The Cost-Effectiveness of Hepatitis C Virus Screening Strategies among Recently Arrived Migrants in the Netherlands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Course of Disease
2.2. Model
2.3. Epidemiology of HCV among Middle Eastern Migrants in The Netherlands
2.4. HCV Screening and Treatment
2.5. Health Outcomes
2.6. Screening and Treatment Strategies
2.7. Cost-Effectiveness and Budget-Impact Analyses
2.8. Sensitivity Analyses
3. Results
3.1. Budget Impact of HCV Screening and Treatment
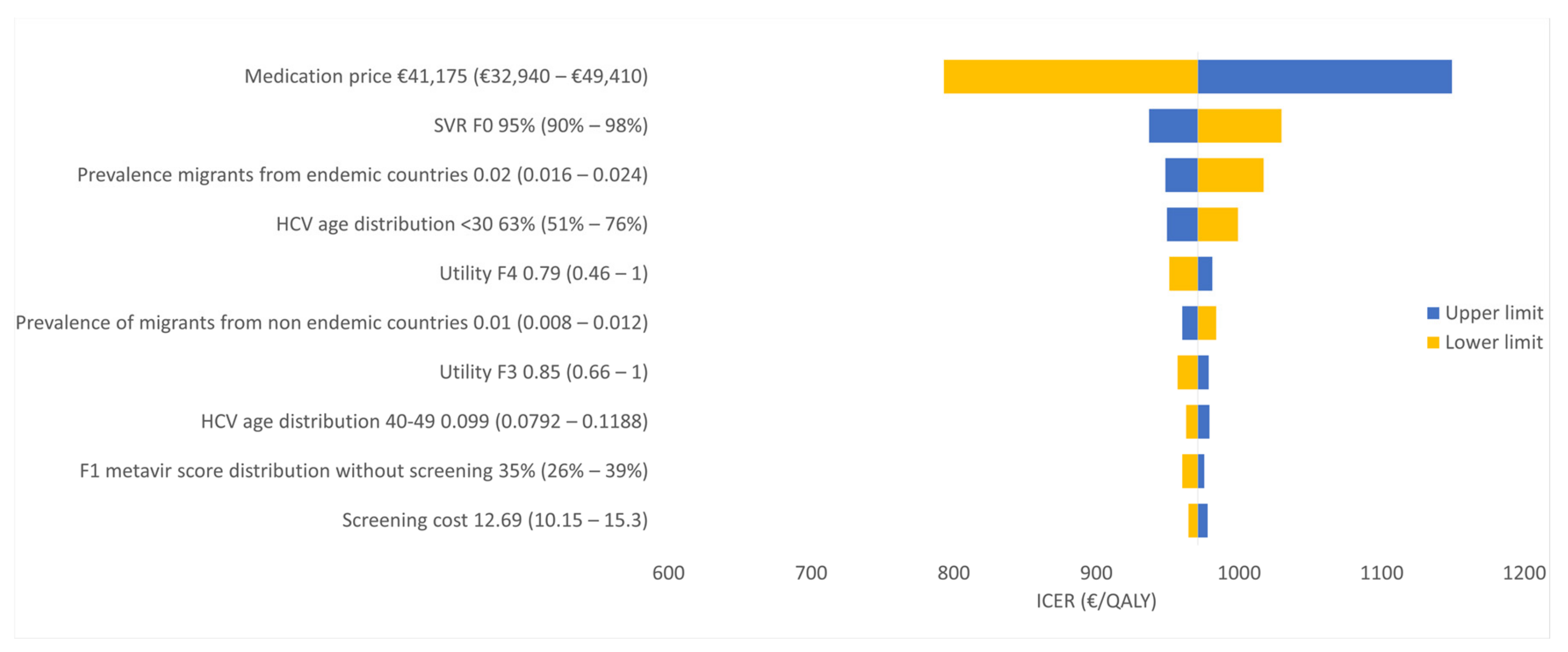
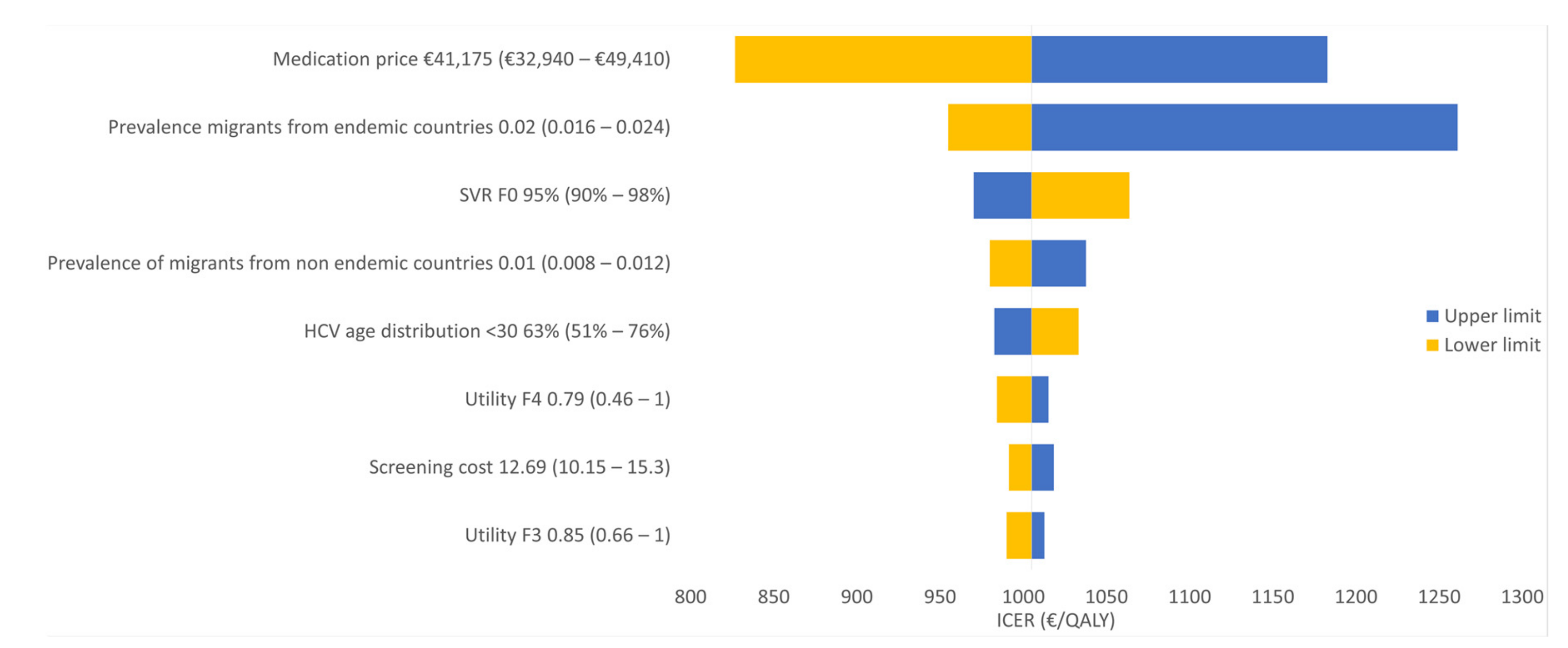
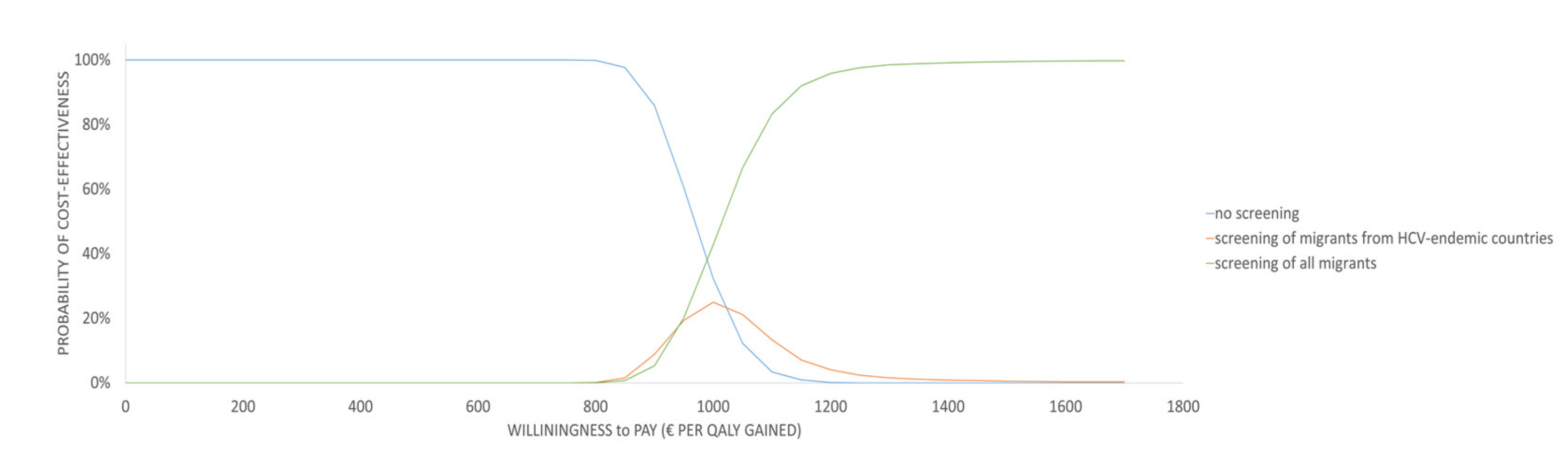
3.2. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Screening van risicogroepen op hepatitis B en C. Advies Gezondheidsraad. pp. 17–22. Available online: https://www.gezondheidsraad.nl/documenten/adviezen/2016/11/01/screening-van-risicogroepen-op-hepatitis-b-en-c (accessed on 17 February 2017).
- CBS. Available online: https://www.cbs.nl/ (accessed on 17 June 2017).
- Baaten, G.G.G.; Sonder, G.J.B.; Dukers, N.H.T.M.; Coutinho, R.A.; Van den Hoek, J.A.R. Population-based study on the seroprevalence of hepatitis A, B, and C virus infection in Amsterdam, 2004. J. Med. Virol. 2007, 79, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Urbanus, A.T.; van de Laar, T.J.W.; van den Hoek, A.; Zuure, F.R.; Speksnijder, A.G.C.L.; Baaten, G.G.G.; Heijman, T.; Vriend, H.J.; Op de Coul, E.L.; Coutinho, R.A.; et al. Hepatitis C in the general population of various ethnic origins living in the Netherlands: Should non-Western migrants be screened? J. Hepatol. 2011, 55, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Is koppeling van vrijwillige screening op hepatitis B en C aan de verplichte tuberculosescreening voor immigranten zinvol? RIVM. Available online: https://www.rivm.nl/publicaties/is-koppeling-van-vrijwillige-screening-op-hepatitis-b-en-c-aan-verplichte (accessed on 17 May 2017).
- The “Arab Spring”: Five years on Amnesty International. Available online: https://www.amnesty.org/en/latest/campaigns/2016/01/arab-spring-five-years-on/ (accessed on 20 February 2017).
- Hepatitis C LCI richtlijnen. Available online: https://lci.rivm.nl/richtlijnen/hepatitis-c (accessed on 17 May 2017).
- Lawitz, E.; Gane, E.; Pearlman, B.; Tam, E.; Ghesquiere, W.; Guyader, D.; Alric, L.; Bronowicki, J.P.; Lester, L.; Sievert, W.; et al. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): A randomised, open-label phase 2 trial. Lancet 2015, 385, 1075–1086. [Google Scholar]
- Afdhal, N.; Zeuzem, S.; Kwo, P.; Chojkier, M.; Gitlin, N.; Puoti, M.; Romero-Gomez, M.; Zarski, J.P.; Agarwal, K.; Buggisch, P.; et al. Ledipasvir and Sofosbuvir for Untreated HCV Genotype 1 Infection. N. Engl. J. Med. 2014, 370, 1889–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manns, M.P.; Foster, G.R.; Rockstroh, J.K.; Zeuzem, S.; Zoulim, F.; Houghton, M. The way forward in HCV treatment—Finding the right path. Nat. Rev. Drug Discov. 2007, 6, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Hepatitis C. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 3 April 2020).
- Khullar, V.; Firpi, R.J. Hepatitis C cirrhosis: New perspectives for diagnosis and treatment. World J. Hepatol. 2015, 7, 1843–1855. [Google Scholar] [CrossRef]
- Westbrook, R.H.; Dusheiko, G. Natural history of hepatitis C. J. Hepatol. 2014, 61, S58–S68. [Google Scholar] [CrossRef] [Green Version]
- Chahal, H.S.; Marseille, E.A.; Tice, J.A.; Pearson, S.D.; Ollendorf, D.A.; Fox, R.K.; Kahn, J.G. Cost-effectiveness of Early Treatment of Hepatitis C Virus Genotype 1 by Stage of Liver Fibrosis in a US Treatment-Naive Population. JAMA Intern. Med. 2016, 176, 65. [Google Scholar] [CrossRef]
- Slavenburg, S.; Verduyn-Lunel, F.M.; Hermsen, J.T.; Melchers, W.J.G.; te Morsche, R.H.M.; Drenth, J.P.H. Prevalence of hepatitis C in the general population in the Netherlands. Neth. J. Med. 2008, 66, 13–17. [Google Scholar]
- Alberti, A.; Noventa, F.; Benvegnù, L.; Boccato, S.; Gatta, A. Prevalence of liver disease in a population of asymptomatic persons with hepatitis C virus infection. Ann. Intern. Med. 2002, 137, 961–964. [Google Scholar] [CrossRef]
- Thein, H.-H.; Yi, Q.; Dore, G.J.; Krahn, M.D. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: A meta-analysis and meta-regression. Hepatology 2008, 48, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Salomon, J.A. Cost-effectiveness of Treatment for Chronic Hepatitis C Infection in an Evolving Patient Population. JAMA 2003, 290, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plunkett, B.A.; Grobman, W.A. Routine hepatitis C virus screening in pregnancy: A cost-effectiveness analysis. Am. J. Obstet. Gynecol. 2005, 192, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.; Jones, J.; Hartwell, D.; Davidson, P.; Waugh, N.; Price, A. Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: A systematic review and economic evaluation. Health Technol. Assess. 2007, 11, 1–205. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for the Screening, Care and Treatment of Persons with Chronic Hepatitis C Infection; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Kosteneffectiviteit in de praktijk ;Rapport Zorginstituut Nederland. Available online: https://www.zorginstituutnederland.nl/publicaties/rapport/2015/06/26/kosteneffectiviteit-in-de-praktijk (accessed on 1 September 2017).
- Zuure, F.R.; Bouman, J.; Martens, M.; Vanhommerig, J.W.; Urbanus, A.T.; Davidovich, U.; van Houdt, R.; Speksnijder, A.G.; Weegink, C.J.; van den Hoek, A.; et al. Screening for hepatitis B and C in first-generation Egyptian migrants living in the Netherlands. Liver Int. 2013, 33, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Daw, M.A.; Dau, A.A. Hepatitis C Virus in Arab World: A State of Concern. Sci. World J. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Yang, J.H.; Lai, J.P.; Douglas, S.D.; Metzger, D.; Zhu, X.H.; Ho, W.Z. Real-time RT-PCR for quantitation of hepatitis C virus RNA. J. Virol. Methods 2002, 102, 119–128. [Google Scholar] [CrossRef]
- Afdhal, N.H. Fibroscan (transient elastography) for the measurement of liver fibrosis. Gastroenterol. Hepatol. 2012, 8, 605–607. [Google Scholar]
- Zhang, F.; Zhu, H.; Wu, Y.; Dou, Z.; Zhang, Y.; Kleinman, N.; Bulterys, M.; Wu, Z.; Ma, Y.; Zhao, D.; et al. HIV, hepatitis B virus, and hepatitis C virus co-infection in patients in the China National Free Antiretroviral Treatment Program, 2010–12: A retrospective observational cohort study. Lancet Infect. Dis. 2014, 14, 1065–1172. [Google Scholar] [CrossRef]
- Do, A.; Mittal, Y.; Liapakis, A.; Cohen, E.; Chau, H.; Bertuccio, C.; Sapir, D.; Wright, J.; Eggers, C.; Drozd, K.; et al. Drug Authorization for Sofosbuvir/Ledipasvir (Harvoni) for Chronic HCV Infection in a Real-World Cohort: A New Barrier in the HCV Care Cascade. PLoS ONE 2015, 10, e0135645. [Google Scholar] [CrossRef]
- Jensen, C.M.; Holle, L.M. Ledipasvir-Sofosbuvir: A Once-Daily Oral Treatment Option for Chronic Hepatitis C Virus Genotype 1 Infection. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2016, 36, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Raedler, L.A. Viekira Pak (Ombitasvir, Paritaprevir, and Ritonavir Tablets; Dasabuvir Tablets): All-Oral Fixed Combination Approved for Genotype 1 Chronic Hepatitis C Infection. Am. Health Drug Benefits 2015, 8, 142. [Google Scholar]
- Na, S.K.; Song, B.C. Development and surveillance of hepatocellular carcinoma in patients with sustained virologic response after antiviral therapy for chronic hepatitis C. Clin. Mol. Hepatol. 2019, 25, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Boerekamps, A.; Newsum, A.M.; Smit, C.; Arends, J.E.; Richter, C.; Reiss, P.; Rijnders, B.J.A.; Brinkman, K.; van der Valk, M.; NVHB-SHM Hepatitis Working Group and the Netherlands ATHENA HIV Observational Cohort. High Treatment Uptake in Human Immunodeficiency Virus/Hepatitis C Virus–Coinfected Patients After Unrestricted Access to Direct-Acting Antivirals in the Netherlands. Clin. Infect. Dis. 2018, 66, 1352–1359. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, A.; Kitson, M.T.; Roberts, S.K. Systematic review: Current concepts and challenges for the direct-acting antiviral era in hepatitis C cirrhosis. Aliment Pharmacol. Ther. 2016, 43, 1276–1292. [Google Scholar] [CrossRef] [Green Version]
- HCV Richtsnoer—Home—Hepatitis C. Available online: https://hcvrichtsnoer.nl/ (accessed on 10 September 2017).
- Welkom bij Medicijnkosten. Available online: https://www.medicijnkosten.nl/ (accessed on 12 September 2017).
- Liu, S.; Cipriano, L.E.; Holodniy, M.; Owens, D.K.; Goldhaber-Fiebert, J.D. New Protease Inhibitors for the Treatment of Chronic Hepatitis C. Ann. Intern. Med. 2012, 156, 279. [Google Scholar] [CrossRef] [PubMed]
- McLernon, D.J.; Dillon, J.; Donnan, P.T. Systematic Review: Health-State Utilities in Liver Disease: A Systematic Review. Med. Decis. Mak. 2008, 28, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Crossan, C.; Tsochatzis, E.A.; Longworth, L.; Gurusamy, K.; Davidson, B.; Rodríguez-Perálvarez, M.; Mantzoukis, K.; O’Brien, J.; Thalassinos, E.; Papastergiou, V.; et al. Cost-effectiveness of non-invasive methods for assessment and monitoring of liver fibrosis and cirrhosis in patients with chronic liver disease: Systematic review and economic evaluation. Health Technol. Assess. 2015, 19, 1–410. [Google Scholar] [CrossRef] [PubMed]
- Juanbeltz, R.; Martínez-Baz, I.; San Miguel, R.; Goñi-Esarte, S.; Cabasés, J.M.; Castilla, J. Impact of successful treatment with directacting antiviral agents on health-related quality of life in chronic hepatitis C patients. PLoS ONE 2018, 13, e0205277. [Google Scholar] [CrossRef] [Green Version]
- Tan-Torres Edejer, T.; Baltussen, R.; Adam, T.; Hutubessy, R.; Acharya, A.; Evans, D.B.; Murray, C.J.L. Making Choices in Health: WHO Guide to Cost Effectiveness Analysis; Book review—Semantic Scholar; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg; Publicatie Zorginstituut Nederland. Available online: https://www.zorginstituutnederland.nl/over-ons/publicaties/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg (accessed on 20 September 2017).
- Luna, L.C.; Costa, M.G.; Tura, B.R.; Correia, M.G.; Santos, M. Guidelines for Budget Impact Analysis: A Literature Review. Value Health 2016, 19, A78. [Google Scholar] [CrossRef]
- Wong, W.W.L.; Tu, H.-A.; Feld, J.J.; Wong, T.; Krahn, M. Cost-effectiveness of screening for hepatitis C in Canada. CMAJ 2015, 187, E110–E121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suijkerbuijk, A.W.M.; van Hoek, A.J.; Koopsen, J.; de Man, R.A.; Mangen, M.-J.J.; de Melker, H.E.; Polder, J.J.; Ardine de Wit, G.; Veldhuijzen, I.K. Cost-effectiveness of screening for chronic hepatitis B and C among migrant populations in a low endemic country. PLoS ONE 2018, 13, e0207037. [Google Scholar] [CrossRef] [PubMed]


| Migrants Age | Percentage [20] |
|---|---|
| 0–3 | 6.8 |
| 4–11 | 12.3 |
| 12–17 | 10.7 |
| 18–29 | 33.8 |
| 30–39 | 20.2 |
| 40–49 | 9.9 |
| 50–59 | 4.1 |
| 60> | 2.2 |
| Country of Origin | Percentage [11] | HCV Prevalence [12,13,14,15] |
|---|---|---|
| Syria | 51% | 1% |
| Eritrea | 13% | 1.9% |
| Iraq | 4.3% | 3.2% |
| Morocco | 2.6% | 7.7% |
| Algeria | 2.4% | 1.8% |
| Weighted Average | 1.5% |
| Scenario | Cohort | Screening Costs | HCV Treatment Costs | HCV Follow up Costs | HCV Complications Costs | Total Costs Discounted | Total Discounted Costs of Both Cohorts | QALYs per Cohort | QALYs per Cohort Discounted | Total Discounted QALYs of Both Cohorts |
|---|---|---|---|---|---|---|---|---|---|---|
| (i) No screening | HCV-endemic countries | €0 | €13,243,572 | €1,872,576 | €4,251,432 | €5,138,945 | €7,708,418 | 12,645 | 6446 | 9669 |
| Non HCV-endemic countries | €0 | €6,621,786 | €936,288 | €1,657,572 | €2,569,473 | 6322 | 3223 | |||
| (ii) Screening of migrants from HCV-endemic countries | HCV-endemic countries | €496,250 | €13,214,500 | €103,286 | €2,461,026 | €14,295,753 | €16,865,226 | 23,063 | 15,875 | 19,098 |
| Non HCV-endemic countries | €0 | €6,621,786 | €936,288 | €1,657,572 | €2,569,473 | 6322 | 3223 | |||
| (iii) Screening of all migrants | HCV-endemic countries | €496,250 | €13,214,500 | €103,286 | €2,461,026 | €14,295,753 | €21,602,254 | 23,063 | 15,875 | 23,812 |
| Non HCV-endemic countries | €406,750 | €6,607,250 | €51,643 | €1,389,138 | €7,306,501 | 11,532 | 7937 |
| Scenario | Total Cost | Incremental Cost | QALYs | QALYs Gained | ICER |
|---|---|---|---|---|---|
| (i) No screening | €7,708,418 | - | 9669 | - | - |
| (ii) Screening of migrants from HCV-endemic countries | €16,865,226 | €9,156,808 | 19,098 | 9429 | €971 |
| (iii) Screening of all migrants | €21,602,254 | €4,737,029 | 23,812 | 4714 | €1005 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Khayat, M.N.M.T.; Eijsink, J.F.H.; Postma, M.J.; Wilschut, J.C.; van Hulst, M. The Cost-Effectiveness of Hepatitis C Virus Screening Strategies among Recently Arrived Migrants in the Netherlands. Int. J. Environ. Res. Public Health 2020, 17, 6091. https://doi.org/10.3390/ijerph17176091
Al Khayat MNMT, Eijsink JFH, Postma MJ, Wilschut JC, van Hulst M. The Cost-Effectiveness of Hepatitis C Virus Screening Strategies among Recently Arrived Migrants in the Netherlands. International Journal of Environmental Research and Public Health. 2020; 17(17):6091. https://doi.org/10.3390/ijerph17176091
Chicago/Turabian StyleAl Khayat, Mohamed N.M.T., Job F.H. Eijsink, Maarten J. Postma, Jan C. Wilschut, and Marinus van Hulst. 2020. "The Cost-Effectiveness of Hepatitis C Virus Screening Strategies among Recently Arrived Migrants in the Netherlands" International Journal of Environmental Research and Public Health 17, no. 17: 6091. https://doi.org/10.3390/ijerph17176091





