Effects of Monopolar Dielectric Radiofrequency Signals on the Symptoms of Fibromyalgia: A Single-Blind Randomized Controlled Trial
Abstract
:1. Introduction
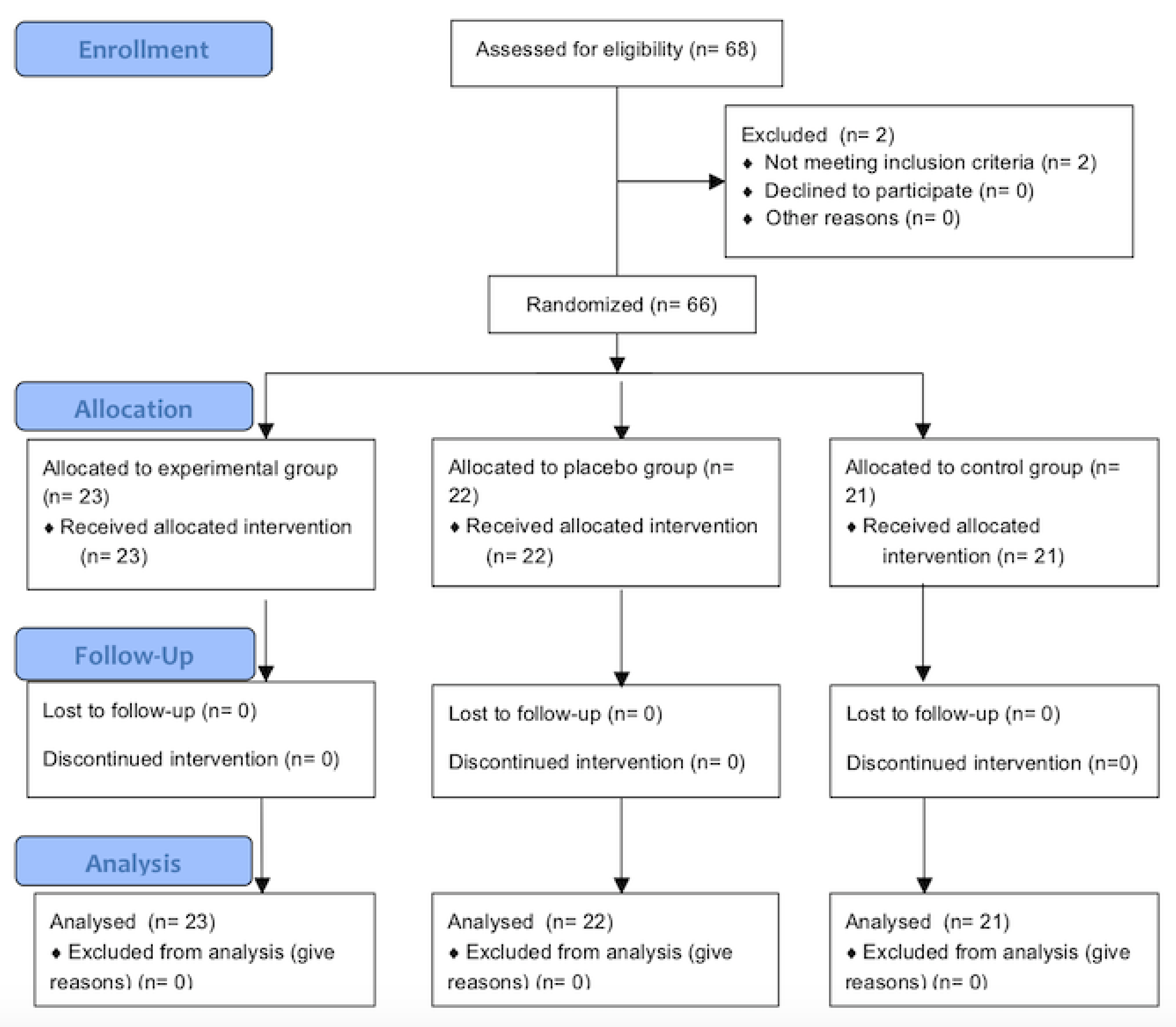
2. Materials and Methods
2.1. Design
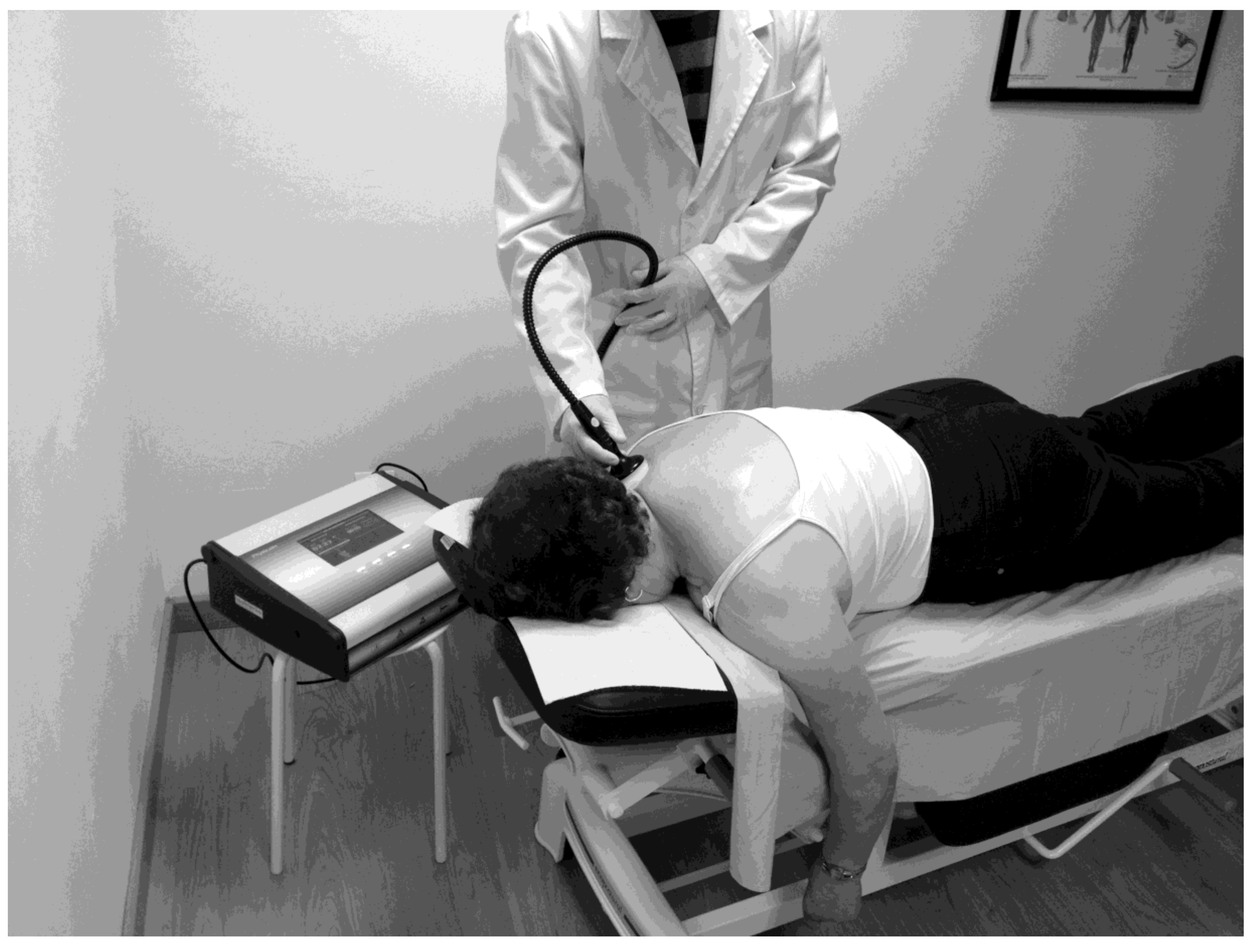
2.2. Intervention
2.3. Outcome Measures
2.3.1. Pain
2.3.2. Quality of Life
2.3.3. Mood
2.3.4. Other measures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wolfe, F.; Brähler, E.; Hinz, A.; Häuser, W. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: results from a survey of the general population. Arthritis Care Res. 2013, 65, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Häuser, W.; Gerhold, K.; Joraschky, P.; Petzke, F.; Tölle, T.; Uçeyler, N.; Winkelmann, A.; Thieme, K. Etiology and pathophysiology of fibromyalgia syndrome and chronic widespread pain. Schmerz. 2008, 22, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, P.J.; Hou, Q.; Argoff, C.E.; Storey, J.R.; Wymer, J.P.; Rice, F.L. Excessive peptidergic sensory innervation of cutaneous arteriole-venule shunts (AVS) in the palmar glabrous skin of fibromyalgia patients: Implications for widespread deep tissue pain and fatigue. Pain Med. 2013, 14, 895–915. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, F.; Talotta, R.; Masala, I.F.; Giacomelli, C.; Conversano, C.; Nucera, V.; Lucchino, B.; Iannuccelli, C.; Di Franco, M.; Bazzichi, L. One year in review 2019: fibromyalgia. Clin. Exp. Rheumatol. 2019, 37, 3–10. [Google Scholar] [PubMed]
- Caro, X.J.; Galbraith, R.G.; Winter, E.F. Evidence of peripheral large nerve involvement in fibromyalgia: a retrospective review of EMG and nerve conduction findings in 55 FM subjects. Eur. J. Rheumatol. 2018, 5, 104–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cagnie, B.; Coppieters, I.; Denecker, S.; Six, J.; Danneels, L.; Meeus, M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin. Arthritis Rheum. 2014, 44, 68–75. [Google Scholar] [CrossRef]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. (Hoboken) 2010, 62, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Rivera, J.; Alegre, C.; Ballina, F.J.; Carbonell, J.; Carmona, L.; Castel, B.; Collado, A.; Esteve, J.J.; Martínez, F.G.; Tornero, J.; et al. Documento de consenso de la Sociedad Española de Reumatología sobre la fibromialgia. Reumatol. Clínica 2006, 2, S55–S66. [Google Scholar] [CrossRef]
- Lacasse, A.; Bourgault, P.; Choinière, M. Fibromyalgia-related costs and loss of productivity: a substantial societal burden. BMC Musculoskelet. Disord. 2016, 17, 168. [Google Scholar] [CrossRef] [Green Version]
- Ablin, J.; Fitzcharles, M.-A.; Buskila, D.; Shir, Y.; Sommer, C.; Häuser, W. Treatment of fibromyalgia syndrome: recommendations of recent evidence-based interdisciplinary guidelines with special emphasis on complementary and alternative therapies. Evid. Based. Complement. Alternat. Med. 2013, 2013, 485272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häuser, W.; Walitt, B.; Fitzcharles, M.-A.; Sommer, C. Review of pharmacological therapies in fibromyalgia syndrome. Arthritis Res. Ther. 2014, 16, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldenberg, D.L.; Clauw, D.J.; Palmer, R.H.; Mease, P.; Chen, W.; Gendreau, R.M. Durability of therapeutic response to milnacipran treatment for fibromyalgia. Results of a randomized, double-blind, monotherapy 6-month extension study. Pain Med. 2010, 11, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, A.; Häuser, W.; Friedel, E.; Moog-Egan, M.; Seeger, D.; Settan, M.; Weiss, T.; Schiltenwolf, M. Physiotherapy and physical therapies for fibromyalgia syndrome. Der Schmerz 2012, 26, 276–286. [Google Scholar] [CrossRef]
- García, D.Á.; Martinez-Nicolas, I.; Saturno-Hernández, P.J. Abordaje clínico de la fibromialgia: síntesis de recomendaciones basadas en la evidencia, una revisión sistemática. Reumatol. Clínica 2016, 12, 65–71. [Google Scholar] [CrossRef]
- Busch, A.J.; Webber, S.C.; Richards, R.S.; Bidonde, J.; Schachter, C.L.; Schafer, L.A.; Danyliw, A.; Sawant, A.; Dal Bello-Haas, V.; Rader, T.; et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst. Rev. 2013, 12, CD010884. [Google Scholar] [CrossRef]
- Häuser, W.; Klose, P.; Langhorst, J.; Moradi, B.; Steinbach, M.; Schiltenwolf, M.; Busch, A. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res. Ther. 2010, 12, R79. [Google Scholar] [CrossRef] [Green Version]
- Dailey, D.L.; Rakel, B.A.; Vance, C.G.T.; Liebano, R.E.; Amrit, A.S.; Bush, H.M.; Lee, K.S.; Lee, J.E.; Sluka, K.A. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain 2013, 154, 2554–2562. [Google Scholar] [CrossRef] [Green Version]
- Carbonario, F.; Matsutani, L.A.; Yuan, S.L.K.; Marques, A.P. Effectiveness of high-frequency transcutaneous electrical nerve stimulation at tender points as adjuvant therapy for patients with fibromyalgia. Eur. J. Phys. Rehabil. Med. 2013, 49, 197–204. [Google Scholar]
- Melzack, R.; Wall, P.D. Pain mechanisms: a new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef]
- Hochsprung, A.; Escudero-Uribe, S.; Ibáñez-Vera, A.J.; Izquierdo-Ayuso, G. Effectiveness of monopolar dielectric transmission of pulsed electromagnetic fields for multiple sclerosis–related pain: A pilot study. Neurologia 2018. [Google Scholar] [CrossRef]
- Albornoz-Cabello, M.; Ibáñez-Vera, A.J.; de la Cruz-Torres, B. Efficacy of monopolar dielectric transmission radio frequency in panniculus adiposus and cellulite reduction Efficacy of monopolar dielectric transmission radio frequency in panniculus adiposus and cellulite reduction. J. Cosmet. laser Ther. 2017, 19, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawant, A.; Dadurka, K.; Overend, T.; Kremenchutzky, M. Systematic review of efficacy of TENS for management of central pain in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2015, 4, 219–227. [Google Scholar] [CrossRef]
- Sutbeyaz, S.T.; Sezer, N.; Koseoglu, F.; Kibar, S. Low-frequency Pulsed Electromagnetic Field Therapy in Fibromyalgia. Clin. J. Pain 2009, 25, 722–728. [Google Scholar] [CrossRef]
- Carrillo-de-la-Peña, M.T.; Triñanes, Y.; González-Villar, A.; Romero-Yuste, S.; Gómez-Perretta, C.; Arias, M.; Wolfe, F. Convergence between the 1990 and 2010 ACR diagnostic criteria and validation of the Spanish version of the Fibromyalgia Survey Questionnaire (FSQ). Rheumatol. Int. 2015, 35, 141–151. [Google Scholar] [CrossRef]
- Plazier, M.; Ost, J.; Stassijns, G.; De Ridder, D.; Vanneste, S. Pain characteristics in fibromyalgia: understanding the multiple dimensions of pain. Clin. Rheumatol. 2015, 34, 775–783. [Google Scholar] [CrossRef]
- Alghadir, A.H.; Anwer, S.; Iqbal, A.; Iqbal, Z.A. Test-retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J. Pain Res. 2018, 11, 851–856. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, M.A.; Rivera, J.; Esteve-Vives, J.; Rejas, J. A confirmatory study of the Combined Index of Severity of Fibromyalgia (ICAF*): factorial structure, reliability and sensitivity to change. Health Qual. Life Outcomes 2011, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, M.A.; Rivera, J.; Esteve-Vives, J.; Rodríguez-Muñoz, M.F. Uso del cuestionario Hospital Anxiety and Depression Scale (HADS) para evaluar la ansiedad y la depresión en pacientes con fibromialgia. Rev. Psiquiatr. Salud Ment. 2012, 5, 107–114. [Google Scholar] [CrossRef]
- Quintana, J.M.; Padierna, A.; Esteban, C.; Arostegui, I.; Bilbao, A.; Ruiz, I. Evaluation of the psychometric characteristics of the Spanish version of the Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 2003, 107, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Löfgren, M.; Norrbrink, C. Pain relief in women with fibromyalgia: a cross-over study of superficial warmth stimulation and transcutaneous electrical nerve stimulation. J. Rehabil. Med. 2009, 41, 557–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutlu, B.; Paker, N.; Bugdayci, D.; Tekdos, D.; Kesiktas, N. Efficacy of supervised exercise combined with transcutaneous electrical nerve stimulation in women with fibromyalgia: a prospective controlled study. Rheumatol. Int. 2013, 33, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Lauretti, G.R.; Chubaci, E.F.; Mattos, A.L. Efficacy of the use of two simultaneously TENS devices for fibromyalgia pain. Rheumatol. Int. 2013, 33, 2117–2122. [Google Scholar] [CrossRef]
- Johnson, M.; Claydon, L.; Herbison, G.; Jones, G.; Paley, C. Transcutaneous electrical nerve stimulation ( TENS ) for fibromyalgia in adults ( Review ). Cochrane Database Syst. Rev. 2017, 10, CD012172. [Google Scholar]
- Crofford, L.J. Psychological aspects of chronic musculoskeletal pain. Best Pract. Res. Clin. Rheumatol. 2015, 29, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Ang, D.C.; Jensen, M.P.; Steiner, J.L.; Hilligoss, J.; Gracely, R.H.; Saha, C. Combining cognitive-behavioral therapy and milnacipran for fibromyalgia: a feasibility randomized-controlled trial. Clin. J. Pain 2013, 29, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Calandre, E.P.; Rico-Villademoros, F.; Slim, M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin. Pharmacother. 2015, 16, 1347–1368. [Google Scholar] [CrossRef]
- Araújo, F.M.; DeSantana, J.M. Physical therapy modalities for treating fibromyalgia. F1000Research 2019, 8, 1–6. [Google Scholar] [CrossRef]
- Andrade, A.; Dominski, F.H.; Sieczkowska, S.M. What we already know about the effects of exercise in patients with fibromyalgia: An umbrella review. Semin. Arthritis Rheum. 2020. [Google Scholar] [CrossRef] [PubMed]
- Vaegter, H.B.; Madsen, A.B.; Handberg, G.; Graven-Nielsen, T. Kinesiophobia is associated with pain intensity but not pain sensitivity before and after exercise: an explorative analysis. Physiotherapy 2018, 104, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaleth, A.S.; Slaven, J.E.; Ang, D.C. Does increasing steps per day predict improvement in physical function and pain interference in adults with fibromyalgia? Arthritis Care Res. (Hoboken) 2014, 66, 1887–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosa-Reina, M.D.; Nunez-Nagy, S.; Gallego-Izquierdo, T.; Pecos-Martín, D.; Monserrat, J.; Álvarez-Mon, M. Effectiveness of Therapeutic Exercise in Fibromyalgia Syndrome: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Biomed Res. Int. 2017, 2017, 2356346. [Google Scholar] [CrossRef]
- da Silva Salazar, A.P.; Stein, C.; Revivo Marchese, R.; Della Méa Plenty, R.; De Souza Pagnussat, A. Electric Stimulation for pain relief in patients with Fibromyalgia: a systematic review and meta-analysis of randomized control trials. Pain Physician 2017, 20, 15–25. [Google Scholar] [CrossRef]
- de Braz, A.S.; de Paula, A.P.; de Diniz, M.F.F.M.; de Almeida, R.N. Non-pharmacological therapy and complementary and alternative medicine in Fibromyalgia. Rev. Bras. Reumatol. 2011, 51, 269–282. [Google Scholar]
- Castro-Sánchez, A.M.; Aguilar-Ferrándiz, M.E.; Matarán-Peñarrocha, G.A.; Sánchez-Joya, M.D.M.; Arroyo-Morales, M.; Fernández-de-las-Peñas, C. Short-term effects of a manual therapy protocol on pain, physical function, quality of sleep, depressive symptoms, and pressure sensitivity in women and men with fibromyalgia syndrome: a randomized controlled trial. Clin. J. Pain 2014, 30, 589–597. [Google Scholar] [CrossRef]
- Sañudo, B.; Carrasco, L.; de Hoyo, M.; McVeigh, J.G. Effects of exercise training and detraining in patients with fibromyalgia syndrome: a 3-yr longitudinal study. Am. J. Phys. Med. Rehabil. 2012, 91, 561–569, quiz 570–3. [Google Scholar] [CrossRef]
- Elkiss, M.; Jerome, J. Touch - More than a basic science. J. Am. Osteopath. Assoc. 2012, 112, 514–517. [Google Scholar]
- Sañudo, B.; Carrasco, L.; De Hoyo, M.; Oliva-Pascual-vaca, Á.; Rodríguez-Blanco, C. Changes in body balance and functional performance following whole-body vibration training in Patients with fibromyalgia syndrome: A Randomized controlled trial. J. Rehabil. Med. 2013, 45, 678–684. [Google Scholar] [CrossRef] [Green Version]
- Häuser, W.; Sarzi-Puttini, P.; Fitzcharles, M.A. Fibromyalgia syndrome: under-, over- and misdiagnosis. Clin. Exp. Rheumatol. 2019, 37, 90–97. [Google Scholar] [PubMed]
| Outcomes | All | Control Group | Sham Group | Experimental Group | ANOVA | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p-Value | |
| Age | 49.00 | 7.17 | 50.63 | 6.69 | 47.78 | 6.32 | 48.57 | 8.25 | 0.461 |
| Height | 1.64 | 0.05 | 1.64 | 0.04 | 1.67 | 0.03 | 1.62 | 0.05 | 0.003 |
| Weight | 65.47 | 4.11 | 65.05 | 4.03 | 64.89 | 2.14 | 66.33 | 5.33 | 0.485 |
| BMI | 24.39 | 2.40 | 24.36 | 2.40 | 23.29 | 1.20 | 25.36 | 2.81 | 0.024 |
| Years diagnosed | 6.58 | 2.53 | 6.43 | 2.66 | 6.82 | 2.08 | 6.48 | 2.87 | 0.861 |
| Pain_G | 6.80 | 1.62 | 6.57 | 1.33 | 7.09 | 1.93 | 6.74 | 1.57 | 0.567 |
| Pain_L | 6.86 | 2.07 | 6.76 | 1.81 | 6.36 | 2.66 | 7.43 | 1.53 | 0.218 |
| HADS | 28.20 | 6.31 | 29.29 | 5.39 | 27.20 | 8.91 | 28.09 | 4.16 | 0.576 |
| ICAF_T | 20.99 | 10.18 | 18.72 | 11.83 | 22.98 | 8.00 | 21.16 | 10.45 | 0.395 |
| ICAF_PF | 60.52 | 11.78 | 55.86 | 14.74 | 64.55 | 8.08 | 60.91 | 10.64 | 0.050 |
| ICAF_EF | 23.42 | 10.40 | 20.71 | 12.17 | 25.18 | 7.26 | 24.22 | 11.13 | 0.340 |
| ICAF_AC | 67.64 | 26.89 | 61.52 | 25.86 | 72.64 | 21.59 | 68.43 | 31.96 | 0.399 |
| ICAF_PC | 37.70 | 15.43 | 35.10 | 17.08 | 40.91 | 12.05 | 37.00 | 16.79 | 0.456 |
| Variable | Group | Pre | Post | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-Value | R2 | Power | ||
| Pain_G | Control | 6.57 | 1.33 | 6.67 | 1.49 | 0.001 | 0.254 | 0.953 |
| Sham | 7.09 | 1.93 | 6.64 | 1.87 | ||||
| Experimental | 6.74 | 1.57 | 4.91 | 2.43 | ||||
| Pain_L | Control | 6.76 | 1.81 | 7.33 | 1.35 | <0.001 | 0.455 | 1.000 |
| Sham | 6.36 | 2.66 | 5.91 | 2.11 | ||||
| Experimental | 7.43 | 1.53 | 3.61 | 2.62 | ||||
| HADS | Control | 29.29 | 5.39 | 32.35 | 5.49 | 0.456 | 0.032 | 0.179 |
| Sham | 27.20 | 8.91 | 29.00 | 5.40 | ||||
| Experimental | 28.09 | 4.16 | 31.91 | 4.69 | ||||
| ICAF_T | Control | 18.72 | 11.83 | 18.05 | 10.63 | <0.001 | 0.422 | 1.000 |
| Sham | 22.98 | 8.00 | 17.07 | 6.92 | ||||
| Experimental | 21.16 | 10.45 | 9.61 | 11.05 | ||||
| ICAF_PF | Control | 55.86 | 14.74 | 56.33 | 14.21 | <0.001 | 0.381 | 0.999 |
| Sham | 64.55 | 8.08 | 53.82 | 12.34 | ||||
| Experimental | 60.91 | 10.64 | 41.17 | 16.24 | ||||
| ICAF_EF | Control | 20.71 | 12.17 | 19.57 | 12.31 | <0.001 | 0.284 | 0.976 |
| Sham | 25.18 | 7.26 | 18.55 | 6.62 | ||||
| Experimental | 24.22 | 11.13 | 14.17 | 8.54 | ||||
| ICAF_AC | Control | 61.52 | 25.86 | 60.43 | 20.23 | 0.032 | 0.131 | 0.651 |
| Sham | 72.64 | 21.59 | 67.55 | 26.13 | ||||
| Experimental | 68.43 | 31.96 | 77.39 | 25.32 | ||||
| ICAF_PC | Control | 35.10 | 17.08 | 31.81 | 17.20 | 0.076 | 0.100 | 0.513 |
| Sham | 40.91 | 12.05 | 44.18 | 12.55 | ||||
| Experimental | 37.00 | 16.79 | 29.65 | 17.22 | ||||
| Outcomes | Control Group | Sham Group | Experimental Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Lower | Upper | p-Value | Mean | Lower | Upper | p-Value | Mean | Lower | Upper | p-Value | |
| Pain_G | 0.26 | −0.38 | 0.89 | 0.418 | 0.09 | −0.70 | 0.88 | 0.818 | 1.81 | 1.20 | 2.42 | <0.001 |
| Pain_L | −0.46 | −1.47 | 0.55 | 0.360 | 0.25 | −1.00 | 1.50 | 0.693 | 3.76 | 2.79 | 4.72 | <0.001 |
| HADS | −3.29 | −7.22 | 0.65 | 0.100 | 0.31 | −4.56 | 5.19 | 0.898 | −3.44 | −7.21 | 0.33 | 0.073 |
| ICAF_T | 0.10 | −2.92 | 3.13 | 0.947 | 5.88 | 2.13 | 9.62 | 0.003 | 12.40 | 9.51 | 15.30 | <0.001 |
| ICAF_PF | −0.82 | −6.39 | 4.75 | 0.768 | 12.59 | 5.69 | 19.48 | 0.001 | 19.69 | 14.37 | 25.02 | <0.001 |
| ICAF_EF | 0.84 | −2.50 | 4.18 | 0.616 | 7.37 | 3.24 | 11.51 | 0.001 | 10.70 | 7.50 | 13.89 | <0.001 |
| ICAF_AC | 3.08 | −5.37 | 11.52 | 0.468 | 5.20 | −5.26 | 15.67 | 0.322 | −10.39 | −18.48 | −2.31 | 0.013 |
| ICAF_PC | 2.94 | −2.75 | 8.63 | 0.304 | −3.05 | −10.09 | 4.00 | 0.389 | 7.75 | 2.31 | 13.19 | 0.006 |
| Outcome | Experimental Group vs. | Mean Difference | Standard Error | 95 % Confidence Interval for Mean Difference | p-Value | |
|---|---|---|---|---|---|---|
| Upper bound | Lower bound | |||||
| Pain_G | Control Group | 1.617 | 0.662 | −0.024 | 3.259 | 0.055 |
| Sham Group | 1.720 | 0.798 | −0.259 | 3.698 | 0.109 | |
| Pain_L | Control Group | 3.659 | 0.677 | 1.982 | 5.337 | <0.001 |
| Sham Group | 2.242 | 0.816 | 0.220 | 4.263 | 0.025 | |
| HADS | Control Group | 0.188 | 1.604 | −3.788 | 4.164 | 1.000 |
| Sham Group | −4.720 | 1.933 | −9.513 | 0.073 | 0.055 | |
| ICAF_T | Control Group | 9.259 | 3.167 | 1.407 | 17.111 | 0.016 |
| Sham Group | 8.170 | 3.818 | −1.295 | 17.635 | 0.112 | |
| ICAF_PF | Control Group | 15.196 | 4.345 | 4.426 | 25.966 | 0.003 |
| Sham Group | 13.980 | 5.237 | 0.998 | 26.963 | 0.031 | |
| ICAF_EF | Control Group | 5.992 | 3.159 | −1.839 | 13.823 | 0.191 |
| Sham Group | 5.031 | 3.808 | −4.409 | 14.470 | 0.578 | |
| ICAF_AC | Control Group | −16.340 | 8.119 | −36.466 | 3.785 | 0.149 |
| Sham Group | −5.932 | 9.786 | −30.192 | 18.328 | 1.000 | |
| ICAF_PC | Control Group | 4.265 | 5.327 | −8.940 | 17.469 | 1.000 |
| Sham Group | 13.451 | 6.421 | −2.466 | 29.368 | 0.124 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibáñez-Vera, A.J.; García-Romero, J.C.; Alvero-Cruz, J.R.; Lomas-Vega, R. Effects of Monopolar Dielectric Radiofrequency Signals on the Symptoms of Fibromyalgia: A Single-Blind Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 2465. https://doi.org/10.3390/ijerph17072465
Ibáñez-Vera AJ, García-Romero JC, Alvero-Cruz JR, Lomas-Vega R. Effects of Monopolar Dielectric Radiofrequency Signals on the Symptoms of Fibromyalgia: A Single-Blind Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2020; 17(7):2465. https://doi.org/10.3390/ijerph17072465
Chicago/Turabian StyleIbáñez-Vera, Alfonso Javier, Jerónimo Carmelo García-Romero, José Ramón Alvero-Cruz, and Rafael Lomas-Vega. 2020. "Effects of Monopolar Dielectric Radiofrequency Signals on the Symptoms of Fibromyalgia: A Single-Blind Randomized Controlled Trial" International Journal of Environmental Research and Public Health 17, no. 7: 2465. https://doi.org/10.3390/ijerph17072465







