Postural Control during Progressively Increased Balance-Task Difficulty in Athletes with Unilateral Transfemoral Amputation: Effect of Ocular Mobility and Visuomotor Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Postural Control Evaluation
2.2. Oculomotor Mobility Evaluation
2.3. Visuomotor Processing Evaluation
2.4. Statistical Analysis
3. Results
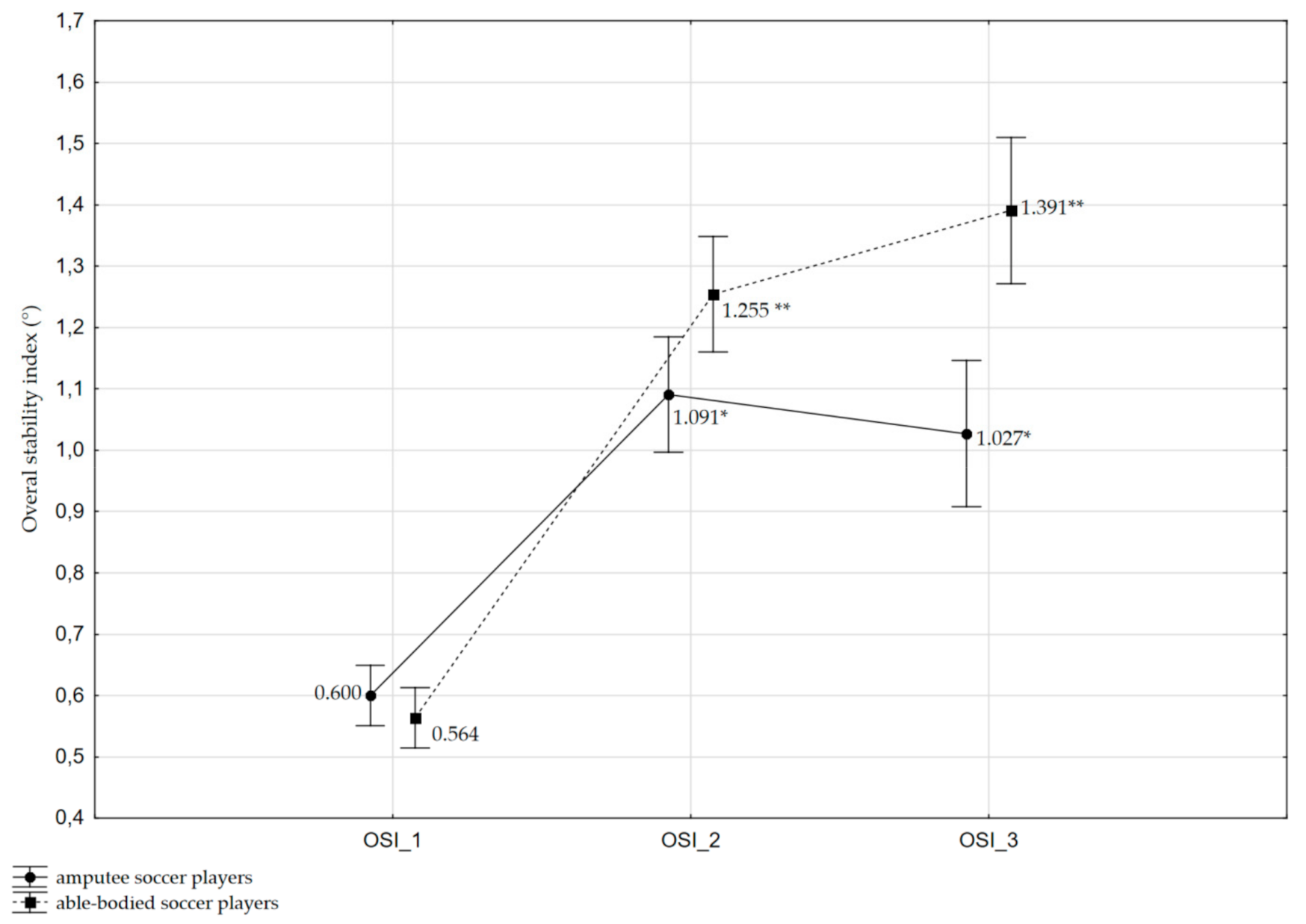
3.1. Effects on OSI
3.2. Effects on API
3.3. Effects on MLI
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Araújo, H.N.d.; Mendes, F.A.d.S.; Fortes, C.E.; Borin, G.; Garcia, P.A.; Macedo, O.G.; Marães, V.R.F.d.S.; Durigan, J.L.Q. Dynamic and Static Postural Control in Volleyball Players with Transfemoral Amputation. Rev. Bras. Med. Esporte 2019, 25, 58–62. [Google Scholar] [CrossRef]
- Beausoleil, S.; Miramand, L.; Turcot, K. Evolution of gait parameters in individuals with a lower-limb amputation during a six-minute walk test. Gait Posture 2019, 72, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Bolger, D.; Ting, L.H.; Sawers, A. Individuals with transtibial limb loss use interlimb force asymmetries to maintain multi-directional reactive balance control. Clin. Biomech. 2014, 29, 1039–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, P.X.; Abu Osman, N.A.; Wan Abas, W.A. Balance control in lower extremity amputees during quiet standing: A systematic review. Gait Posture 2014, 39, 672–682. [Google Scholar] [CrossRef] [Green Version]
- Pollock, A.; Baer, G.; Langhorne, P.; Pomeroy, V. Physiotherapy treatment approaches for the recovery of postural control and lower limb function following stroke: A systematic review. Clin. Rehabil. 2007, 21, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.; Sawamura, S.; Fujita, H.; Nakajima, S.; Oyabu, H.; Nagakura, Y.; Ojima, I.; Otsuka, H.; Nakagawa, A. Physical fitness of lower limb amputees. Am. J. Phys. Med. Rehabil. 2002, 81, 321–325. [Google Scholar] [CrossRef]
- Van Velzen, J.M.; van Bennekom, C.A.; Polomski, W.; Slootman, J.R.; van der Woude, L.H.; Houdijk, H. Physical capacity and walking ability after lower limb amputation: A systematic review. Clin. Rehabil. 2006, 20, 999–1016. [Google Scholar] [CrossRef] [Green Version]
- De-Rosende Celeiro, I.; Simon Sanjuan, L.; Santos-Del-Riego, S. Activities of daily living in people with lower limb amputation: Outcomes of an intervention to reduce dependence in pre-prosthetic phase. Disabil. Rehabil. 2017, 39, 1799–1806. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.; van Den Heuvel, W.J. A systematic literature review of quality of life in lower limb amputees. Disabil. Rehabil. 2011, 33, 883–899. [Google Scholar] [CrossRef]
- Buckley, J.G.; O’Driscoll, D.; Bennett, S.J. Postural sway and active balance performance in highly active lower-limb amputees. Am. J. Phys. Med. Rehabil. 2002, 81, 13–20. [Google Scholar] [CrossRef]
- Viton, J.M.; Mouchnino, L.; Mille, M.L.; Cincera, M.; Delarque, A.; Pedotti, A.; Bardot, A.; Massion, J. Equilibrium and movement control strategies in trans-tibial amputees. Prosthet. Orthot. Int. 2000, 24, 108–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnett, C.T.; Vanicek, N.; Polman, R.C. Postural responses during volitional and perturbed dynamic balance tasks in new lower limb amputees: A longitudinal study. Gait Posture 2013, 37, 319–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arifin, N.; Abu Osman, N.A.; Ali, S.; Wan Abas, W.A. The effects of prosthetic foot type and visual alteration on postural steadiness in below-knee amputees. Biomed. Eng. Online 2014, 13, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanicek, N.; Strike, S.; McNaughton, L.; Polman, R. Postural responses to dynamic perturbations in amputee fallers versus nonfallers: A comparative study with able-bodied subjects. Arch. Phys. Med. Rehabil. 2009, 90, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Li, C.; Wu, J.; Jiang, T.; Zhang, Y.; Zhao, L.; Evans, A.C.; Li, L.; Ran, S.; Yin, X.; et al. Progressive Thinning of Visual Motion Area in Lower Limb Amputees. Front. Hum. Neurosci. 2016, 10, 79. [Google Scholar] [CrossRef]
- Aytar, A.; Pekyavas, N.O.; Ergun, N.; Karatas, M. Is there a relationship between core stability, balance and strength in amputee soccer players? A pilot study. Prosthet. Orthot. Int. 2012, 36, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Simim, M.A.M.; da Mota, G.R.; Marocolo, M.; da Silva, B.V.C.; de Mello, M.T.; Bradley, P.S. The Demands of Amputee Soccer Impair Muscular Endurance and Power Indices But Not Match Physical Performance. Adapt. Phys. Act. Q. 2018, 35, 76–92. [Google Scholar] [CrossRef]
- Yazicioglu, K.; Taskaynatan, M.A.; Guzelkucuk, U.; Tugcu, I. Effect of playing football (soccer) on balance, strength, and quality of life in unilateral below-knee amputees. Am. J. Phys. Med. Rehabil. 2007, 86, 800–805. [Google Scholar] [CrossRef]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35, ii7–ii11. [Google Scholar] [CrossRef] [Green Version]
- Laurens, J.; Awai, L.; Bockisch, C.J.; Hegemann, S.; van Hedel, H.J.; Dietz, V.; Straumann, D. Visual contribution to postural stability: Interaction between target fixation or tracking and static or dynamic large-field stimulus. Gait Posture 2010, 31, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, S.T.; Polastri, P.F.; Carvalho, J.C.; Barela, J.A.; Moraes, R.; Barbieri, F.A. Saccadic and smooth pursuit eye movements attenuate postural sway similarly. Neurosci. Lett. 2015, 584, 292–295. [Google Scholar] [CrossRef]
- Thomas, N.M.; Bampouras, T.M.; Donovan, T.; Dewhurst, S. Eye Movements Affect Postural Control in Young and Older Females. Front. Aging Neurosci. 2016, 8, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatzitaki, V.; Zisi, V.; Kollias, I.; Kioumourtzoglou, E. Perceptual-motor contributions to static and dynamic balance control in children. J. Mot. Behav. 2002, 34, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Rydalch, G.; Bell, H.B.; Ruddy, K.L.; Bolton, D.A.E. Stop-signal reaction time correlates with a compensatory balance response. Gait Posture 2019, 71, 273–278. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.W. The importance of a priori sample size estimation in strength and conditioning research. J. Strength Cond. Res. 2013, 27, 2323–2337. [Google Scholar] [CrossRef] [PubMed]
- Poiroux, E.; Cavaro-Menard, C.; Leruez, S.; Lemee, J.M.; Richard, I.; Dinomais, M. What Do Eye Gaze Metrics Tell Us about Motor Imagery? PLoS ONE 2015, 10, e0143831. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge Academic: New York, NY, USA, 1988. [Google Scholar]
- Chin, T.; Sawamura, S.; Fujita, H.; Nakajima, S.; Ojima, I.; Oyabu, H.; Nagakura, Y.; Otsuka, H.; Nakagawa, A. Effect of endurance training program based on anaerobic threshold (AT) for lower limb amputees. J. Rehabil. Res. Dev. 2001, 38, 7–11. [Google Scholar]
- Guchan, Z.; Bayramlar, K.; Ergun, N. Determination of the effects of playing soccer on physical fitness in individuals with transtibial amputation. J. Sports Med. Phys. Fit. 2017, 57, 879–886. [Google Scholar]
- Bragaru, M.; Dekker, R.; Geertzen, J.H.; Dijkstra, P.U. Amputees and sports: A systematic review. Sports Med. 2011, 41, 721–740. [Google Scholar] [CrossRef]
- Yanci, A.; Banu, H. Comparison of the quality of life of footballer and non footballer amputees. Int. J. Acad. Res. 2014, 6, 350–355. [Google Scholar] [CrossRef]
- Monteiro, R.; Pfeifer, L.; Santos, A.; Sousa, N. Soccer practice and functional and social performance of men with lower limb amputations. J. Hum. Kinet. 2014, 43, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Kasińska, Z.; Tasiemski, T. Determinants of sports injuries in amputee football: Initial analysis. Trends Sport Sci. 2017, 2, 73–79. [Google Scholar]
- Ozkan, A.; Kayihan, G.; Koklu, Y.; Ergun, N.; Koz, M.; Ersoz, G.; Dellal, A. The relationship between body composition, anaerobic performance and sprint ability of amputee soccer players. J. Hum. Kinet. 2012, 35, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Simim, M.A.; Bradley, P.S.; da Silva, B.V.; Mendes, E.L.; de Mello, M.T.; Marocolo, M.; da Mota, G.R. The quantification of game-induced muscle fatigue in amputee soccer players. J. Sports Med. Phys. Fit. 2017, 57, 766–772. [Google Scholar]
- Gunduz, M.E.; Pinto, C.B.; Saleh Velez, F.G.; Duarte, D.; Pacheco-Barrios, K.; Lopes, F.; Fregni, F. Motor Cortex Reorganization in Limb Amputation: A Systematic Review of TMS Motor Mapping Studies. Front. Neurosci. 2020, 14, 314. [Google Scholar] [CrossRef] [Green Version]
- Kavounoudias, A.; Tremblay, C.; Gravel, D.; Iancu, A.; Forget, R. Bilateral changes in somatosensory sensibility after unilateral below-knee amputation. Arch. Phys. Med. Rehabil. 2005, 86, 633–640. [Google Scholar] [CrossRef]
- Rodrigues, S.T.; Gotardi, G.C.; Aguiar, S.A. Effects of vision on postural control in neurologically healthy individuals. In Locomotion and Posture in Older Adults; Springer International Publishing: New York, NY, USA, 2017; pp. 219–236. [Google Scholar]
- Fujishita, H.; Urabe, Y.; Maeda, N.; Komiya, M.; Sakai, S.; Hirata, K.; Sakamitsu, T.; Kimura, H. Biomechanics of single-leg running using lofstrand crutches in amputee soccer. J. Phys. Sci. 2018, 30, 1483–1487. [Google Scholar] [CrossRef] [Green Version]
- Saverino, A.; Waller, D.; Rantell, K.; Parry, R.; Moriarty, A.; Playford, E.D. The Role of Cognitive Factors in Predicting Balance and Fall Risk in a Neuro-Rehabilitation Setting. PLoS ONE 2016, 11, e0153469. [Google Scholar] [CrossRef] [Green Version]
- Caetano, M.J.D.; Menant, J.C.; Schoene, D.; Pelicioni, P.H.S.; Sturnieks, D.L.; Lord, S.R. Sensorimotor and Cognitive Predictors of Impaired Gait Adaptability in Older People. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1257–1263. [Google Scholar] [CrossRef] [Green Version]
- Piras, A.; Lobietti, R.; Squatrito, S. Response time, visual search strategy, and anticipatory skills in volleyball players. J. Ophthalmol. 2014, 2014, 189268. [Google Scholar] [CrossRef] [PubMed]
- Zwierko, T.; Osiński, W.; Lubiński, W.; Czepita, D.; Florkiewicz, B. Speed of visual sensorimotor processes and conductivity in visual pathway in volleyball players. J. Hum. Kinet. 2010, 23, 21–27. [Google Scholar] [CrossRef]
- Zwierko, T.; Lubinski, W.; Lesiakowski, P.; Steciuk, H.; Piasecki, L.; Krzepota, J. Does athletic training in volleyball modulate the components of visual evoked potentials? A preliminary investigation. J. Sports Sci 2014, 32, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Static Postural Control | Postural Control during Decreasing Platform Stability at Levels 8 to 4 | Postural Control during Platform Stability at Level 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OSI_1 (°) | API_1 (°) | MLI_1 (°) | OSI_2 (°) | API_2 (°) | MLI_2 (°) | OSI_3 (°) | API_3 (°) | MLI_3 (°) | |
| Oculomotor Function | |||||||||
| Saccade acceleration (°/s2) | 0.281 | 0.310 | 0.224 | −0.059 | −0.083 | −0.011 | −0.031 | 0.076 | −0.132 |
| Saccade velocity (°/s) | 0.303 | 0.337 | 0.261 | −0.046 | −0.098 | −0.077 | −0.007 | −0.053 | −0.125 |
| OMI (%) | 0.064 | −0.028 | 0.107 | −0.656 * | −0.624 * | −0.549 | −0.587 | −0.648 * | −0.427 |
| Simple Reaction Time Test | |||||||||
| Reaction time (ms) | 0.234 | 0.216 | 0.224 | 0.384 | 0.271 | 0.416 | 0.193 | 0.230 | 0.124 |
| Motor time (ms) | 0.230 | 0.260 | 0.231 | 0.427 | 0.243 | 0.607* | 0.310 | 0.206 | 0.344 |
| Speed of eye-hand reaction (ms) | 0.363 | 0.268 | 0.258 | 0.457 | 0.295 | 0.571 | 0.280 | 0.248 | 0.256 |
| Choice Reaction Time Test | |||||||||
| Reaction time (ms) | 0.190 | 0.145 | 0.195 | 0.183 | 0.104 | 0.265 | 0.154 | 0.039 | 0.220 |
| Motor time (ms) | 0.209 | 0.197 | 0.183 | 0.514 | 0.373 | 0.519 | 0.260 | 0.202 | 0.274 |
| Speed of eye-hand reaction (ms) | 0.204 | 0.168 | 0.198 | 0.308 | 0.204 | 0.365 | 0.198 | 0.100 | 0.247 |
| Peripheral Perception Test | |||||||||
| Visual field(°) | −0.232 | −0.189 | −0.234 | 0.313 | 0.182 | 0.302 | −0.156 | −0.042 | −0.233 |
| Speed of eye-foot reaction (ms) | 0.214 | 0.196 | 0.222 | 0.683 * | 0.691 * | 0.478 | 0.621* | 0.544 | 0.634 * |
| Tracking deviation (pixels) | −0.088 | −0.039 | −0.156 | 0.032 | 0.021 | −0.043 | −0.119 | −0.097 | −0.181 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwierko, M.; Lesiakowski, P.; Zwierko, T. Postural Control during Progressively Increased Balance-Task Difficulty in Athletes with Unilateral Transfemoral Amputation: Effect of Ocular Mobility and Visuomotor Processing. Int. J. Environ. Res. Public Health 2020, 17, 6242. https://doi.org/10.3390/ijerph17176242
Zwierko M, Lesiakowski P, Zwierko T. Postural Control during Progressively Increased Balance-Task Difficulty in Athletes with Unilateral Transfemoral Amputation: Effect of Ocular Mobility and Visuomotor Processing. International Journal of Environmental Research and Public Health. 2020; 17(17):6242. https://doi.org/10.3390/ijerph17176242
Chicago/Turabian StyleZwierko, Michał, Piotr Lesiakowski, and Teresa Zwierko. 2020. "Postural Control during Progressively Increased Balance-Task Difficulty in Athletes with Unilateral Transfemoral Amputation: Effect of Ocular Mobility and Visuomotor Processing" International Journal of Environmental Research and Public Health 17, no. 17: 6242. https://doi.org/10.3390/ijerph17176242





