Water Quality and Brain Function
Abstract
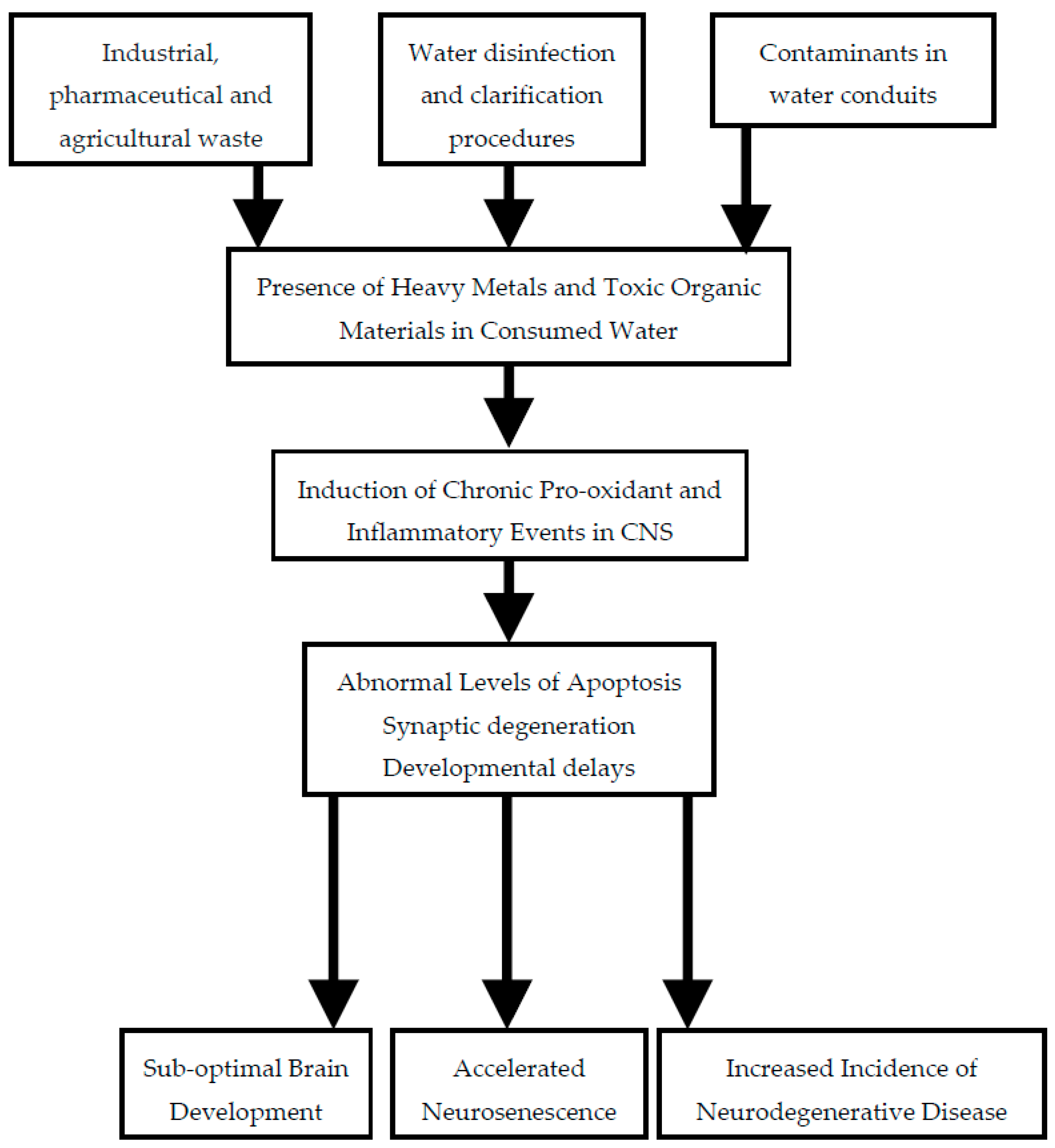
:1. Introduction
2. Sources of Contamination of Drinking Water
2.1. Industrial Waste
2.2. Agricultural Runoff
2.3. Water Treatment
2.4. Water Conduits
2.5. Consumer Products
3. Consideration of Neurotoxic Potential of Individual Contaminants
3.1. Metals
3.1.1. Lead (Pb)
3.1.2. Aluminum (Al)
3.1.3. Copper (Cu)
3.1.4. Arsenic and Cadmium
3.2. Organic Materials
3.2.1. Halogenated Residues
3.2.2. Acrylamide
3.2.3. Bisphenol A
3.2.4. Other Organic Contaminants of Anthropogenic Origin
3.3. Mixtures of Contaminants
4. Mechanisms of Neurotoxicity
4.1. Neuroinflammation
4.2. Oxidative Stress
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Vengosh, A.; Jackson, R.B.; Warner, N.; Darrah, T.H.; Kondash, A. A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ. Sci. Technol. 2014, 48, 8334–8348. [Google Scholar] [CrossRef] [PubMed]
- Pérez, H.L.; Osterman-Golkar, S. A sensitive gas chromatographic-tandem mass spectrometric method for detection of alkylating agents in water: Application to acrylamide in drinking water, coffee and snuff. Analyst 2003, 128, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mo, Z.; Qin, J.; Li, Q.; Wei, Y.; Ma, S.; Xiong, Y.; Liang, G.; Qing, L.; Chen, Z.; et al. Change of water sources reduces health risks from heavy metals via ingestion of water, soil, and rice in a riverine area, South China. Sci. Total Environ. 2015, 530–531, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Romić, D.; Akber, M.A.; Romić, M. Trace metals accumulation in soil irrigated with polluted water and assessment of human health risk from vegetable consumption in Bangladesh. Environ. Geochem. Health 2017. [Google Scholar] [CrossRef] [PubMed]
- Brender, J.D.; Weyer, P.J. Agricultural compounds in water and birth defects. Curr. Environ. Health Rep. 2016, 3, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Croen, L.A.; Todoroff, K.; Shaw, G.M. Maternal exposure to nitrate from drinking water and diet and risk for neural tube defects. Am. J. Epidemol. 2001, 153, 325–331. [Google Scholar] [CrossRef]
- Costa, L.G.; Giordano, G.; Guizzetti, M.; Vitalone, A. Neurotoxicity of pesticides: A brief review. Front. Biosci. 2008, 13, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jennings, A. Worldwide regulations of standard values of pesticides for human health risk control: A review. Int. J. Environ. Res. Public Health 2017, 14, 826. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jennings, A.A. Implied maximum dose analysis of standard values of 25 pesticides based on major human exposure pathways. AIMS Public Health 2017, 4, 383–396. [Google Scholar] [CrossRef]
- Liu, S.; Gunawan, C.; Barraud, N.; Rice, S.A.; Harry, E.J.; Amal, R. Understanding, monitoring, and controlling biofilm growth in drinking water distribution systems. Environ. Sci. Technol. 2016, 50, 8954–8976. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wu, Q.Y.; Lu, Y.; Hu, H.Y.; Yang, Y.; Liu, R.; Liu, F. Increase of cytotoxicity during wastewater chlorination: Impact factors and surrogates. J. Hazard. Mater. 2017, 324, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Lytle, D.A.; Liggett, J. Impact of water quality on chlorine demand of corroding copper. Water Res. 2016, 92, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Gopal, K.; Srivastava, S.B.; Shukla, S.; Bersillon, J.L. Contaminants in drinking water and its mitigation using suitable adsorbents: An overview. J. Environ. Biol. 2004, 25, 469–475. [Google Scholar] [PubMed]
- Rondeau, V.; Jacqmin-Gadda, H.; Commenges, D.; Helmer, C.; Dartigues, J.F. Aluminum and silica in drinking water and the risk of Alzheimer’s disease or cognitive decline: Findings from 15-year follow-up of the PAQUID cohort. Am. J. Epidemiol. 2009, 169, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Becaria, A.; Lahiri, D.K.; Sharman, K.; Bondy, S.C. Chronic exposure to aluminum in drinking water increases inflammatory parameters selectively in the brain. J. Neurosci. Res. 2004, 75, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Bondy, S.C. Low levels of aluminum can lead to behavioral and morphological changes associated with Alzheimer’s disease and age-related neurodegeneration. Neurotoxicology 2016, 52, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez, M.; Bolumar, F.; García, A.M. Occupational risk factors in Alzheimer’s disease: A review assessing the quality of published epidemiological studies. Occup. Environ. Med. 2007, 64, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, C.M.; Gracia-Lavedan, E.; Julvez, J.; Santa-Marina, L.; Lertxundi, N.; Ibarluzea, J.; Llop, S.; Ballester, F.; Fernández-Somoano, A.; Tardón, A.; et al. Drinking water disinfection by-products during pregnancy and child neuropsychological development in the INMA Spanish cohort study. Environ. Int. 2018, 110, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, D.; Chen, Y.; Jing, J.; Hu, Q.; Chen, Y. Lead exposure at each stage of pregnancy and neurobehavioral development of neonates. Neurotoxicology 2014, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.L.; Schreurs, B. Trace amounts of copper in water induce beta-amyloid plaques and learning deficits in a rabbit model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 11065–11069. [Google Scholar] [CrossRef] [PubMed]
- Becaria, A.; Lahiri, D.K.; Bondy, S.C.; Chen, D.; Hamadeh, A.; Li, H.; Taylor, R.; Campbell, A. Aluminum and copper in drinking water enhance inflammatory or oxidative events specifically in the brain. J. Neuroimmunol. 2006, 176, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, M.; Cheng, D.; Laferla, F.M. Chronic copper exposure exacerbates both amyloid and tau pathology and selectively dysregulates cdk5 in a mouse model of AD. J. Neurochem. 2009, 108, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, M.; Hsu, H.W.; Medeiros, R. Copper exposure perturbs brain inflammatory responses and impairs clearance of amyloid-beta. Toxicol. Sci. 2016, 152, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Blair, B.D. Potential upstream strategies for the mitigation of pharmaceuticals in the aquatic environment: A brief review. Curr. Environ. Health Rep. 2016, 3, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Brezina, E.; Prasse, C.; Meyer, J.; Muckter, H.; Ternes, T.A. Investigation and risk evaluation of the occurrence of carbamazepine, oxcarbazepine, their human metabolites and transformation products in the urban water cycle. Environ. Pollut. 2017, 225, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Schaider, L.A.; Rudel, R.A.; Ackerman, J.M.; Dunagan, S.C.; Brody, J.G. Pharmaceuticals, perfluorosurfactants, and other organic wastewater compounds in public drinking water wells in a shallow sand and gravel aquifer. Sci. Total Environ. 2014, 468–469, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Brodin, T.; Fisk, J.; Josnsson, M.; Klaminder, J. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science 2013, 339, 814–815. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, E.R.; Money, J.E.; Green, P.G.; Young, T.M. Metals associated with stormwater-relevant brake and tire samples. Sci. Total Environ. 2009, 407, 5855–5860. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Gupta, S. Impact of waste dump on surface water quality and aquatic insect diversity of Deepor Beel (Ramsar site), Assam, North-east India. Environ. Monit. Assess. 2017, 189, 540. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, Y.; Markowitz, M.E.; Rosen, J.F. Low-level lead-induced neurotoxicity in children: An update on central nervous system effects. Brain Res. Rev. 1998, 27, 168–176. [Google Scholar] [CrossRef]
- Zahran, S.; McElmurry, S.P.; Sadler, R.C. Four phases of the Flint Water Crisis: Evidence from blood lead levels in children. Environ. Res. 2017, 157, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M. Our Sampling of 252 Homes Demonstrates a High Lead in Water Risk: Flint Should Be Failing to Meet the EPA Lead and Copper Rule. 2015. Available online: http://flintwaterstudy.org/2015/09/our-sampling-of-252-homes-demonstrates-a-high-lead-in-water-risk-flint-should-be-failing-to-meet-the-epa-lead-and-copper-rule (accessed on 23 June 2017).
- Hanna-Attisha, M.; LaChance, J.; Sadler, R.C.; Schnepp, A.C. Elevated blood lead levels in children associated with the flint drinking water crisis: A spatial analysis of risk and public health response. Am. J. Public Health 2016, 106, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Barboza, T.; Poston, B. Brain-Damaging Lead Levels near Battery Plant Found as High as 100 Times above Health Limits. 2016. Available online: http://www.latimes.com/local/california/la-me-ln-dangerous-lead-levels-20160714-snap-story.html (accessed on 23 June 2017).
- Department of Toxic Substances Control (DTSC). An Analysis of Children’s Blood Lead Levels in the Area around the Exide Site. 2016. Available online: http://dtsc.ca.gov/HazardousWaste/Projects/upload/An-Analysis-of-Children-s-Blood-Lead-Levels-in-the-Area-Around-the-Exide-Site.pdf (accessed on 23 June 2017).
- Mazumdar, M.; Xia, W.; Hofmann, O.; Gregas, M.; Ho Sui, S.; Hide, W.; Yang, T.; Needleman, H.L.; Bellinger, D.C. Prenatal lead levels, plasma amyloid β levels, and gene expression in young adulthood. Environ. Health Perspect. 2012, 120, 702–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahiri, D.K.; Maloney, B. The “LEARn” (Latent Early-life Associated Regulation) model integrates environmental risk factors and the developmental basis of Alzheimer’s disease, and proposes remedial steps. Exp. Gerontol. 2010, 45, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Basha, M.R.; Wei, W.; Bakheet, S.A.; Benitez, N.; Siddiqi, H.K.; Ge, Y.W.; Lahiri, D.K.; Zawia, N.H. The fetal basis of amyloidogenesis: Exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. J. Neurosci. 2005, 25, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Basha, M.R.; Brock, B.; Cox, D.P.; Cardozo-Pelaez, F.; McPherson, C.A.; Harry, J.; Rice, D.C.; Maloney, B.; Chen, D.; et al. Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): Evidence for a developmental origin and environmental link for AD. J. Neurosci. 2008, 28, 3–9. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality, Second Edition. Volume 2: Health Criteria and Other Supporting Information; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- Perl, D.P.; Brody, A.R. Alzheimer’s disease: X-ray spectrometric evidence of aluminum accumulation in neurofibrillary tangle-bearing neurons. Science 1980, 208, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Majidi, V.; Markesbery, W.R.; Ehmann, W.D. Brain aluminum in Alzheimer’s disease using an improved GFAAS method. Neurotoxicology 1992, 13, 735–743. [Google Scholar] [PubMed]
- Mirza, A.; King, A.; Troakes, C.; Exley, C. Aluminium in brain tissue in familial Alzheimer’s disease. J. Trace Elem. Med. Biol. 2017, 40, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Zhao, Y.; Hill, J.M.; Culicchia, F.; Kruck, T.P.; Percy, M.E.; Pogue, A.I.; Walton, J.R.; Lukiw, W.J. Selective accumulation of aluminum in cerebral arteries in Alzheimer’s disease (AD). J. Inorg. Biochem. 2013, 126, 35–77. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, F.; Sgarlata, C.; Francis, M.; Maurizi, N.; Faragli, A.; Perna, S.; Rondanelli, M.; Rollone, M.; Ricevuti, G. Neuroinflammation, immune system and Alzheimer disease: Searching for the missing link. Aging Clin. Exp. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, D.R.; Bergeron, C.; Smith, J.E.; Boomer, D.; Rifat, S.L. Risk for neuropathologically confirmed Alzheimer’s disease and residual aluminum in municipal drinking water employing weighted residential histories. Neurology 1996, 46, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Baron, M.; Schäper, M.; Knapp, G.; van Thriel, C. Occupational aluminum exposure: Evidence in support of its neurobehavioral impact. Neurotoxicology 2007, 28, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Gillette-Guyonnet, S.; Andrieu, S.; Nourhashemi, F.; de La Guéronnière, V.; Grandjean, H.; Vellas, B. Cognitive impairment and composition of drinking water in women: Findings of the EPIDOS Study. Am. J. Clin. Nutr. 2005, 81, 897–902. [Google Scholar] [PubMed]
- Foglio, E.; Buffoli, B.; Exley, C.; Rezzani, R.; Rodella, L.F. Regular consumption of a silicic acid-rich water prevents aluminium-induced alterations of nitrergic neurons in mouse brain: Histochemical and immunohistochemical studies. Histol. Histopathol. 2012, 27, 1055–1066. [Google Scholar] [PubMed]
- Bondy, S.C.; Campbell, A. Aluminum and neurodegenerative diseases. In Advances in Neurotoxicology; Aschner, A., Costa, L.G., Eds.; Elsevier: Atlanta, GA, USA, 2017; Volume 1, pp. 131–156. [Google Scholar]
- Campbell, A. The role of aluminum and copper on neuroinflammation and Alzheimer’s disease. J. Alzheimers Dis. 2006, 10, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Hébert, C.D.; Elwell, M.R.; Travlos, G.S.; Fitz, C.J.; Bucher, J.R. Subchronic toxicity of cupric sulfate administered in drinking water and feed to rats and mice. Fundam. Appl. Toxicol. 1993, 21, 461–475. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Copper in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking Water Quality. 2004. Available online: http://www.who.int/water_sanitation_health/dwq/chemicals/copper.pdf (accessed on 23 June 2017).
- Environmental Protection Agency (EPA) Lead and Copper Rule. Available online: https://www.epa.gov/dwreginfo/lead-and-copper-rule (accessed on 23 June 2017).
- Spitalny, K.C.; Brondum, J.; Vogt, R.L.; Sargent, H.E.; Kappel, S. Drinking-water-induced copper intoxication in a Vermont family. Pediatrics 1984, 74, 1103–1106. [Google Scholar] [PubMed]
- Araya, M.; Olivares, M.; Pizarro, F.; González, M.; Speisky, H.; Uauy, R. Gastrointestinal symptoms and blood indicators of copper load in apparently healthy adults undergoing controlled copper exposure. Am. J. Clin. Nutr. 2003, 77, 646–650. [Google Scholar] [PubMed]
- Squitti, R.; Pasqualetti, P.; Dal Forno, G.; Moffa, F.; Cassetta, E.; Lupoi, D.; Vernieri, F.; Rossi, L.; Baldassini, M.; Rossini, P.M. Excess of serum copper not related to ceruloplasmin in Alzheimer disease. Neurology 2005, 64, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, I.; Hasegawa, T.; Honda, A.; Ozawa, K.; Hayashi, Y.; Hashimoto, K.; Yamada, M.; Koumura, A.; Sakurai, T.; Kimura, A.; et al. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J. Neurol. Sci. 2011, 303, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Gorell, J.M.; Johnson, C.C.; Rybicki, B.A.; Peterson, E.L.; Kortsha, G.X.; Brown, G.G.; Richardson, R.J. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson’s disease. Neurotoxicology 1999, 20, 239–247. [Google Scholar] [PubMed]
- Salustri, C.; Barbati, G.; Ghidoni, R.; Quintiliani, L.; Ciappina, S.; Binetti, G.; Squitti, R. Is cognitive function linked to serum free copper levels? A cohort study in a normal population. Clin. Neurophysiol. 2010, 121, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J. Copper toxicity in Alzheimer’s disease: Cognitive loss from ingestion of inorganic copper. J. Trace Elem. Med. Biol. 2012, 26, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; McKinstry, W.J.; Multhaup, G.; Galatis, D.; Morton, C.J.; Curtain, C.C.; Williamson, N.A.; White, A.R.; Hinds, M.G.; Norton, R.S.; et al. Structure of the Alzheimer’s disease amyloid precursor protein copper binding domain. J. Biol. Chem. 2003, 278, 17401–17407. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Atwood, C.S.; Anderson, V.E.; Siedlak, S.L.; Smith, M.A.; Perry, G.; Carey, P.R. Metal binding and oxidation of amyloid-beta within isolated senile plaque cores: Raman microscopic evidence. Biochemistry 2003, 42, 2768–2773. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.M.; Wang, Q.; Telivala, T.P.; Smith, R.J.; Lanzirotti, A.; Miklossy, J. Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with beta-amyloid deposits in Alzheimer’s disease. J. Struct. Biol. 2006, 155, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Vitek, M.P.; Mason, R.P. Cupric-amyloid beta peptide complex stimulates oxidation of ascorbate and generation of hydroxyl radical. Free Radic. Med. Biol. 2004, 36, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Guilloreau, L.; Combalbert, S.; Sournia-Saquet, A.; Mazarguil, H.; Faller, P. Redox chemistry of copper-amyloid-beta: The generation of hydroxyl radical in the presence of ascorbate is linked to redox-potentials and aggregation state. Chem. Biochem. 2007, 8, 1317–1325. [Google Scholar]
- Lung, S.; Li, H.; Bondy, S.C.; Campbell, A. Low concentrations of copper in drinking water increase AP-1 binding in the brain. Toxicol. Ind. Health 2015, 31, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; White, C.; Lee, J.; Peterson, T.S.; Bush, A.I.; Sun, G.Y.; Weisman, A.; Petris, M.J. Altered microglial copper homeostasis in a mouse model of Alzheimer’s disease. J. Neurochem. 2010, 114, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Sagare, A.P.; Coma, M.; Perlmutter, D.; Gelein, R.; Bell, R.D.; Deane, R.J.; Zhong, E.; Parisi, M.; Ciszewski, J.; et al. Low levels of copper disrupt brain amyloid-β homeostasis by altering its production and clearance. Proc. Natl. Acad. Sci. USA 2013, 110, 14771–14776. [Google Scholar] [CrossRef] [PubMed]
- Frisbie, S.H.; Ortega, R.; Maynard, D.M.; Sarkar, B. The concentrations of arsenic and other toxic elements in Bangladesh’s drinking water. Environ. Health Perspect. 2002, 110, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, D.N.; Ghosh, A.; Majumdar, K.K.; Ghosh, N.; Saha, C.; Mazumder, R.N. Arsenic contamination of ground water and its health impact on population of district of Nadia, West Bengal, India. Indian J. Community Med. 2010, 35, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Tauheed, J.; Sanchez-Guerra, M.; Lee, J.J.; Paul, L.; Ibne Hasan, M.O.S.; Quamruzzaman, Q.; Selhub, J.; Wright, R.O.; Christiani, D.C.; Coull, B.A.; et al. Associations between post translational histone modifications, myelomeningocele risk, environmental arsenic exposure, and folate deficiency among participants in a case control study in Bangladesh. Epigenetics 2017, 12, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Chou, H.Y.; The, H.W.; Chen, C.M.; Chen, C.J. The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology 2003, 24, 747–753. [Google Scholar] [CrossRef]
- Wasserman, G.A.; Liu, X.; Parvez, F.; Ahsan, H.; Factor-Litvak, P.; Kline, J.; van Geen, A.; Slavkovich, V.; Loiacono, N.J.; Levy, D.; et al. Water arsenic exposure and intellectual function in 6-year-old children in Araihazar, Bangladesh. Environ. Health Perspect. 2007, 115, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Parvez, F.; Wasserman, G.A.; Factor-Litvak, P.; Liu, X.; Slavkovich, V.; Siddique, A.B.; Sultana, R.; Sultana, R.; Islam, T.; Levy, D.; et al. Arsenic exposure and motor function among children in Bangladesh. Environ. Health Perspect. 2011, 119, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Cholanians, A.B.; Phan, A.V.; Ditzel, E.J.; Camenisch, T.D.; Lau, S.S.; Monks, T.J. From the Cover: Arsenic Induces Accumulation of α-Synuclein: Implications for Synucleinopathies and Neurodegeneration. Toxicol. Sci. 2016, 153, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Dani, S.U. Arsenic for the fool: An exponential connection. Sci. Total Environ. 2010, 408, 1842–1846. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Lourdes, C. Toxicity mechanisms of arsenic that are shared with neurodegenerative diseases and cognitive impairment: Role of oxidative stress and inflammatory responses. Neurotoxicology 2016, 53, 223–235. [Google Scholar] [CrossRef] [PubMed]
- US Geological Service. Arsenic in Groundwater of the United States. Available online: http://water.usgs.gov/nawqa/trace/arsenic/index.html (accessed on 17 November 2011).
- Agnihotri, S.K.; Agrawal, U.; Ghosh, I. Brain most susceptible to cadmium induced oxidative stress in mice. J. Trace Elem. Med. Biol. 2015, 30, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Stolakis, V.; Tsakiris, S.; Kalafatakis, K.; Zarros, A.; Skandali, N.; Gkanti, V.; Kyriakaki, A.; Liapi, C. Developmental neurotoxicity of cadmium on enzyme activities of crucial offspring rat brain regions. Biometals 2013, 26, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Mandour, R.A.; Azab, Y.A. The prospective toxic effects of some heavy metals overload in surface drinking water of Dakahlia Governorate, Egypt. Int. J. Occup. Environ. Med. 2011, 2, 245–253. [Google Scholar] [PubMed]
- Fakhri, F.; Jafarzadeh, S.; Moradi, B.; Zandsalimi, Y.; Langarizadeh, G.; Amirhajeloo, L.R.; Mirzaei, M. The non-carcinogenic risk of cadmium in bottled water in different age groups humans: Bandar Abbas City, Iran. Mater. Sociomed. 2015, 27, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Needham, L.L.; Barr, D.B.; Caudill, S.P.; Pirkle, J.L.; Turner, W.E.; Osterloh, J.; Jones, R.L.; Sampson, E.J. ConIcentrations of environmental chemicals associated with neurodevelopmental effects in U.S. population. Neurotoxicology 2005, 26, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Manasfi, T.; Coulomb, B.; Boudenne, J.L. Occurrence, origin, and toxicity of disinfection byproducts in chlorinated swimming pools: An overview. Int. J. Hyg. Environ. Health 2017, 220, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Moser, V.C.; Phillips, P.M.; Levine, A.B.; McDaniel, K.L.; Sills, R.C.; Jortner, B.S.; Butt, M.T. Neurotoxicity produced by dibromoacetic acid in drinking water of rats. Toxicol. Sci. 2004, 79, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, F.; Slevin, J.T. Trichloroethylene and Parkinson disease. Neurol. Clin. 2011, 29, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Igisu, H.; Goto, I.; Kawamura, Y.; Kato, M.; Izumi, K. Acrylamide encephaloneuropathy due to well water pollution. J. Neurol. Neurosurg. Psychiatry 1975, 38, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Gökmen, V. Acrylamide in Food: Analysis, Content and Potential Health Effects; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Lee, Y.M.; Seong, M.J.; Lee, J.W.; Lee, Y.K.; Kim, T.M.; Nam, S.Y.; Kim, D.J.; Yun, Y.W.; Kim, T.S.; Han, S.Y.; et al. Estrogen receptor independent neurotoxic mechanism of bisphenol A, an environmental estrogen. J. Vet. Sci. 2007, 8, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Stump, D.G.; Beck, M.J.; Radovsky, A.; Garman, R.H.; Freshwater, L.L.; Sheets, L.P.; Marty, M.S.; Waechter, J.M., Jr.; Dimond, S.S.; Van Miller, J.P.; et al. Developmental neurotoxicity study of dietary bisphenol A in Sprague-Dawley rats. Toxicol. Sci. 2010, 115, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Karalius, V.P.; Harbison, J.E.; Plange-Rhule, J.; van Breemen, R.B.; Li, G.; Huang, K.; Durazo-Arvizu, R.A.; Mora, N.; Dugas, L.R.; Vail, L.; et al. Bisphenol A (BPA) found in humans and water in three geographic regions with distinctly different levels of economic development. Environ. Health Insights 2014, 8, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Q.; Man, S.; Zeng, X.; Yu, Y.; Pang, Y.; Sheng, G.; Fu, J. Tissue concentrations, bioaccumulation, and biomagnification of synthetic musks in freshwater fish from Taihu Lake, China. Environ. Sci. Pollut. Res. Int. 2013, 20, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.J.; Pan, C.G.; Zhang, M.; Zhang, N.S.; Windfeld, R.; Salvito, D.; Selck, H.; Van den Brink, P.J.; Ying, G.G. Occurrence and ecological risk assessment of emerging organic chemicals in urban rivers: Guangzhou as a case study in China. Sci. Total Environ. 2107, 589, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Litwa, E.; Rzemieniec, J.; Wnuk, A.; Lason, W.; Krzeptowski, W.; Kajta, M. Apoptotic and neurotoxic actions of 4-para-nonylphenol are accompanied by activation of retinoid X receptor and impairment of classical estrogen receptor signaling. J. Steroid Biochem. Mol. Biol. 2014, 144, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Mallela, M.K.; Were, S.R.; Hrubec, T.C. Neural tube defects in mice exposed to tap water. Environ. Toxicol. 2011, 26, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Karri, V.; Schuhmacher, M.; Kumar, V. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol. 2016, 48, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.S.; Gennings, C.; Carter, W.H., Jr.; Yang, R.S.; Campain, J.A. Toxicological interactions among arsenic, cadmium, chromium, and lead in human keratinocytes. Toxicol. Sci. 2001, 63, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, M.H.; Wang, G.; Chen, X.Q.; Lipsky, M.; Smith, D.; Gwiazda, R.; Fowler, B.A. Exposure to Pb, Cd, and As mixtures potentiates the production of oxidative stress precursors: 30-day, 90-day, and 180-day drinking water studies in rats. Toxicol. Appl. Pharmacol. 2011, 254, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Valeri, L.; Mazumdar, M.M.; Bobb, J.F.; Henn, B.C.; Rodrigues, E.; Sharif, O.I.A.; Kile, M.L.; Quamruzzaman, Q.; Afroz, S.; Golam, M.; et al. The joint effect of prenatal exposure to metal mixtures on neurodevelopmental outcomes at 20–40 months of age: Evidence from rural Bangladesh. Environ. Health Perspect. 2017, 125, 067015. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Rai, N.K.; Tripathi, S.; Bandyopadhyay, S. Exposure to As-, Cd-, and Pb-mixture induces Aβ, amyloidogenic APP processing and cognitive impairments via oxidative stress-dependent neuroinflammation in young rats. Toxicol. Sci. 2015, 143, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Parham, P. The Immune System, 4th ed.; Garland Sciences: New York, NY, USA, 2014. [Google Scholar]
- Carson, M.J.; Doose, J.M.; Melchior, B.; Schmid, C.D.; Ploix, C.C. CNS immune privilege: Hiding in plain sight. Immunol. Rev. 2006, 213, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jiang, L. Neuroinflammation in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2015, 11, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef] [PubMed]
- McGeer, E.G.; McGeer, P.L. Innate immunity in Alzheimer’s disease: A model for local inflammatory reactions. Mol. Interv. 2001, 1, 22–29. [Google Scholar] [PubMed]
- Steinman, L. Inflammatory cytokines at the summits of pathological signal cascades in brain diseases. Sci. Signal. 2013, 6, pe3. [Google Scholar] [CrossRef] [PubMed]
- Lustbader, J.W.; Cirilli, M.; Lin, C.; Xu, H.W.; Takuma, K.; Wang, N.; Caspersen, C.; Chen, X.; Pollak, S.; Chaney, M.; et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science 2004, 304, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.; Wang, N.; Yao, J.; Sosunov, A.; Chen, X.; Lustbader, J.W.; Xu, H.W.; Stern, D.; McKhann, G.; Yan, S.D. Mitochondrial Abeta: A potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J. 2005, 19, 2040–2041. [Google Scholar] [PubMed]
- Gibson, G.E.; Karuppagounder, S.S.; Shi, Q. Oxidant-induced changes in mitochondria and calcium dynamics in the pathophysiology of Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2008, 1147, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Coskun, P.E.; Wyrembak, J.; Derbereva, O.; Melkonian, G.; Doran, E.; Lott, I.T.; Head, E.; Cotman, C.W; Wallace, D.C. Systemic mitochondrial dysfunction and the etiology of Alzheimer’s disease and down syndrome dementia. J. Alzheimers Dis. 2010, 20, S293–S310. [Google Scholar] [CrossRef] [PubMed]
- Lubik, N. Opening the “green pharmacy”. Environ. Sci. Technol. 2008, 42, 8620–8621. [Google Scholar] [CrossRef]
- Rastogi, T.; Leder, C.; Kümmerer, K. Re-Designing of Existing Pharmaceuticals for Environmental Biodegradability: A Tiered Approach with β-Blocker Propranolol as an Example. Environ. Sci. Technol. 2015, 49, 11756–11763. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.A.; Hoffman, S.; Luthi, C.; Truffer, B.; Maurer, M. Emerging solutions to the water challenges of an urbanizing world. Science 2016, 352, 928–933. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondy, S.C.; Campbell, A. Water Quality and Brain Function. Int. J. Environ. Res. Public Health 2018, 15, 2. https://doi.org/10.3390/ijerph15010002
Bondy SC, Campbell A. Water Quality and Brain Function. International Journal of Environmental Research and Public Health. 2018; 15(1):2. https://doi.org/10.3390/ijerph15010002
Chicago/Turabian StyleBondy, Stephen C., and Arezoo Campbell. 2018. "Water Quality and Brain Function" International Journal of Environmental Research and Public Health 15, no. 1: 2. https://doi.org/10.3390/ijerph15010002




