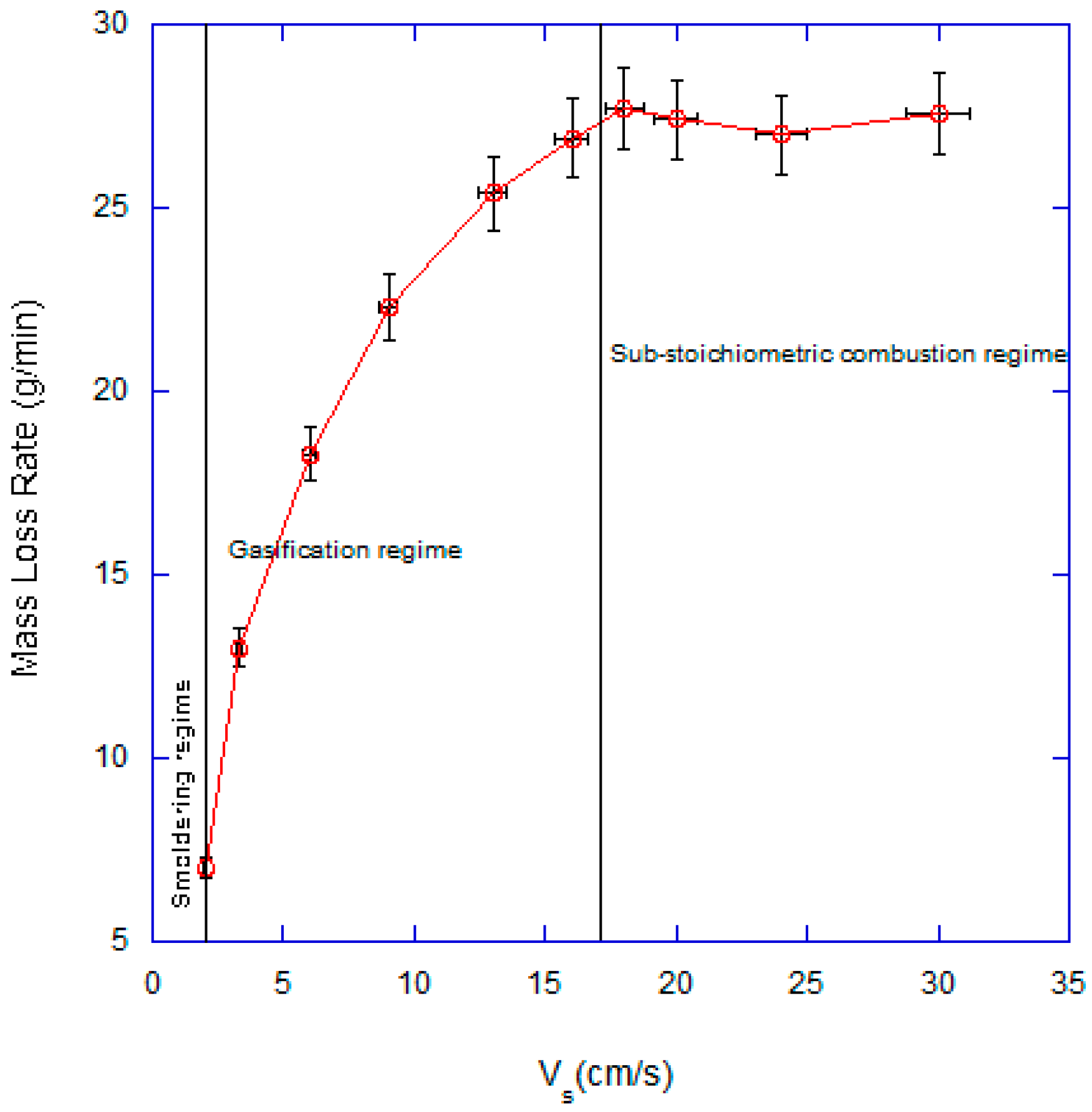
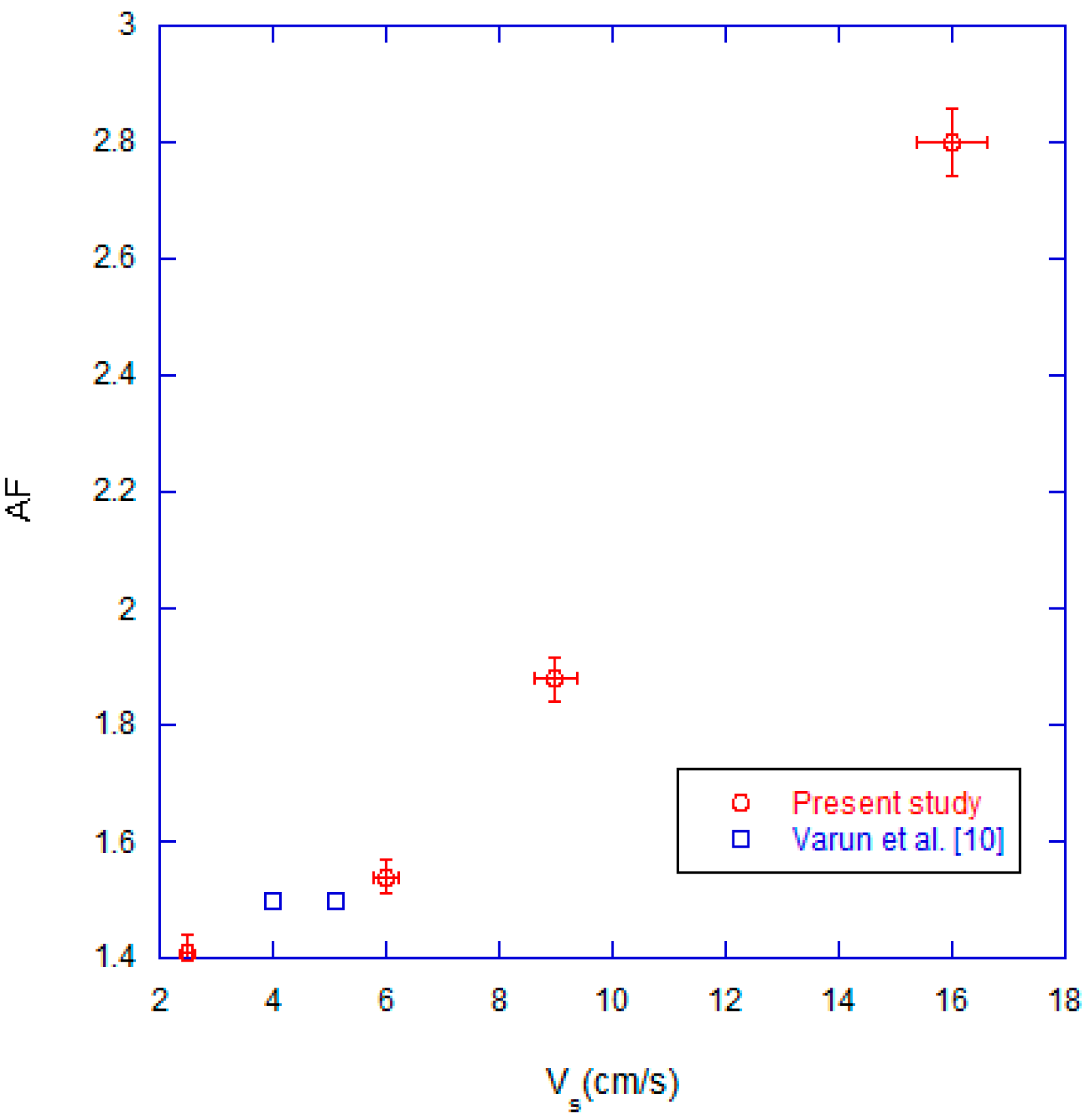
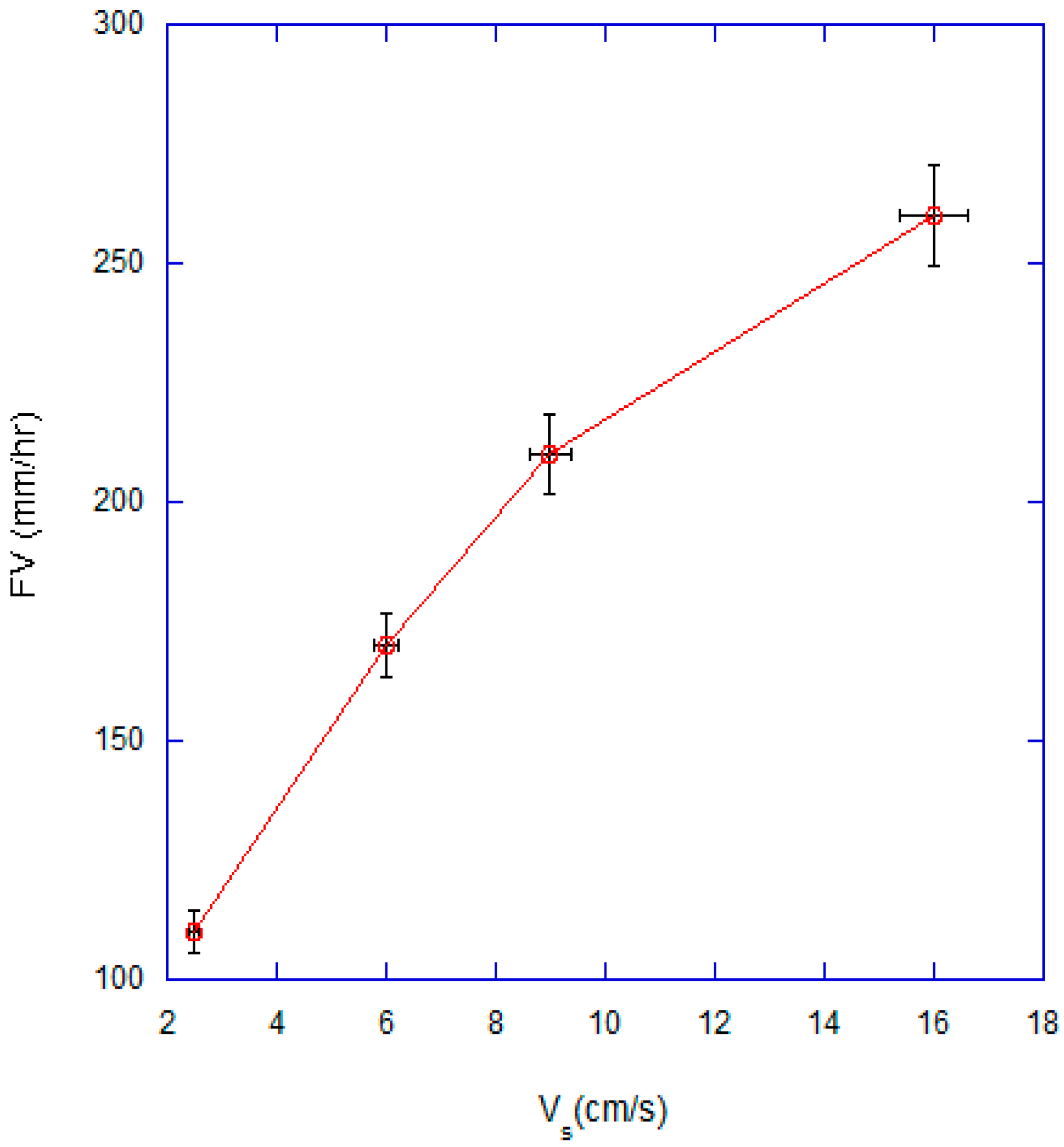
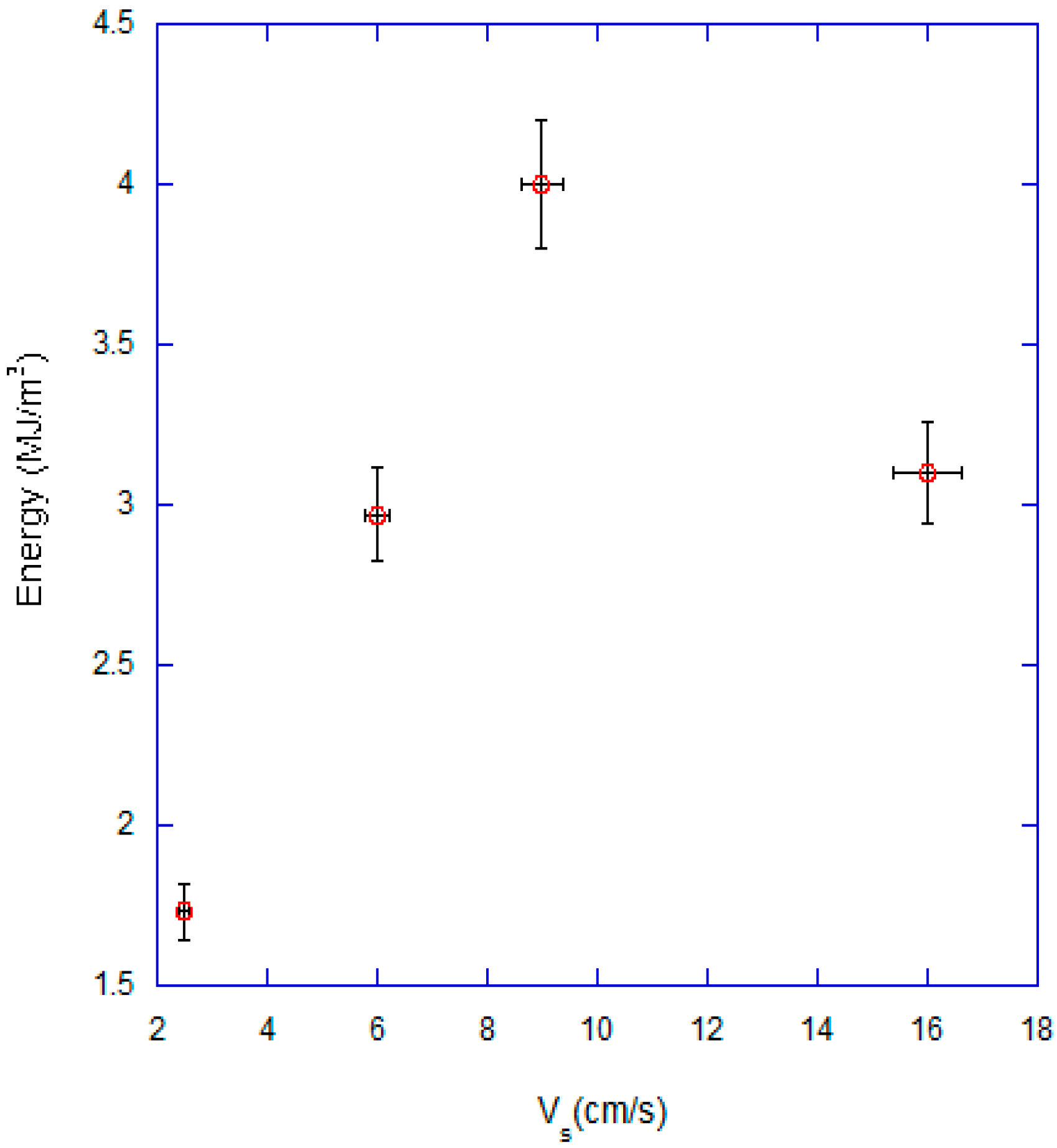
3.1. Effect of Superficial Velocity
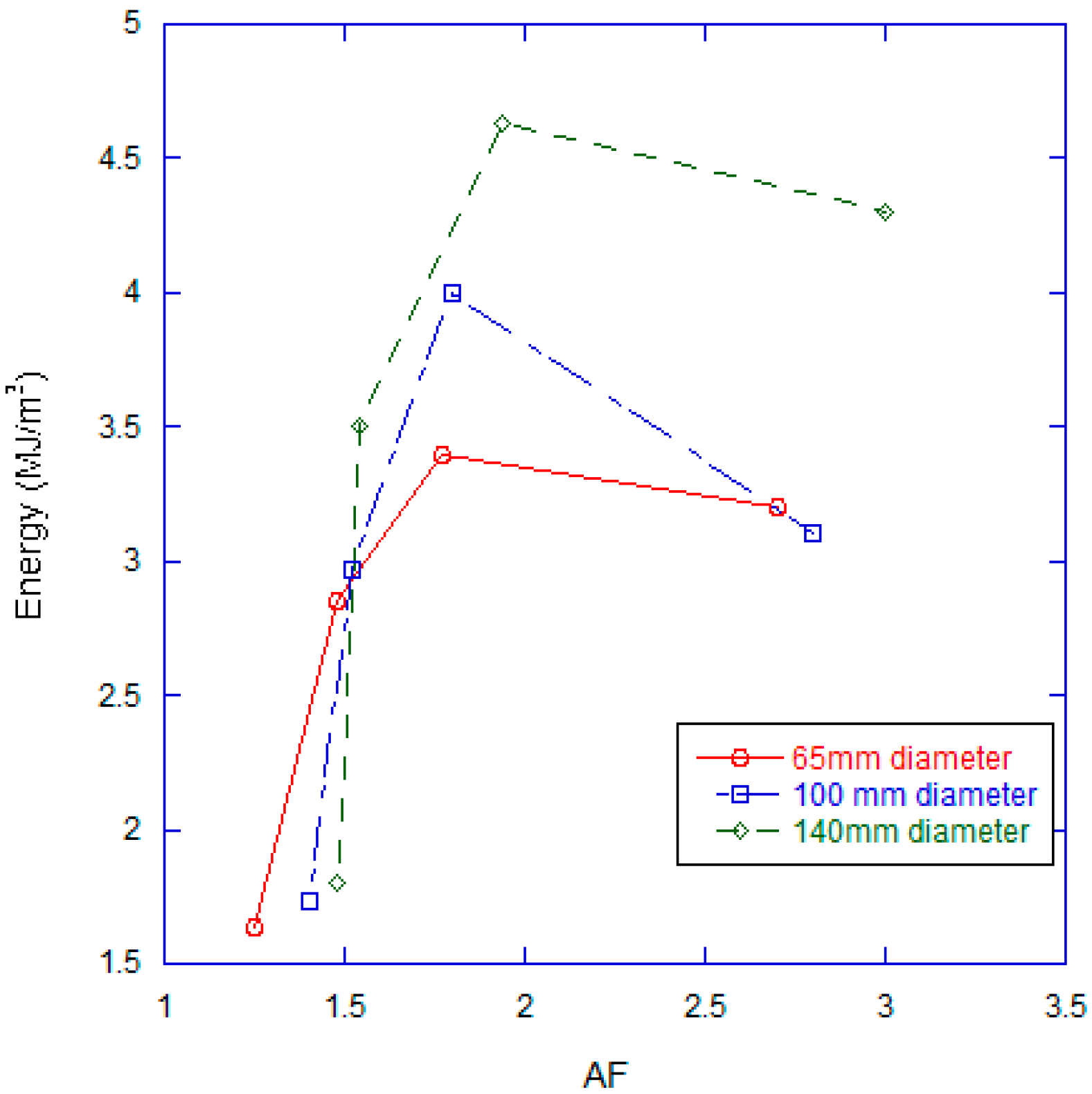
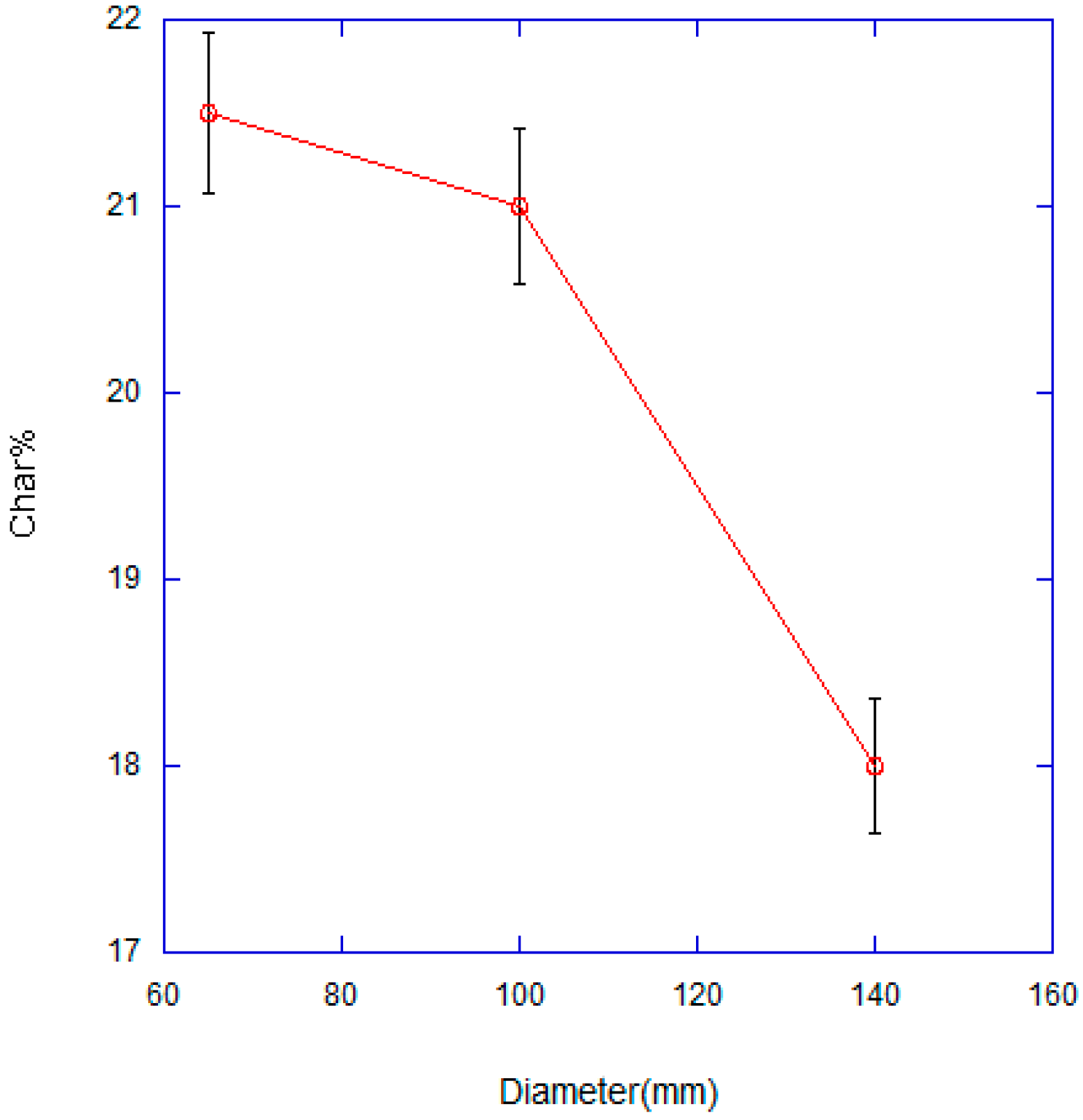
3.2. Effect of Combustion Chamber Geometry
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Kshirsagar, M.P.; Kalamkar, V.R. A comprehensive review on biomass cookstoves and a systematic approach for modern cookstove design. Renew. Sustain. Energy Rev. 2014, 30, 580–603. [Google Scholar] [CrossRef]
- Arora, P.; Das, P.; Jain, S.; Kishore, V.V.N. A laboratory based comparative study of Indian biomass cookstove testing protocol and Water Boiling Test. Energy Sustain. Dev. 2014, 21, 81–88. [Google Scholar] [CrossRef]
- MacCarty, N.A.; Bryden, K.M. Modeling of household biomass cookstoves: A review. Energy Sustain. Dev. 2015, 26, 1–13. [Google Scholar] [CrossRef]
- Obeng, G.Y.; Mensah, E.; Ashiagbor, G.; Boahen, O.; Sweeney, D.J. Watching the smoke rise up: Thermal efficiency, pollutant emissions and global warming impact of three biomass Cookstoves in Ghana. Energies 2017, 10, 641. [Google Scholar] [CrossRef]
- MacCarty, N.A.; Bryden, K.M. A unified set of experimental data for cylindrical, natural draft, shielded single pot wood-fired cookstoves. Energy Sustain. Dev. 2015, 26, 62–71. [Google Scholar] [CrossRef]
- Kar, A.; Rehman, I.H.; Burney, J.; Puppala, S.P.; Suresh, R.; Singh, L.; Singh, V.K.; Ahmed, T.; Ramanathan, N.; Ramanathan, V. Real-time assessment of black carbon pollution in Indian households due to traditional and improved biomass cookstoves. Environ. Sci. Technol. 2012, 46, 2993–3000. [Google Scholar] [CrossRef] [PubMed]
- Jetter, J.; Zhao, Y.; Smith, K.R.; Khan, B.; Yelverton, T.; Decarlo, P.; Hays, M.D. Pollutant emissions and energy efficiency under controlled conditions for household biomass cookstoves and implications for metrics useful in setting international test standards. Environ. Sci. Technol. 2012, 46, 10827–10834. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Leavey, A.; He, S.; Fang, J.; O’Malley, K.; Biswas, P. Characterization of gaseous and particulate pollutants from gasification-based improved cookstoves. Energy Sustain. Dev. 2016, 32, 130–139. [Google Scholar] [CrossRef]
- James R., A.M.; Yuan, W.; Boyette, M.D. The effect of biomass physical properties on top-lit updraft gasification of woodchips. Energies 2016, 9, 283. [Google Scholar] [CrossRef]
- Reed, T.; Walt, R.; Ellis, S.; Das, A.; Deutch, S. Superficial velocity—The key to downdraft gasification. In Proceedings of the Fourth Biomass Conference of the Americas, Oakland, CA, USA, 29 August–2 September 1999; pp. 343–356. [Google Scholar]
- Fatehi, M.; Kaviany, M. Adiabatic reverse combustion in a packed bed. Combust. Flame 1994, 99, 1–17. [Google Scholar] [CrossRef]
- Porteiro, J.; Patino, D.; Collazo, J.; Granada, E.; Moran, J.; Miguez, J. Experimental analysis of the ignition front propagation of several biomass fuels in a fixed-bed combustor. Fuel 2010, 89, 26–35. [Google Scholar] [CrossRef]
- Ronnback, M.; Axell, M.; Gustavsson, L.; Thunman, H.; Leckner, B. Combustion processes in a biomass fuel bed—Experimental results. In Progress in Thermochemical Biomass Conversion; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Varunkumar, S.; Rajan, N.K.S.; Mukunda, H.S. Experimental and computational studies on a gasifier based stove. Energy Convers. Manag. 2012, 53, 135–141. [Google Scholar] [CrossRef]
- Adkins, E.; Tyler, E.; Wang, J.; Siriri, D.; Modi, V. Field testing and survey evaluation of household biomass cookstoves in rural sub-Saharan Africa. Energy Sustain. Dev. 2010, 14, 172–185. [Google Scholar] [CrossRef]
- Jetter, J.J.; Kariher, P. Solid fuel household cook stoves: Characterization of performance and emissions. Biomass Bioenergy 2009, 33, 294–305. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, G.; Su, S.; Du, W.; Huangfu, Y.; Liu, G.; Wang, X.; Xing, B.; Smith, K.R.; Tao, S. Efficiencies and pollutant emissions from forced-draft biomass-pellet semi-gasifier stoves: Comparison of International and Chinese water boiling test protocols. Energy Sustain. Dev. 2016, 32, 22–30. [Google Scholar] [CrossRef]
- Caballero, M.A.; Corella, J.; Aznar, M.P.; Gil, J. Biomass gasification with air in fluidized bed. Hot gas cleanup with selected commercial and full-size nickel based catalysts. Ind. Eng. Chem. Res. 2000, 39, 1143–1154. [Google Scholar] [CrossRef]
- Warnecke, R. Gasification of biomass: Comparison of fixed bed and fluidized bed gasifier. Biomass Bioenergy 2000, 18, 489–497. [Google Scholar] [CrossRef]
- Kirubakaran, V.; Sivaramakrishnan, V.; Nalini, R.; Sekar, T.; Premalatha, M.; Subramanian, P. A review on gasification of biomass. Renew. Sustain. Energy Rev. 2009, 13, 179–186. [Google Scholar] [CrossRef]
- Ryu, C.; Yang, Y.; Khor, A.; Yates, N.; Sharifi, V.; Swithenbank, J. Effect of fuel properties on biomass combustion: Part I. Experiments—Fuel type, equivalence ratio and particle size. Fuel 2006, 85, 1039–1046. [Google Scholar] [CrossRef]
- Ohlemiller, T.J. Smoldering combustion. In SFPE Handbook of Fire Protection Engineering, 2nd ed.; National Fire Protection Association, Quincy, M.A., DiNenno, P.J., Beyler, C.L., Custer, R.L.P., Walton, W.D., Eds.; SFPE: Gaithersburg, MD, USA, 1995; Section 2, Chapter 11; Volume 2, pp. 171–179. [Google Scholar]
- Torero, J.L.; Kitano, M.; Femandez-Pello, A.C. Opposed forced flow smoldering of polyurethane foam. Combust. Sci. Technol. 1993, 91, 95–117. [Google Scholar] [CrossRef]
- Bäfver, L.S.; Leckner, B.; Tullin, C.; Berntsen, M. Particle emissions from pellets stoves and modern and old-type wood stoves. Biomass Bioenergy 2011, 35, 3648–3655. [Google Scholar] [CrossRef]
- Patil, R.; Shinde, Y.; Pandit, A. Optimization and development of solid biomass burning cookstoves. Int. J. Chem. Phys. Sci. 2013, 2, 1–10. [Google Scholar]
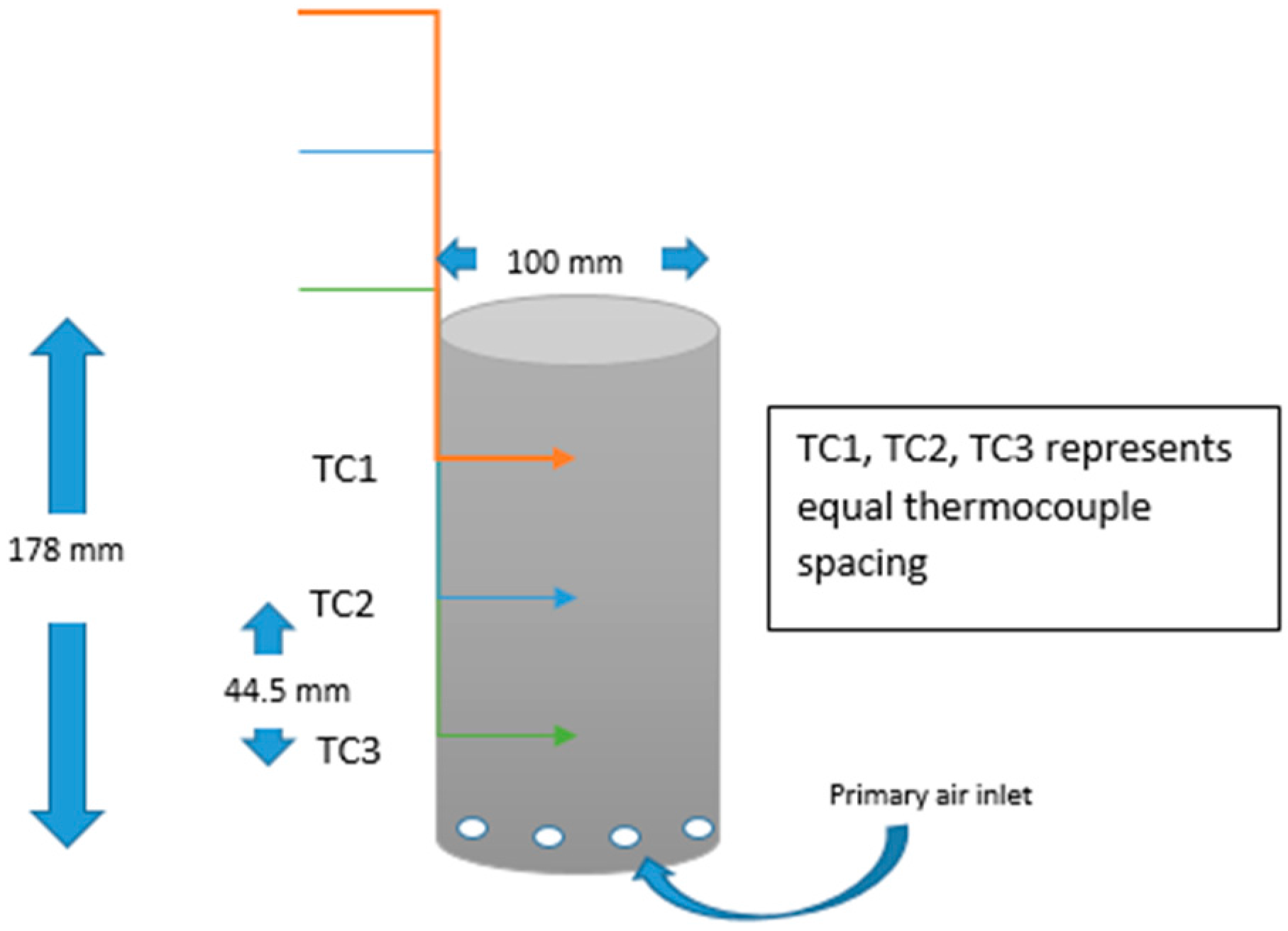



| # | Length (mm) | Diameter (mm) | Fuel Capacity (g) |
|---|---|---|---|
| 1 | 178 | 100 | 800 |
| 2 | 305 | 100 | 1400 |
| 3 | 140 | 140 | 1200 |
| 4 | 140 | 65 | 250 |
| Fuel Properties | Values |
|---|---|
| Size (mm) | 5–35 |
| Moisture Content (% D.B) | 10 ± 3 |
| Lower heating value (MJ/kg) | 15 |
| Ash (%) | <1 |
| Volatile Matter (%) | 77 |
| Fixed Carbon (%) | 23 |
| Vs (cm/s) | CO (%v/v) | H2 (%v/v) | CH4 (%v/v) | C2H6 (%v/v) | C3H8 (%v/v) |
|---|---|---|---|---|---|
| 2.5 | 5.4 ± 1.2 | 3.50 ± 1.2 | 1.74 ± 0.84 | 0.12 ± 0.01 | 0.0013 ± 0.0002 |
| 6 | 10.20 ± 0.82 | 8.44 ± 1.51 | 2.27 ± 0.51 | 0.13 ± 0.04 | 0.0027 ± 0.0007 |
| 9 | 13.45 ± 0.48 | 11.95 ± 2.2 | 2.34 ± 0.58 | 0.15 ± 0.02 | 0.024 ± 0.001 |
| 16 | 12.3 ± 1.5 | 10.1 ± 1.7 | 1.21 ± 0.52 | 0.02 ± 0.01 | 0.0032 ± 0.0005 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehta, Y.; Richards, C. Gasification Performance of a Top-Lit Updraft Cook Stove. Energies 2017, 10, 1529. https://doi.org/10.3390/en10101529
Mehta Y, Richards C. Gasification Performance of a Top-Lit Updraft Cook Stove. Energies. 2017; 10(10):1529. https://doi.org/10.3390/en10101529
Chicago/Turabian StyleMehta, Yogesh, and Cecilia Richards. 2017. "Gasification Performance of a Top-Lit Updraft Cook Stove" Energies 10, no. 10: 1529. https://doi.org/10.3390/en10101529




