Prognosis of Hypothermic Patients Undergoing ECLS Rewarming—Do Alterations in Biochemical Parameters Matter?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Data Collection
2.3. Clinical Procedure
2.4. Statistical Analysis
3. Results
3.1. Changes of Concentrations in the Biochemical Parameters
3.2. Predictors of Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pasquier, M.; Hugli, O.; Paal, P.; Darocha, T.; Blancher, M.; Husby, P.; Silfvast, T.; Carron, P.-N.; Rousson, V. Hypothermia outcome prediction after extracorporeal life support for hypothermic cardiac arrest patients: The HOPE score. Resuscitation 2018, 126, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Podsiadło, P.; Darocha, T.; Svendsen, Ø.S.; Kosiński, S.; Silfvast, T.; Blancher, M.; Sawamoto, K.; Pasquier, M. Outcomes of patients suffering unwitnessed hypothermic cardiac arrest rewarmed with extracorporeal life support: A systematic review. Artif. Organs 2021, 45, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Lott, C.; Truhlář, A.; Alfonzo, A.; Barelli, A.; González-Salvado, V.; Hinkelbein, J.; Nolan, J.P.; Paal, P.; Perkins, G.D.; Thies, K.-C.; et al. European Resuscitation Council Guidelines 2021: Cardiac arrest in special circumstances. Resuscitation 2021, 161, 152–219. [Google Scholar] [CrossRef]
- Cheng, R.; Hachamovitch, R.; Kittleson, M.; Patel, J.; Arabia, F.; Moriguchi, J.; Esmailian, F.; Azarbal, B. Complications of Extracorporeal Membrane Oxygenation for Treatment of Cardiogenic Shock and Cardiac Arrest: A Meta-Analysis of 1,866 Adult Patients. Ann. Thorac. Surg. 2014, 97, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Mair, P.; Kornberger, E.; Furtwaengler, W.; Balogh, D.; Antretter, H. Prognostic markers in patients with severe accidental hypothermia and cardiocirculatory arrest. Resuscitation 1994, 27, 47–54. [Google Scholar] [CrossRef]
- Darocha, T.; Podsiadło, P.; Polak, M.; Hymczak, H.; Krzych, Ł.; Skalski, J.; Witt-Majchrzak, A.; Nowak, E.; Toczek, K.; Waligórski, S.; et al. Prognostic Factors for Nonasphyxia-Related Cardiac Arrest Patients Undergoing Extracorporeal Rewarming—HELP Registry Study. J. Cardiothorac. Vasc. Anesth. 2020, 34, 365–371. [Google Scholar] [CrossRef]
- Podsiadło, P.; Smoleń, A.; Kosiński, S.; Hymczak, H.; Waligórski, S.; Witt-Majchrzak, A.; Drobiński, D.; Nowak, E.; Barteczko-Grajek, B.; Toczek, K.; et al. Impact of rescue collapse on mortality rate in severe accidental hypothermia: A matched-pair analysis. Resuscitation 2021, 164, 108–113. [Google Scholar] [CrossRef]
- Mungan, I.; Kazancı, D.; Bektaş, Ş.; Ademoglu, D.; Turan, S. Does lactate clearance prognosticates outcomes in ECMO therapy: A retrospective observational study. BMC Anesthesiol. 2018, 18, 152. [Google Scholar] [CrossRef]
- Haas, S.A.; Lange, T.; Saugel, B.; Petzoldt, M.; Fuhrmann, V.; Metschke, M.; Kluge, S. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensiv. Care Med. 2016, 42, 202–210. [Google Scholar] [CrossRef]
- Nichol, A.D.; Egi, M.; Pettila, V.; Bellomo, R.; French, C.; Hart, G.; Davies, A.; Stachowski, E.; Reade, M.C.; Bailey, M.; et al. Relative hyperlactatemia and hospital mortality in critically ill patients: A retrospective multi-centre study. Crit. Care 2010, 14, R25. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.-L.; E Silva, A.Q.; Couto, L.; Taccone, F.S. The value of blood lactate kinetics in critically ill patients: A systematic review. Crit. Care 2016, 20, 257. [Google Scholar] [CrossRef] [Green Version]
- Sawamoto, K.; Bird, S.B.; Katayama, Y.; Maekawa, K.; Uemura, S.; Tanno, K.; Narimatsu, E. Outcome from severe accidental hypothermia with cardiac arrest resuscitated with extracorporeal cardiopulmonary resuscitation. Am. J. Emerg. Med. 2014, 32, 320–324. [Google Scholar] [CrossRef]
- Saczkowski, R.S.; Brown, D.J.; Abu-Laban, R.B.; Fradet, G.; Schulze, C.J.; Kuzak, N.D. Prediction and risk stratification of survival in accidental hypothermia requiring extracorporeal life support: An individual patient data meta-analysis. Resuscitation 2018, 127, 51–57. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Dufaye, P.; Berré, J.; Leeman, M.; Degaute, J.-P.; Kahn, R.J. Serial lactate determinations during circulatory shock. Crit. Care Med. 1983, 11, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Umemoto, N.; Taniguchi, T.; Ishii, H.; Hiramatsu, Y.; Arata, K.; Takuya, H.; Inoue, S.; Sugiura, T.; Asai, T.; et al. The lactate clearance calculated using serum lactate level 6 h after is an important prognostic predictor after extracorporeal cardiopulmonary resuscitation: A single-center retrospective observational study. J. Intensiv. Care 2018, 6, 33. [Google Scholar] [CrossRef]
- Debaty, G.; Babaz, V.; Durand, M.; Gaide-Chevronnay, L.; Fournel, E.; Blancher, M.; Bouvaist, H.; Chavanon, O.; Maignan, M.; Bouzat, P.; et al. Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis. Resuscitation 2017, 112, 1–10. [Google Scholar] [CrossRef]
- Mégarbane, B.; Deye, N.; Malissin, I.; Baud, F.J. Usefulness of the serum lactate concentration for predicting mortality in acute beta-blocker poisoning. Clin. Toxicol. 2010, 48, 974–978. [Google Scholar] [CrossRef]
- Gunnerson, K.J.; Saul, M.; He, S.; Kellum, J.A. Lactate versus non-lactate metabolic acidosis: A retrospective outcome evaluation of critically ill patients. Crit. Care 2006, 10, R22. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, G.; Bellomo, R.; Bakker, J. The ten pitfalls of lactate clearance in sepsis. Intensiv. Care Med. 2019, 45, 82–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, B.J. Severe lactic acidosis and hypothermia. West. J. Med. 1981, 134, 162–166. [Google Scholar] [PubMed]
- Pirnes, J.; Ala-Kokko, T. Accidental hypothermia: Factors related to long-term hospitalization. A retrospective study from northern Finland. Intern. Emerg. Med. 2016, 12, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Van der Ploeg, G.-J.; Goslings, J.C.; Walpoth, B.H.; Bierens, J.J. Accidental hypothermia: Rewarming treatments, complications and outcomes from one university medical centre. Resuscitation 2010, 81, 1550–1555. [Google Scholar] [CrossRef]
- Debaty, G.; Moustapha, I.; Bouzat, P.; Maignan, M.; Blancher, M.; Rallo, A.; Brun, J.; Chavanon, O.; Danel, V.; Carpentier, F.; et al. Outcome after severe accidental hypothermia in the French Alps: A 10-year review. Resuscitation 2015, 93, 118–123. [Google Scholar] [CrossRef]
- Okada, Y.; Kiguchi, T.; Irisawa, T.; Yoshiya, K.; Yamada, T.; Hayakawa, K.; Noguchi, K.; Nishimura, T.; Ishibe, T.; Yagi, Y.; et al. Predictive accuracy of biomarkers for survival among cardiac arrest patients with hypothermia: A prospective observational cohort study in Japan. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 75. [Google Scholar] [CrossRef]
- Ahmad, S.; Beckett, M. Recovery from ph 6.38: Lactic acidosis complicated by hypothermia. Emerg. Med. J. 2002, 19, 169–171. [Google Scholar] [CrossRef]
- Frei, C.; Darocha, T.; Debaty, G.; Dami, F.; Blancher, M.; Carron, P.; Oddo, M.; Pasquier, M. Clinical characteristics and outcomes of witnessed hypothermic cardiac arrest: A systematic review on rescue collapse. Resuscitation 2019, 137, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Soar, J.; Nolan, J.P.; Böttiger, B.W.; Perkins, G.D.; Lott, C.; Carli, P.; Pellis, T.; Sandroni, C.; Skrifvars, M.B.; Smith, G.B.; et al. European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation 2015, 95, 100–147. [Google Scholar] [CrossRef] [Green Version]
- Tveita, T. Pharmacodynamics in hypothermia. Crit. Care 2012, 16, A6. [Google Scholar] [CrossRef] [Green Version]

| Overall (n = 50) | Survival at ICU Discharge (n = 29) | Death (n = 21) | p | ||
|---|---|---|---|---|---|
| Age, Mean | 55.30 | ±12.96 | 56.50 | ±16.87 | 0.783 |
| Men, n (%) | 24.00 | 82.8% | 15.00 | 71.4% | 0.336 |
| pH | 6.99 | ±0.17 | 6.89 | ±0.21 | 0.067 |
| pCO2 (mmHg) | 51.50 | ±20.03 | 56.50 | ±24.24 | 0.424 |
| pO2 (mmHg) | 92.00 | (53.8–317.0) | 73.70 | (53.9–130) | 0.265 |
| HCO3 (mmol/L) | 12.00 | ±4.37 | 10.50 | ±4.63 | 0.258 |
| BE (mmol/L) | −19.30 | ±6.29 | −23.20 | ±7.4 | 0.054 |
| K+ (mmol/L) | 3.90 | ±1.21 | 5.00 | ±1.61 | 0.008 * |
| Haemoglobin (g/dL) | 12.30 | ±3.3 | 11.80 | ±3.07 | 0.566 |
| Glucose (mmol/L) | 8.10 | (4.5–11.5) | 6.30 | (4.1–10.2) | 0.520 |
| Lactate (mmol/L) | 9.80 | ±4.96 | 13.20 | ±5.73 | 0.028 * |
| Tc (°C) | 23.90 | ±2.64 | 25.20 | ±2.41 | 0.090 |
| CA | 19 | 66.5% | 17 | 81.0% | 0.230 |
| Time from CA Onset to v-a ECMO Implantation (min) | 144.00 | (120–195) | 120.00 | (67.0–240) | 0.350 |
| Concentration | Survival | Death | p | ||
|---|---|---|---|---|---|
| T1 (n = 50) | 9.80 | ±4.96 | 13.20 | ±5.73 | 0.028 * |
| T2 (n = 49) | 9.40 | ±4.58 | 13.90 | ±5.94 | 0.004 * |
| T3 (n = 48) | 8.50 | ±4.65 | 13.30 | ±6.50 | 0.005 * |
| T4 (n = 44) | 7.20 | ±4.83 | 14.30 | ±6.90 | <0.001 * |
| Difference in Concentration | |||||
| T2-T1 (n = 49) | −0.36 | ±2.06 | 0.73 | ±3.82 | 0.204 |
| T3-T1 (n = 48) | −1.29 | ±3.46 | 0.44 | ±4.16 | 0.126 |
| T4-T1 (n = 44) | −2.42 | ±4.49 | 1.44 | ±6.41 | 0.024 * |
| Lactate kinetics | |||||
| T2/T1 (n = 49) | 0.78 | (−11.3–15.5) | 1.08 | (−11.5–10.1) | 0.490 |
| T3/T1 (n = 48) | 5.90 | (−10.5–37.1) | 1.70 | (−26.1–21.1) | 0.320 |
| T4/T1 (n = 44) | 21.20 | (−14.1–56.8) | −2.60 | (−16.0–13.5) | 0.048 * |
| T3/T2 (n = 48) | 0.00 | (−8.8–33.1) | −1.77 | (−14.3–19.7) | 0.310 |
| T4/T3 (n = 44) | 24.00 | (−4.5–40.0) | 0.00 | (−8.1–8.1) | 0.008 * |
| Variable | Survival (n = 29) | Death (n = 21) | p | ||
|---|---|---|---|---|---|
| NaHCO3 8.4% Therapy 0–24 h (mL) | 80 | (40–80) | 140 | (100–200) | 0.070 |
| Transfusions of RCC (n) ≤4 units >4 units | 18 11 | (62) (38) | 10 11 | (48) (52) | 0.310 |
| Rewarming Rate (°C/h) | 1.78 | (1.35–2.90) | 2.07 | (1.55–2.60) | 0.350 |
| Duration of v-a ECMO Therapy (h) | 23 | (21–34) | 9 | (6–21) | <0.001 * |
| Length of ICU Hospitalisation (Days) | 13 | (8–22) | 1 | (1–3) | <0.001 * |
| Mechanical Ventilation (h) | 164 | (74–298) | 29 | (9–51) | <0.001 * |
| Diuresis 0–24 h (mL) | 3300.00 | (2400–4300) | 650.00 | (50–1050) | <0.001 * |
| Fluid therapy 0–24 h (mL) | 10,889.70 | (3338.56) | 9431.00 | (3881.64) | 0.160 |
| Variable | T1 | T2 | T3 | T4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Age | 0.966 | (0.917–1.019) | 0.205 | 0.954 | (0.900–1.010) | 0.104 | 0.955 | (0.902–1.011) | 0.115 | 0.965 | (0.901–1.034) | 0.315 |
| Sex | 0.573 | (0.124–2.642) | 0.475 | 0.384 | (0.073–2.016) | 0.258 | 0.272 | (0.045–1.654) | 0.157 | 0.043 | (0.003–0.632) | 0.022 * |
| CA (yes) | 0.213 | (0.036–1.267) | 0.089 | 0.341 | (0.057–2.061) | 0.241 | 0.404 | (0.063–2.611) | 0.341 | 0.659 | (0.079–5.469) | 0.699 |
| Tc | 0.754 | (0.557–1.019) | 0.066 | 0.781 | (0.569–1.072) | 0.126 | 0.797 | (0.569–1.116) | 0.186 | 0.711 | (0.450–1.126) | 0.146 |
| Rewarming Rate | 0.728 | (0.444–1.457) | 0.472 | 0.728 | (0.375–1.412) | 0.347 | 0.790 | (0.403–1.548) | 0.492 | 0.400 | (0.468–0.875) | 0.107 |
| Lactate concentration T1 | 0.851 | (0.735–0.986) | 0.032 * | |||||||||
| Lactate concentration T2 | 0.779 | (0.649–0.934) | 0.007 * | |||||||||
| Lactate concentration T3 | 0.779 | (0.653–0.929) | 0.005 * | |||||||||
| Lactate concentration T4 | 0.640 | (0.468–0.875) | 0.005 * | |||||||||
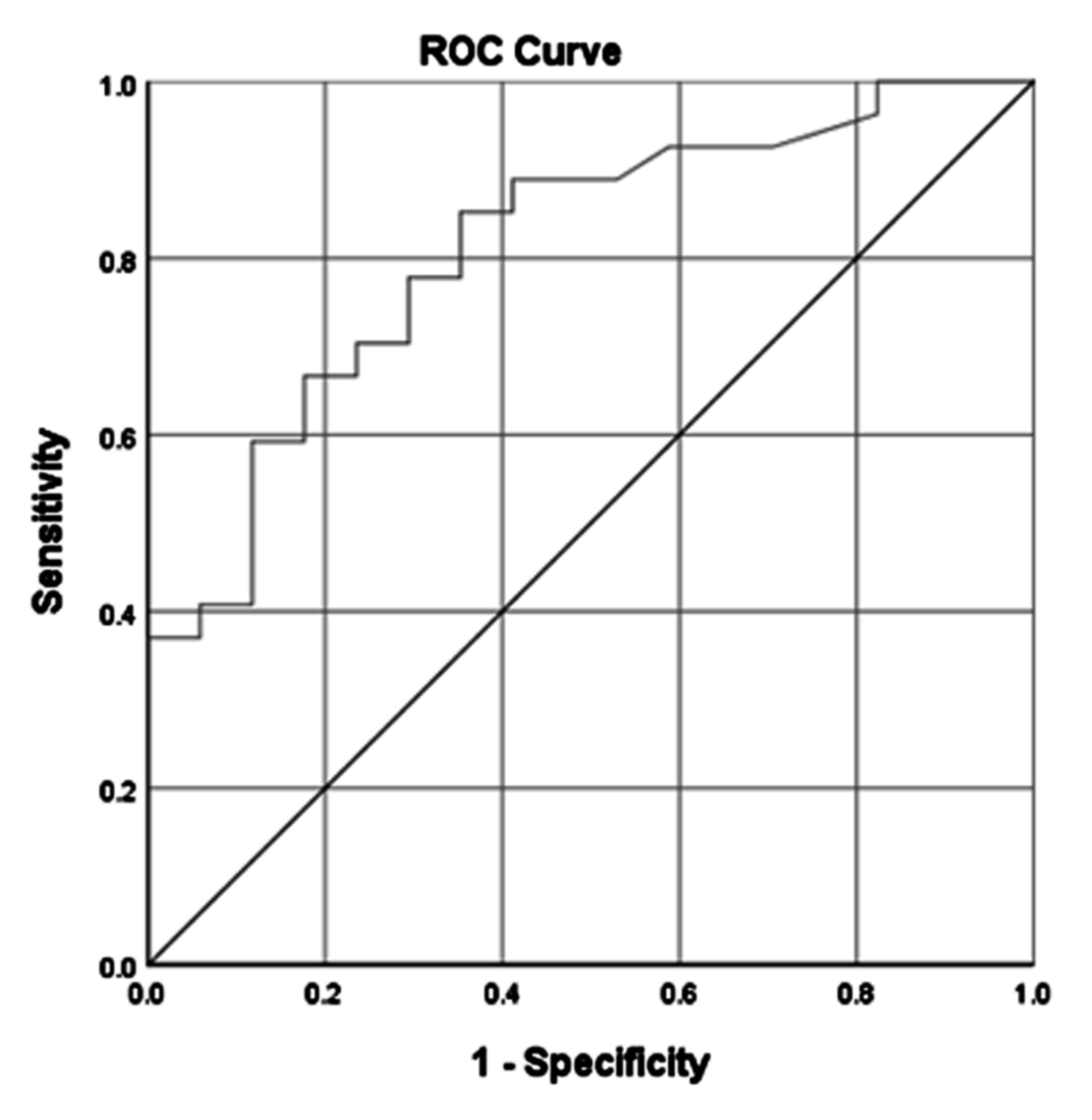
| AUC | 0.770 | (0.630–0.899) | 0.806 | (0.685–0.926) | 0.797 | (0.671–0.922) | 0.911 | (0.829–0.993) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hymczak, H.; Podsiadło, P.; Kosiński, S.; Pasquier, M.; Mendrala, K.; Hudziak, D.; Gocoł, R.; Plicner, D.; Darocha, T. Prognosis of Hypothermic Patients Undergoing ECLS Rewarming—Do Alterations in Biochemical Parameters Matter? Int. J. Environ. Res. Public Health 2021, 18, 9764. https://doi.org/10.3390/ijerph18189764
Hymczak H, Podsiadło P, Kosiński S, Pasquier M, Mendrala K, Hudziak D, Gocoł R, Plicner D, Darocha T. Prognosis of Hypothermic Patients Undergoing ECLS Rewarming—Do Alterations in Biochemical Parameters Matter? International Journal of Environmental Research and Public Health. 2021; 18(18):9764. https://doi.org/10.3390/ijerph18189764
Chicago/Turabian StyleHymczak, Hubert, Paweł Podsiadło, Sylweriusz Kosiński, Mathieu Pasquier, Konrad Mendrala, Damian Hudziak, Radosław Gocoł, Dariusz Plicner, and Tomasz Darocha. 2021. "Prognosis of Hypothermic Patients Undergoing ECLS Rewarming—Do Alterations in Biochemical Parameters Matter?" International Journal of Environmental Research and Public Health 18, no. 18: 9764. https://doi.org/10.3390/ijerph18189764






