Modifiable Maternal Factors and Their Relationship to Postpartum Depression
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic Characteristics
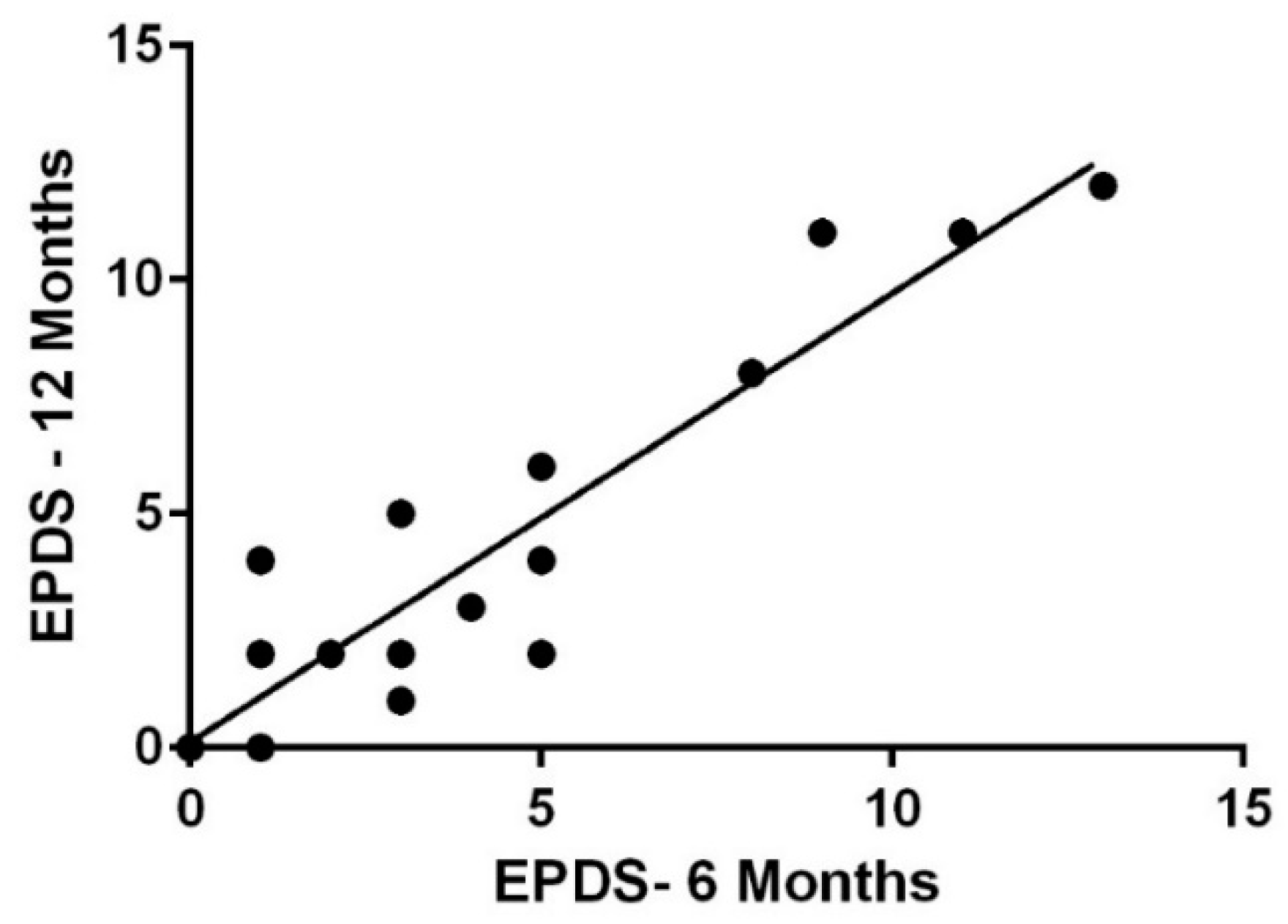
3.2. EPDS
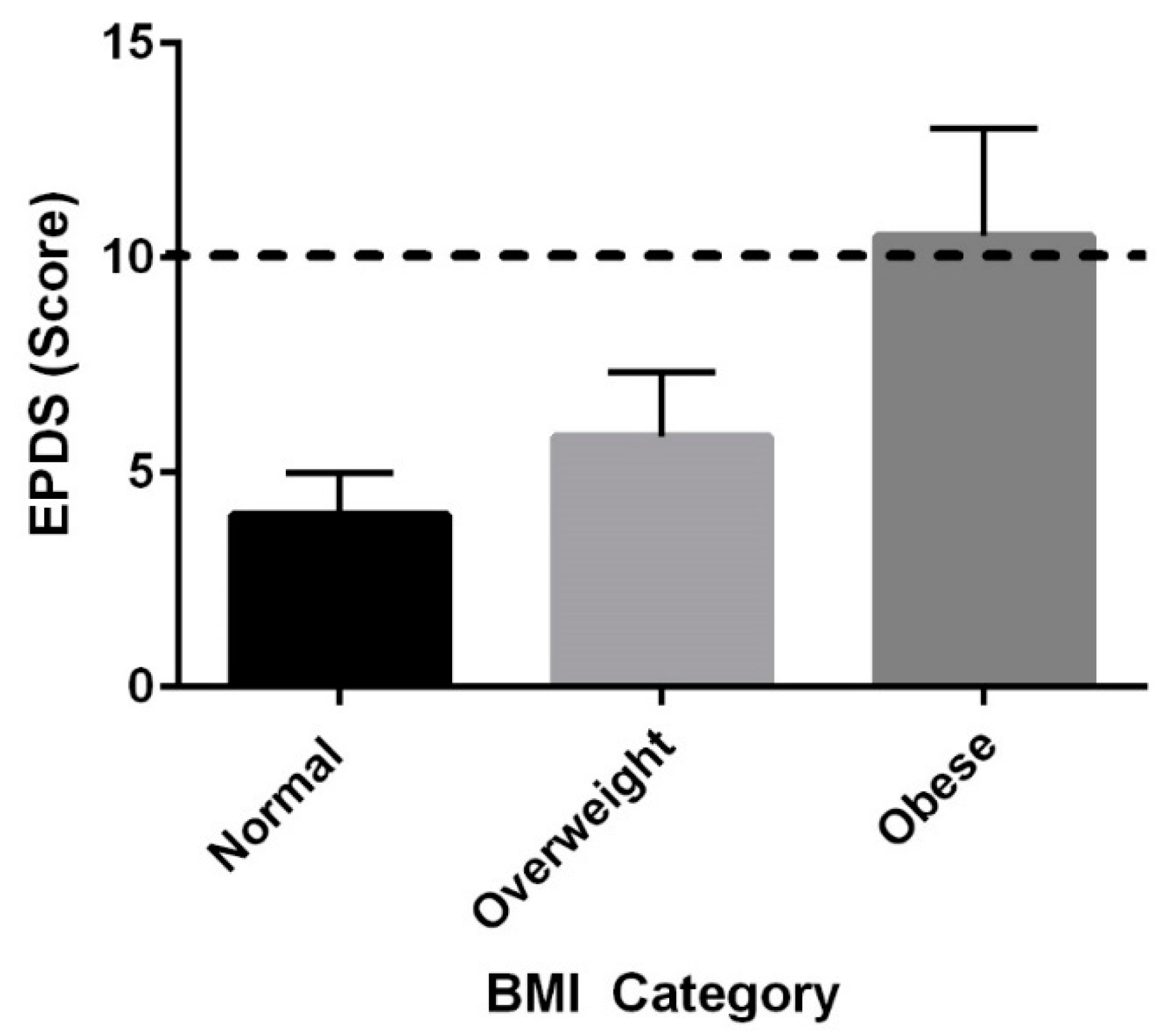
3.3. BMI
3.4. Household Income
3.5. Fatigue
3.6. Sleep
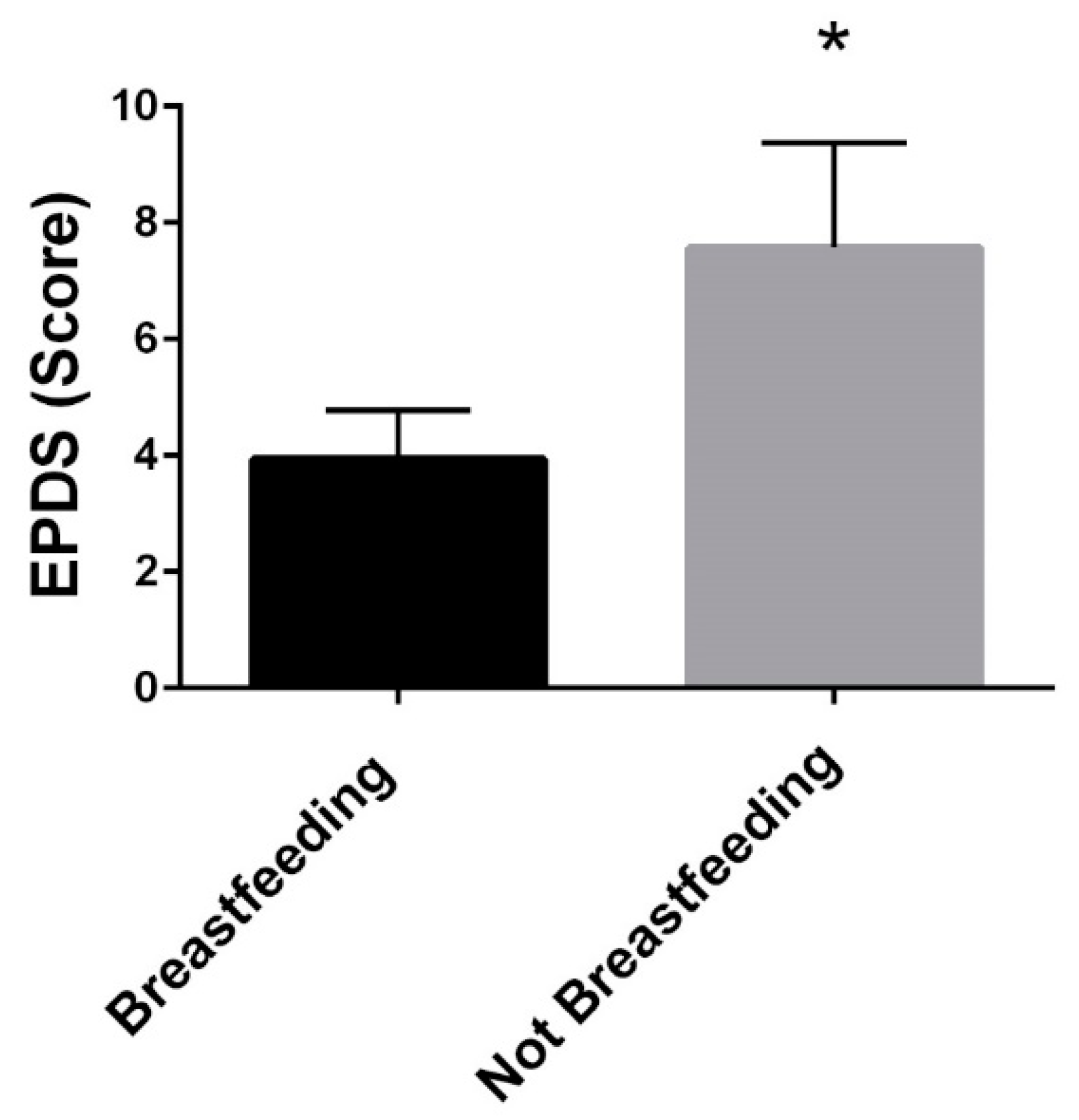
3.7. Breastfeeding
3.8. Physical Activity
3.9. Dietary Intake
3.10. Simple and Multiple Regression
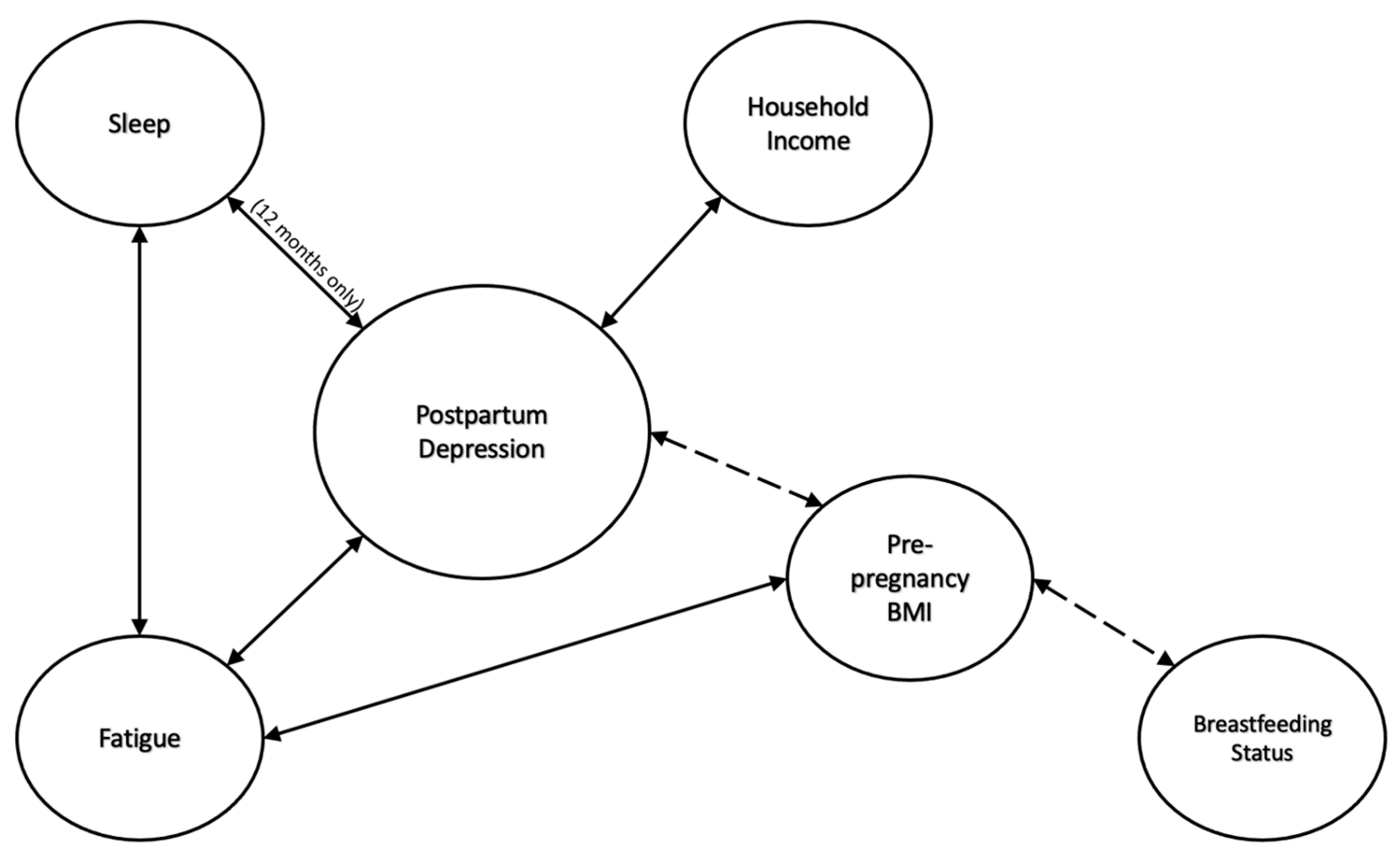
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wendland, C. The Vanishing Mother: Cesarean Section and “Evidence-Based Obstetrics”. Med. Anthr. Q. 2007, 21, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.M.; Østbye, T.; Swamy, G.K. Occurrence and Correlates of Postpartum Depression in Overweight and Obese Women: Results from the Active Mothers Postpartum (AMP) Study. Matern. Child Health J. 2008, 13, 832–838. [Google Scholar] [CrossRef]
- Vieira, E.D.S.; Caldeira, N.T.; Eugênio, D.S.; Di Lucca, M.M.; Silva, I.A. Breastfeeding self-efficacy and postpartum depression: A cohort study. Rev. Lat. -Am. Enferm. 2018, 26, e3035. [Google Scholar] [CrossRef] [PubMed]
- Alba, B.M. Postpartum Depression: A Nurse’s Guide. Am. J. Nurs. 2021, 121, 32–43. [Google Scholar] [CrossRef]
- Tinius, R.A.; Yoho, K.; Blankenship, M.M.; Maples, J.M. Postpartum Metabolism: How Does It Change from Pregnancy and What are the Potential Implications? Int. J. Womens Health 2021, 13, 591–599. [Google Scholar] [CrossRef]
- Tinius, R.A.; Blankenship, M.M.; Furgal, K.E.; Cade, W.T.; Pearson, K.J.; Rowland, N.S.; Pearson, R.; Hoover, D.L.; Maples, J. Metabolic flexibility is impaired in women who are pregnant and overweight/obese and related to insulin resistance and inflammation. Metabolism 2020, 104, 154142. [Google Scholar] [CrossRef] [PubMed]
- Qiu-Yue, Z.; Gelaye, B.; Zhong, Q.-Y.; Enquobahrie, D.A.; Frederick, I.O.; Williams, M.A. Construct validity and factor structure of the Pittsburgh Sleep Quality Index among pregnant women in a Pacific-Northwest cohort. Sleep Breath. 2016, 20, 293–301. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of Postnatal Depression. Development of the 10-item EdinBurgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef]
- Keen, K.; Maples, J.; Cooley, B.; Olenick, A.; Blankenship, M.; Hoover, D. Acute High-Intensity Exercise Improves Mood during the Second Trimester of Pregnancy. Int. J. Womens Health Wellness 2017, 3, 53. [Google Scholar] [CrossRef]
- Brandt, R.; Herrero, D.; Massetti, T.; Crocetta, T.B.; Guarnieri, R.; Monteiro, C.B.D.M.; Viana, M.D.S.; Bevilacqua, G.G.; de Abreu, L.C.; Andrade, A. The Brunel Mood Scale Rating in Mental Health for Physically Active and Apparently Healthy Populations. Health 2016, 8, 125–132. [Google Scholar] [CrossRef] [Green Version]
- MeAuley, E.; Courneya, K.S. The Subjective Exercise Experiences Scale (SEES): Development and Preliminary Validation. J. Sport Exerc. Psychol. 1994, 16, 163–177. [Google Scholar] [CrossRef]
- Subar, A.F.; Thompson, F.E.; Kipnis, V.; Midthune, D.; Hurwitz, P.; McNutt, S.; McIntosh, A.; Rosenfeld, S. Comparative validation of the block, willett, and national cancer institute food frequency questionnaires the eating at America’s table study. Am. J. Epidemiol. 2001, 154, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Ghaedrahmati, M.; Kazemi, A.; Kheirabadi, G.; Ebrahimi, A.; Bahrami, M. Postpartum depression risk factors: A narrative review. J. Educ. Health Promot. 2017, 6, 60. [Google Scholar] [PubMed]
- Guintivano, J.; Manuck, T.; Meltzer-Brody, S. Predictors of Postpartum Depression: A Comprehensive Review of the Last Decade of Evidence. Clin. Obstet. Gynecol. 2018, 61, 591–603. [Google Scholar] [CrossRef]
- Wilson, N.; Lee, J.J.; Bei, B. Postpartum fatigue and depression: A systematic review and meta-analysis. J. Affect. Disord. 2018, 246, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Okun, M.L.; Mancuso, R.A.; Hobel, C.J.; Schetter, C.D.; Coussons-Read, M. Poor sleep quality increases symptoms of depression and anxiety in postpartum women. J. Behav. Med. 2018, 41, 703–710. [Google Scholar] [CrossRef]
- Dias, C.C.; Figueiredo, B. Breastfeeding and depression: A systematic review of the literature. J. Affect. Disord. 2015, 171, 142–154. [Google Scholar] [CrossRef]
- ACOG Committee Opinion No. 736: Optimizing Postpartum Care. Obstet. Gynecol. 2018, 131, e140–e150. [CrossRef]
- ACOG Committee Opinion No. 757 Summary: Screening for Perinatal Depression. Obstet. Gynecol. 2018, 132, 1314–1316. [CrossRef]
- Holopainen, A.; Hakulinen, T. New parents’ experiences of postpartum depression: A systematic review of qualitative evidence. JBI Database Syst. Rev. Implement. Rep. 2019, 17, 1731–1769. [Google Scholar] [CrossRef] [Green Version]
- Silverman, M.E.; Smith, L.; Lichtenstein, P.; Reichenberg, A.; Sandin, S. The association between body mass index and postpartum depression: A population-based study. J. Affect. Disord. 2018, 240, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Lacoursiere, D.Y.; Baksh, L.; Bloebaum, L.; Varner, M. Maternal Body Mass Index and Self-Reported Postpartum Depressive Symptoms. Matern. Child Health J. 2006, 10, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhang, S.; Yan, J. Gestational weight gain and risk of postpartum depression: A meta-analysis of observational studies. Psychiatry Res. 2022, 310, 114448. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, N.-A.; Kersting, A.; Riedel-Heller, S.G.; Luck-Sikorski, C. Body Dissatisfaction in Individuals with Obesity Compared to Normal-Weight Individuals: A Systematic Review and Meta-Analysis. Obes. Facts 2016, 9, 424–441. [Google Scholar] [CrossRef]
- Roomruangwong, C.; Kanchanatawan, B.; Sirivichayakul, S.; Maes, M. High incidence of body image dissatisfaction in pregnancy and the postnatal period: Associations with depression, anxiety, body mass index and weight gain during pregnancy. Sex. Reprod. Health 2017, 13, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Lee, A.M.; Koh, Y.W.; Lam, S.K.; Lee, C.P.; Leung, K.Y.; Tang, C.S.K. Associations of body dissatisfaction with anxiety and depression in the pregnancy and postpartum periods: A longitudinal study. J. Affect. Disord. 2019, 263, 582–592. [Google Scholar] [CrossRef]
- Riquin, E.; Lamas, C.; Nicolas, I.; Lebigre, C.D.; Curt, F.; Cohen, H.; Legendre, G.; Corcos, M.; Godart, N. A key for perinatal depression early diagnosis: The body dissatisfaction. J. Affect. Disord. 2019, 245, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Gay, C.; Lee, K.A. How Much Does Low Socioeconomic Status Increase the Risk of Prenatal and Postpartum Depressive Symptoms in First-Time Mothers? Women’s Health Issues 2010, 20, 96–104. [Google Scholar] [CrossRef]
- Wilson, N.; Wynter, K.; Fisher, J.; Bei, B. Related but different: Distinguishing postpartum depression and fatigue among women seeking help for unsettled infant behaviours. BMC Psychiatry 2018, 18, 309. [Google Scholar] [CrossRef]
- Negron, R.; Martin, A.; Almog, M.; Balbierz, A.; Howell, E.A. Social Support During the Postpartum Period: Mothers’ Views on Needs, Expectations, and Mobilization of Support. Matern. Child Health J. 2012, 17, 616–623. [Google Scholar] [CrossRef] [Green Version]
- Montgomery-Downs, H.E.; Insana, S.P.; Clegg-Kraynok, M.M.; Mancini, L.M. Normative longitudinal maternal sleep: The first 4 postpartum months. Am. J. Obstet. Gynecol. 2010, 203, 465.e1–465.e7. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; Krämer, M.; Tang, N.K.Y.; Montgomery-Downs, H.E.; Lemola, S. Long-term effects of pregnancy and childbirth on sleep satisfaction and duration of first-time and experienced mothers and fathers. Sleep 2019, 42, zsz015. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.A.; Gjerdingen, D.; Schuver, K.; Avery, M.; Marcus, B.H. The effect of sleep pattern changes on postpartum depressive symptoms. BMC Womens Health 2018, 18, 12. [Google Scholar] [CrossRef]
- Gregory, E.F.; Butz, A.M.; Ghazarian, S.R.; Gross, S.M.; Johnson, S.B. Are Unmet Breastfeeding Expectations Associated With Maternal Depressive Symptoms? Acad. Pediatr. 2015, 15, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Pezley, L.; Cares, K.; Duffecy, J.; Koenig, M.D.; Maki, P.; Odoms-Young, A.; Withington, M.H.C.; Oliveira, M.L.; Loiacono, B.; Prough, J.; et al. Efficacy of behavioral interventions to improve maternal mental health and breastfeeding outcomes: A systematic review. Int. Breastfeed. J. 2022, 17, 67. [Google Scholar] [CrossRef]
- Poyatos-León, R.; García-Hermoso, A.; Sanabria-Martínez, G.; Alvarez-Bueno, C.; Cavero-Redondo, I.; Martínez-Vizcaíno, V. Effects of exercise-based interventions on postpartum depression: A meta-analysis of randomized controlled trials. Birth 2017, 44, 200–208. [Google Scholar] [CrossRef]
- Silverman, M.E.; Reichenberg, A.; Savitz, D.A.; Cnattingius, S.; Lichtenstein, P.; Hultman, C.M.; Larsson, H.; Sandin, S. The risk factors for postpartum depression: A population-based study. Depress. Anxiety 2017, 34, 178–187. [Google Scholar] [CrossRef]
- US Census Data. Available online: https://www.census.gov/quickfacts/bowlinggreencitykentucky (accessed on 22 September 2022).
- Bryant, A.S.; Worjoloh, A.; Caughey, A.B.; Washington, A.E. Racial/ethnic disparities in obstetric outcomes and care: Prevalence and determinants. Am. J. Obstet. Gynecol. 2010, 202, 335–343. [Google Scholar] [CrossRef]
- Howell, E.A.; Mora, P.A.; Horowitz, C.R.; Leventhal, H. Racial and Ethnic Differences in Factors Associated With Early Postpartum Depressive Symptoms. Obstet. Gynecol. 2005, 105, 1442–1450. [Google Scholar] [CrossRef]
- Slomian, J.; Honvo, G.; Emonts, P.; Reginster, J.-Y.; Bruyère, O. Consequences of maternal postpartum depression: A systematic review of maternal and infant outcomes. Women’s Health 2019, 15, 1745506519844044. [Google Scholar] [CrossRef] [Green Version]


| Pre-Pregnancy (n = 26) | 6 months Postpartum (n = 26) | 12 months Postpartum (n = 17) | |
|---|---|---|---|
| BMI | 24.5 ± 4.0 | 25.2 ± 4.4 | 24.2 ± 4.5 |
| BMI Classification | |||
| Underweight < 18.5 | 0 (0%) | 0 (0%) | 0 (0%) |
| Normal Weight 18.5–24.9 | 15 (63%) | 15 (58%) | 12 (70%) |
| Overweight 25–29.9 | 7 (29%) | 7 (27%) | 3 (18%) |
| Obese ≥ 3 | 2 (8%) | 4 (15%) | 2 (12%) |
| Weight | 68.1 ± 11.7 kg | 70.3 ± 13.2 kg | 67.6± 13.6 kg |
| Body Fat Percentage | 26.9 ± 6.9 % | 23.8 ± 5.9% | |
| Age | 32.0 ± 4.3 years | ||
| Race White | 100% | ||
| Parity | |||
| Nulliparous | 10 (38%) | ||
| Multiparous | 16 (62%) | ||
| Breastfeeding Status | |||
| Breastfeeding | 15 (57%) | 6 (35%) | |
| Formula-Feeding | 7 (27%) | 5 (29%) | |
| Both/Combination | 3 (12%) | 3 (18%) | |
| Did Not Report | 1 (4%) | 3 (18%) | |
| Physical Activity Levels | |||
| Sedentary (%) | 54.8 ± 9.4% | 55.2 ± 15.8% | |
| Light (%) | 32.1 ± 7.3% | 31.0 ± 10.5% | |
| Moderate (%) | 12.9 ± 3.9% | 13.5 ± 7.09 | |
| Edinburg Postnatal Depression Scale Scores | |||
| Median | 4.0 | 3.0 | |
| Interquartile Range | 6.5 | 5.5 | |
| Household Income | Average: USD 99,583 Range: USD 28,000–USD 240,000 | ||
| Relationship between Dietary Variable and Postpartum Depression Scores (EPDS) | ||
|---|---|---|
| 6 months | 12 months | |
| Total kilocalories | r = 0.010, p = 0.965 | r = −0.150, p = 0.609 |
| Vitamin B6 | r = 0.100, p = 0.657 | r = −0.116, p = 0.693 |
| Zinc | r = 0.051, p = 0.818 | r = −0.020, p = 0.945 |
| Selenium | r = −0.089, p = 0.695 | r = 0.050, p = 0.925 |
| Simple Linear Regression | |||||
|---|---|---|---|---|---|
| Predictors | B | SE | β | p-value | 95% CI |
| Pre-pregnancy BMI * | 28.670 | 11.542 | 0.468 | 0.021 * | 4.733–52.607 |
| Household Income | −6.631 | 3.406 | −0.391 | 0.065 | −13.714–0.452 |
| Fatigue * | 13.686 | 3.564 | 0.642 | <0.001 * | 6.274–21.099 |
| Breastfeeding Status * | 3.630 | 1.736 | 0.407 | 0.048 * | 0.030–7.230 |
| Multiple Linear Regression | |||||
| Predictors | B | SE | β | p-value | 95% CI |
| Pre-pregnancy BMI | 4.269 | 12.269 | 0.072 | 0.732 | −21.615–30.153 |
| Household Income * | −6.405 | 2.827 | −0.375 | 0.037 * | −12.370–−0.440 |
| Fatigue * | 10.854 | 3.562 | 0.524 | 0.007 * | 3.338–18.369 |
| Breastfeeding Status | 1.692 | 1.760 | 0.193 | 0.350 | −2.022–5.406 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howard, K.; Maples, J.M.; Tinius, R.A. Modifiable Maternal Factors and Their Relationship to Postpartum Depression. Int. J. Environ. Res. Public Health 2022, 19, 12393. https://doi.org/10.3390/ijerph191912393
Howard K, Maples JM, Tinius RA. Modifiable Maternal Factors and Their Relationship to Postpartum Depression. International Journal of Environmental Research and Public Health. 2022; 19(19):12393. https://doi.org/10.3390/ijerph191912393
Chicago/Turabian StyleHoward, Kathryn, Jill M. Maples, and Rachel A. Tinius. 2022. "Modifiable Maternal Factors and Their Relationship to Postpartum Depression" International Journal of Environmental Research and Public Health 19, no. 19: 12393. https://doi.org/10.3390/ijerph191912393





