A Longitudinal Study of Association between Heavy Metals and Itchy Eyes, Coughing in Chronic Cough Patients: Related with Non-Immunoglobulin E Mediated Mechanism
Abstract
:1. Introduction
2. Experimental Section
2.1. Participant Recruitment
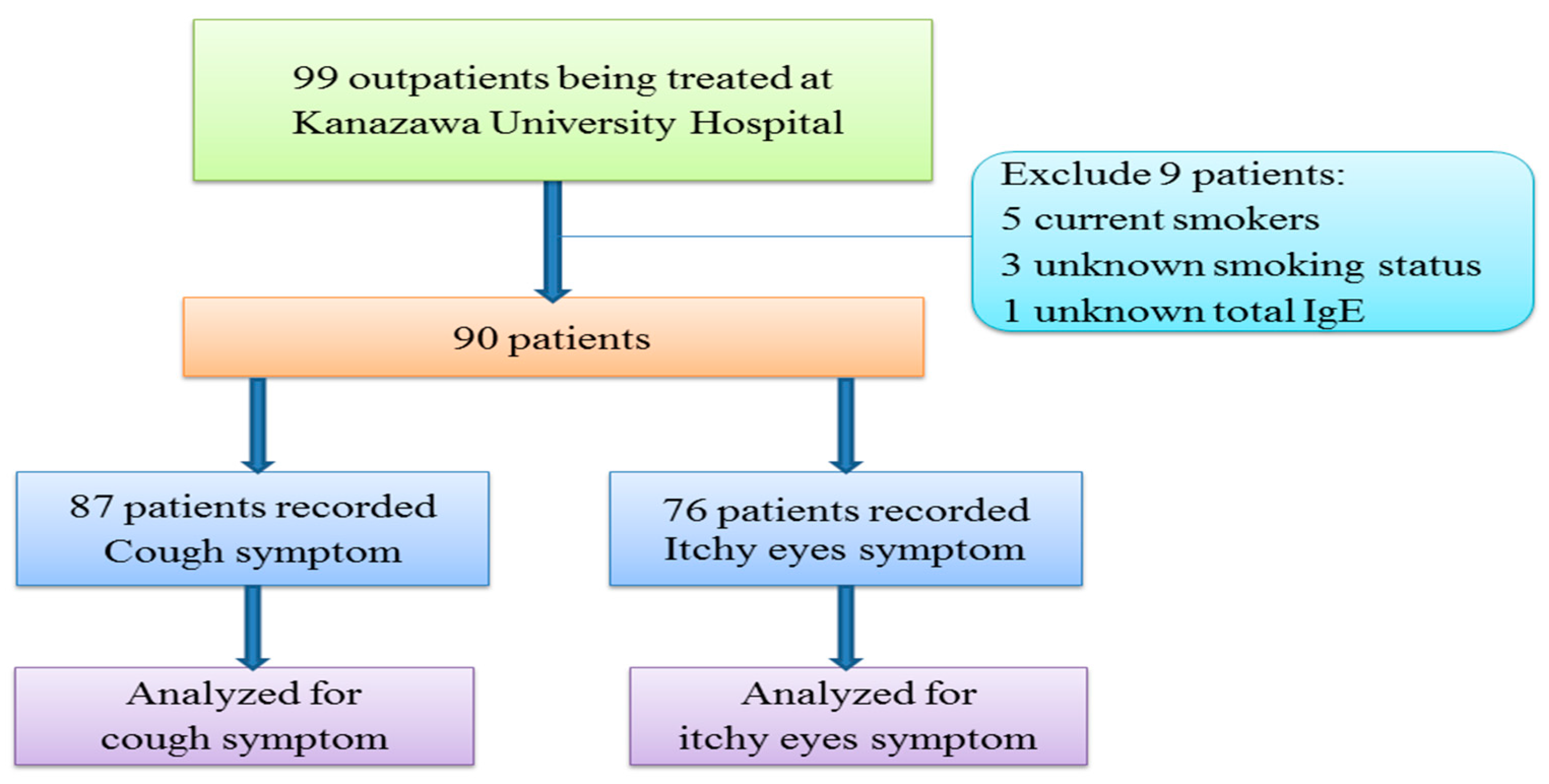
| Characteristics | Patient-Recorded Itchy Eyes Symptoms (n = 76) | Patient-Recorded Cough Symptoms (n = 87) | ||
|---|---|---|---|---|
| IgE < 250 IU/m (n = 59) | IgE ≥ 250 IU/mL (n = 17) | IgE < 250 IU/mL (n = 65) | IgE ≥ 250 IU/Ml (n = 22) | |
| Gender (Male/Female) | 18/41 | 13/4 | 20/45 | 8/14 |
| Smoking status | ||||
| Non-Smoker | 44 | 13 | 47 | 14 |
| Ex-Smoker | 15 | 4 | 18 | 8 |
| Age (years) (Mean ± SD) | 63.8 ± 11.9 | 56.8 ± 15.4 | 64.8 ± 11.2 | 61.6 ± 16.3 |
| BMI (kg/m2) (Mean ± SD) | 22.9 ± 3.8 | 23.2 ± 2.6 | 22.6 ± 3.6 | 22.8 ± 2.5 |
| Duration of disease (years) | 15.0 ± 8.8 | 18.1 ± 8.1 | 15.7 ± 10.8 | 18.8 ± 10.0 |
| Onset (age) | 49.0 ± 13.3 | 38.9 ± 18.8 | 64.3 ± 11.2 | 61.1 ± 16.2 |
| BA patients | 31 | 12 | 37 | 17 |
| CVA patients | 13 | 5 | 13 | 5 |
| AC patients a,b | 26 | 3 | 27 | 3 |
2.2. Health Outcomes
2.3. Measurement of Total Serum IgE Levels
2.4. Measurement of Heavy Metals in Total Suspended Particulates
2.5. Statistical Analysis
3. Results
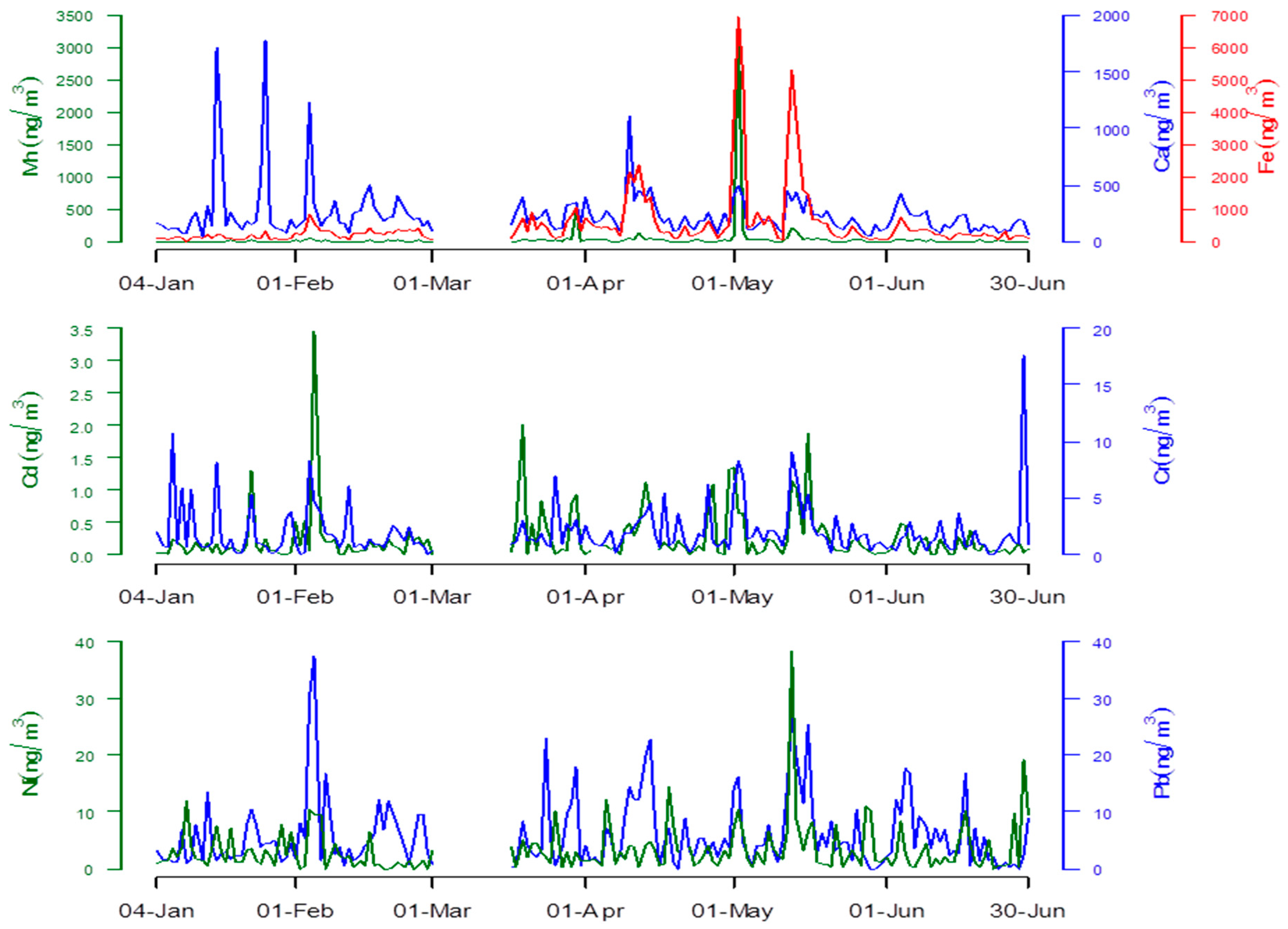
3.1. Heavy Metal Concentrations
| Heavy Metals | Days of Observation | Mean | IQR | Minimum | Maximum |
|---|---|---|---|---|---|
| Ca (ng/m3) | 163 | 238.53 ± 232.06 | 184.817 | 41.29 | 1777.49 |
| Cd (ng/m3) | 161 | 0.26 ± 0.42 | 0.122 | 0.00 | 3.44 |
| Cr (ng/m3) | 163 | 2.03 ± 2.30 | 1.279 | 0.00 | 17.54 |
| Fe (ng/m3) | 163 | 503.23 ± 920.15 | 247.813 | 15.50 | 6926.79 |
| Mn (ng/m3) | 163 | 37.54 ± 254.06 | 8.326 | 0.00 | 3211.78 |
| Ni (ng/m3) | 162 | 3.31 ± 4.33 | 1.866 | 0.00 | 38.33 |
| Pb (ng/m3) | 163 | 5.98 ± 6.25 | 3.956 | 0.00 | 37.33 |
3.2. Relationship between Heavy Metals and Itchy Eyes and Coughing
| Metals | Itchy Eyes Symtom | Cough Symptom | ||||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) a | p-Value a | OR (95% CI) b | p-Value b | OR (95% CI) a | p-Value a | OR (95% CI) b | p-Value b | |
| Ca | 1.49 (1.20–1.84) | <0.001 | 1.22 (1.02–1.45) | 0.026 | 1.11 (0.99–1.25) | 0.084 | 1.03 (0.93–1.13) | 0.663 |
| Cd | 1.34 (1.15–1.57) | <0.001 | 1.27 (1.09–1.47) | 0.002 | 1.15 (1.04–1.26) | 0.005 | 1.13 (1.03–1.24) | 0.012 |
| Cr | 1.29 (1.11–1.49) | 0.001 | 1.17 (1.03–1.33) | 0.019 | 1.12 (1.01–1.25) | 0.027 | 1.10 (0.99–1.02) | 0.053 |
| Fe | 1.83 (1.40–2.40) | <0.001 | 1.56 (1.20–2.02) | 0.001 | 1.26 (1.07–1.48) | 0.007 | 1.22 (1.05–1.42) | 0.010 |
| Mn | 1.77 (1.43–2.18) | <0.001 | 1.60 (1.31–1.95) | <0.001 | 1.17 (1.03–1.33) | 0.015 | 1.13 (1.01–1.27) | 0.030 |
| Ni | 1.18 (0.99–1.41) | 0.068 | 1.23 (1.02–1.49) | 0.031 | 1.01 (0.92–1.11) | 0.886 | 1.02 (0.93–1.13) | 0.663 |
| Pb | 1.25 (1.05–1.50) | 0.015 | 1.20 (1.01–1.41) | 0.038 | 1.05 (0.97–1.14) | 0.227 | 1.04 (0.96v1.13) | 0.376 |
3.3. Relationship between Heavy Metals and Itchy Eyes, Coughing, and Serum IgE Levels
| Metals | IgE < 250 IU/mL | IgE ≥ 250 IU/mL | ||||||
|---|---|---|---|---|---|---|---|---|
| Itchy Eyes Symtom | Cough Symptom | Itchy Eyes Symtom | Cough Symptom | |||||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Ca | 1.16 (0.96–1.40) | 0.118 | 1.06 (0.95–1.19) | 0.315 | 1.56 (0.99–2.47) | 0.056 | 0.91 (0.75–1.12) | 0.388 |
| Cd | 1.30 (1.12–1.51) | 0.001 | 1.14 (1.03–1.27) | 0.014 | 1.13 (0.72–1.79) | 0.599 | 1.08 (0.88–1.32) | 0.458 |
| Cr | 1.18 (1.03–1.35) | 0.015 | 1.08 (0.97–1.21) | 0.178 | 1.11 (0.73–1.69) | 0.620 | 1.16 (0.97–1.39) | 0.100 |
| Fe | 1.61 (1.22–2.12) | 0.001 | 1.22 (1.02–1.45) | 0.027 | 1.34 (0.73–2.48) | 0.347 | 1.27 (0.89–1.81) | 0.195 |
| Mn | 1.65 (1.32–2.05) | <0.001 | 1.15 (1.00–1.31) | 0.046 | 1.39 (0.92–2.10) | 0.122 | 1.11 (0.87–1.41) | 0.414 |
| Ni | 1.23 (1.00–1.52) | 0.048 | 1.03 (0.92–1.17) | 0.599 | 1.21 (0.70–2.07) | 0.494 | 1.00 (0.84–1.20) | 0.992 |
| Pb | 1.21 (1.03–1.42) | 0.019 | 1.07 (0.97–1.17) | 0.194 | 1.14 (0.64–2.05) | 0.657 | 0.97 (0.81–1.16) | 0.703 |
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IgE | Immunoglobulin E |
| TSP | total suspended particles |
| PM | particulate matter |
| Ca | calcium |
| Cd | cadmium |
| Co | cobalt |
| Cu | copper |
| Cr | chromium |
| Fe | iron |
| Mn | manganese |
| Ni | nickel |
| Pb | lead |
| BMI | body mass index |
| SO2 | sulfur dioxide |
| OR | Odds ratio |
| CI | Confidence interval |
| SD | Standard deviation |
| SPSS | Statistical Package for Social Sciences |
| GEE | generalized estimating equation |
References
- Bae, S.; Pan, X.-C.; Kim, S.-Y.; Park, K.; Kim, Y.-H.; Kim, H.; Hong, Y.-C. Exposures to particulate matter and polycyclic aromatic hydrocarbons and oxidative stress in schoolchildren. Environ. Health Perspect. 2010, 118, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Han, I.-K.; Shao, M.; Hu, M.; Zhang, J.; Tang, X. PM2.5 constituents and oxidative DNA damage in humans. Environ. Sci. Technol. 2009, 43, 4757–4762. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Feng, W.; Kuang, D.; Deng, Q.; Zhang, W.; Wang, S.; He, M.; Zhang, X.; Wu, T.; Guo, H. The effects of heavy metals and their interactions with polycyclic aromatic hydrocarbons on the oxidative stress among coke-oven workers. Environ. Res. 2015, 140, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Eeftens, M.; Beelen, R.; De Hoogh, K.; Bellander, T.; Cesaroni, G.; Cirach, M.; Declercq, C.; Dėdelė, A.; Dons, E.; de-Nazelle, A.; et al. Development of land use regression models for PM2.5, PM2.5 absorbance, PM10 and PM coarse in 20 European study areas; Results of the ESCAPE project. Environ. Sci. Technol. 2012, 46, 11195–11205. [Google Scholar] [CrossRef] [PubMed]
- De Hoogh, K.; Wang, M.; Adam, M.; Badaloni, C.; Beelen, R.; Birk, M.; Cesaroni, G.; Cirach, M.; Declercq, C.; Dėdelė, A.; et al. Development of land use regression models for particle composition in twenty study areas in Europe. Environ. Sci. Technol. 2013, 47, 5778–5786. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, V.; Zutavern, A.; Cyrys, J.; Brockow, I.; Koletzko, S.; Krämer, U.; Behrendt, H.; Herbarth, O.; von Berg, A.; Bauer, C.P.; et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am. J. Respir. Crit. Care. Med. 2008, 177, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Gasana, J.; Dillikar, D.; Mendy, A.; Forno, E.; Ramos Vieira, E. Motor vehicle air pollution and asthma in children: A meta-analysis. Environ. Res. 2012, 117, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.H.; Brunekreef, B.; Van Vliet, P.; Aarts, F.; Meliefste, K.; Harssema, H.; Fischer, P. The relationship between air pollution from heavy traffic and allergic sensitization, bronchial hyperresponsiveness, and respiratory symptoms in Dutch schoolchildren. Environ. Health Perspect. 2003, 111, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Gehring, U.; Beelen, R.; Eeftens, M.; Hoek, G.; De Hoogh, K.; De Jongste, J.C.; Keuken, M.; Koppelman, G.H.; Meliefste, K.; Oldenwening, M.; et al. Particulate matter composition and respiratory health: The PIAMA Birth Cohort study. Epidemiology 2015, 26, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Hoepner, L.; Garfinkel, R.; Chillrud, S.; Reyes, A.; Quinn, J.W.; Perera, F.; Miller, R.L. Ambient metals, elemental carbon, and wheeze and cough in New York city children through 24 months of age. Am. J. Respir. Crit. Care. Med. 2009, 180, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Mutti, A.; Corradi, M.; Goldoni, M.; Vettori, M.V.; Bernard, A.; Apostoli, P. Exhaled metallic elements and serum pneumoproteins in asymptomatic smokers and patients with COPD or asthma. Chest 2006, 129, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Braman, S.S.; Corrao, W.M. Chronic cough: Diagnosis and treatment. Prim. Care 1985, 12, 217–225. [Google Scholar] [PubMed]
- Chung, K.F.; Pavord, I.D. Prevalence, pathogenesis, and causes of chronic cough. Lancet 2008, 371, 1364–1374. [Google Scholar] [CrossRef]
- Yamada, T.; Yoshimura, M.; Nago, N.; Asai, Y.; Koga, Y.; Inoue, Y.; Hamasaki, K.; Mise, J.; Lamberts, H.; Okkes, I.M. What is the common diseases and common health problems?—The use of ICPC in the community-based project. JpN J. Prim. Care 2000, 23, 80–89. [Google Scholar]
- Fujimura, M. Cough variant asthma and related disorders: Atopic cough. Arerugi no Rinsho 1996, 16, 22–27. [Google Scholar]
- Fujimura, M. Eosinophilic bronchitis is an important cause of chronic cough. Am. J. Respir Crit. Care Med. 2000, 161, 1764–1765. [Google Scholar] [PubMed]
- Carney, I.K.; Gibson, P.G.; Murree-Allen, K.; Saltos, N.; Olson, L.G.; Hensley, M.J. A systematic evaluation of mechanisms in chronic cough. Am. J. Respir Crit. Care Med. 1997, 156, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ballaney, M.; Al-Alem, U.; Quan, C.; Jin, X.; Perera, F.; Chen, L.C.; Miller, R.I. Combined inhaled diesel exhaust particles and allergen exposure alter methylation of T helper genes and IgE production in vivo. Toxicol. Sci. 2008, 102, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Herr, C.E.W.; Ghosh, R.; Dostal, M.; Skokanova, V.; Ashwood, P.; Lipsett, M.; Joad, J.P.; Pinkerton, K.E.; Yap, P.-S.; Frost, J.D.; et al. Exposure to air pollution in critical prenatal time windows and IgE levels in newborns. Pediatr. Allergy. Immunol. 2011, 22, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-N.; Hsieh, C.-C.; Kuo, H.-F.; Lee, M.-S.; Huang, M.-Y.; Kuo, C.-H.; Hung, C.-H. The effects of environmental toxins on allergic inflammation. Allergy Asthma Immunol. Res. 2014, 6, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; von Schirnding, Y.E.; Prapamontol, T. Environmental lead exposure: A public health problem of global dimensions. Bull. World Health Organ. 2000, 78, 1068–1077. [Google Scholar] [PubMed]
- Mishra, K.P. Lead exposure and its impact on immune system: A review. Toxicol. Vitr. 2009, 23, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Marth, E.; Jelovcan, S.; Kleinhappl, B.; Gutschi, A.; Barth, S. The effect of heavy metals on the immune system at low concentrations. Int. J. Occup. Med. Environ. Health 2001, 14, 375–386. [Google Scholar] [PubMed]
- Ashley-martin, J.; Levy, A.R.; Arbuckle, T.E.; Platt, R.W.; Marshall, J.S.; Dodds, L. Maternal exposure to metals and persistent pollutants and cord blood immune system biomarkers. Environ. Health 2015. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Yamaguchi, M.; Akiyama, K.; Adachi, M.; Ichinose, M.; Takahashi, K.; Nishimuta, T.; Morikawa, A.; Nishima, S. Japanese guideline for adult asthma. Allergol. Int. 2011, 60, 115–145. [Google Scholar] [CrossRef] [PubMed]
- Kohno, S.; Ishida, T.; Uchida, Y.; Kishimoto, H.; Sasaki, H.; Shioya, T.; Tokuyama, K.; Niimi, A.; Nishi, K.; Fujimura, M.; et al. The Japanese Respiratory Society guidelines for management of cough. Respirology 2006, 11, S135–S186. [Google Scholar] [PubMed]
- Fujimura, M.; Sakamoto, S.; Kamio, Y.; Matsuda, T. Effects of methacholine induced bronchoconstriction and procaterol induced bronchodilation on cough receptor sensitivity to inhaled capsaicin and tartaric acid. Thorax 1992, 47, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Kambayashi, Y.; Ohkura, N.; Fujimura, M.; Nakanishi, S.; Yoshizaki, T.; Saijoh, K.; Hayakawa, K.; Kobayashi, F.; Michigami, Y.; et al. Exacerbation of daily cough and allergic symptoms in adult patients with chronic cough by Asian dust: A hospital-based study in Kanazawa. Atmos. Environ. 2014, 97, 537–543. [Google Scholar] [CrossRef]
- Ikeda, Y.; Makino, S. Measurement of total IgE in sera from normal subjects and allergic patients by chemiluminescent immunoassay. Arerugi 1994, 43, 134–141. [Google Scholar] [PubMed]
- Higashi, T.; Kambayashi, Y.; Ohkura, N.; Fujimura, M.; Nakai, S.; Honda, Y.; Saijoh, K.; Hayakawa, K.; Kobayashi, F.; Michigami, Y.; et al. Effects of Asian dust on daily cough occurrence in patients with chronic cough: A panel study. Atmos. Environ. 2014, 92, 506–513. [Google Scholar] [CrossRef]
- Yang, F.; Tan, J.; Zhao, Q.; Du, Z.; He, K.; Ma, Y.; Duan, F.; Chen, G. Characteristics of PM2.5 speciation in representative megacities and across China. Atmos. Chem. Phys. 2011, 11, 5207–5219. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Liu, H.; Zhou, H.; Fan, Z.; Lin, M.; Wu, D.; Xia, B. Characteristics, sources and health risk assessment of toxic heavy metals in PM2.5 at a megacity of southwest China. Environ. Geochem. Health 2015. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Kim, D.; Park, K. Monitoring of ambient particles and heavy metals in a residential area of Seoul, Korea. Environ. Monit. Assess. 2008, 137, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Laraqui, C.H.; Caubet, A.; Laraqui, O.; Rahhali, A.E.; Curtes, J.P.; Verger, C. Health risks study in a pottery environment in Morocco. Sante 2000, 10, 249–254. [Google Scholar] [PubMed]
- Wieloch, M.; Kamiński, P.; Ossowska, A.; Koim-Puchowska, B.; Stuczyński, T.; Kuligowska-Prusińska, M.; Dymek, G.; Mańkowska, A.; Odrowąż-Sypniewska, G. Do toxic heavy metals affect antioxidant defense mechanisms in humans? Ecotoxicol. Environ. Safety 2012, 78, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Majestic, B.J.; Anbar, A.D.; Herckes, P. Elemental and iron isotopic composition of aerosols collected in a parking structure. Sci. Total Environ. 2009, 407, 5104–5109. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yamasaki, A.; Burioka, N.; Kurai, J.; Yoneda, K.; Yoshida, A.; Igishi, T.; Fukuoka, Y.; Nakamoto, M.; Takeuchi, H.; et al. Correlation between Asian dust storms and worsening asthma in western Japan. Allergol. Int. 2011, 60, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Fujimori, E.; Hatoya, K.; Takada, H.; Kumata, H.; Nakajima, F.; Hatakeyama, S.; Uchida, M.; Tanaka, S.; He, K.; et al. Rapid and simple determination of multi-elements in aerosol samples collected on quartz fiber filters by using EDXRF coupled with fundamental parameter quantification technique. Aerosol. Air Qual. Res. 2013, 13, 1864–1876. [Google Scholar] [CrossRef]
- Lough, G.C.; Schauer, J.J.; Park, J.-S.; Shafer, M.M.; DeMinter, J.T.; Weinstein, J.P. Emissions of metals associated with motor vehicle roadways. Environ. Sci. Technol 2005, 39, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Salma, I.; Maenhaut, W. Changes in elemental composition and mass of atmospheric aerosol pollution between 1996 and 2002 in a Central European city. Environ. Pollut. 2006, 143, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Schaumann, F.; Borm, P.J.A.; Herbrich, A.; Knoch, J.; Pitz, M.; Schins, R.P.F.; Luettig, B.; Hohlfeld, J.M.; Heinrich, J.; Krug, N. Metal-rich ambient particles (particulate matter 2.5) cause airway inflammation in healthy subjects. Am. J. Respir. Crit. Care Med. 2004, 170, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Frye, C.; Hoelscher, B.; Cyrys, J.; Wjst, M.; Wichmann, H.-E.; Heinrich, J. Association of lung function with declining ambient air pollution. Environ. Health Perspect. 2003, 111, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Porebski, G.; Woźniak, M.; Czarnobilska, E. Residential proximity to major roadways is associated with increased prevalence of allergic respiratory symptoms in children. Ann. Agric. Environ. Med. 2014, 21, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Boojar, M.M.A.; Goodarzi, F. A longitudinal follow-up of pulmonary function and respiratory symptoms in workers exposed to manganese. J. Occup. Environ. Med. 2002, 44, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Hedmer, M.; Karlsson, J.-E.; Andersson, U.; Jacobsson, H.; Nielsen, J.; Tinnerberg, H. Exposure to respirable dust and manganese and prevalence of airways symptoms, among Swedish mild steel welders in the manufacturing industry. Int. Arch. Occup. Environ. Health 2014, 87, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.A.M.; Novaes, P.; Matsuda, M.; Braga, A.; Saldiva, P.H.N.; Alves, M.R.; Monteiro, M.L.R. Correlation between signs and symptoms of ocular surface dysfunction and tear osmolarity with ambient levels of air pollution in a large metropolitan area. Cornea 2013, 32, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Seidman, M.D.; Gurgel, R.K.; Lin, S.Y.; Schwartz, S.R.; Baroody, F.M.; Bonner, J.R.S.; Dawson, D.E.; Dykewicz, M.S.; Hackell, J.M.; Han, J.K.; et al. Clinical practice guideline: Allergic rhinitis. Otolaryngol. Head Neck Surg. 2015, 152, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Canonica, G.W.; Van Weel, C.; Agache, I.; et al. Allergic rhinitis and its impact on asthma (ARIA) 2008. Allergy 2008, 63, 8–160. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Igishi, T.; Burioka, N.; Yamasaki, A.; Kurai, J.; Takeuchi, H.; Sako, T.; Yoshida, A.; Yoneda, K.; Fukuoka, Y.; et al. Pollen augments the influence of desert dust on symptoms of adult asthma patients. Allergol. Int. 2011, 60, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Miyagawa, K.; Ogino, K.; Endo, T.; Imai, T.; Ozasa, K.; Motohashi, Y.; Matsuzaki, I.; Sasahara, S.; Hatta, K.; et al. High contribution contrast between the genes of eosinophil peroxidase and IL-4 receptor alpha-chain in Japanese cedar pollinosis. J. Allergy Clin. Immunol. 2003, 112, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y. Japanese cedar pollinosis: Discovery, nomenclature, and epidemiological trends. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2014, 90, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Horiguchi, S.; Yamamoto, H.; Yonekura, S.; Hanazawa, T. Present situation of cedar pollinosis in Japan and its immune responses. Allergol. Int. 2009, 58, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, S.; Minami, H.; Abe, M.; Hasei, T.; Sera, N.; Yamamoto, S.; Funasaka, K.; Asakawa, D.; Watanabe, M.; Honda, N.; et al. Seasonal fluctuations in air pollution in Dazaifu, Japan, and effect of long-range transport from mainland East Asia. Biol. Pharm. Bull. 2015, 38, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Gould, H.J.; Sutton, B.J. IgE in allergy and asthma today. Nat. Rev. Immunol. 2008, 8, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Platts-Mills, T.A. The role of immunoglobulin E in allergy and asthma. Am. J. Respir. Crit. Care Med. 2001, 164, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, N.; Goshima, H.; Nabe, T.; Yoshino, S. Complement C3a-induced IL-17 plays a critical role in an IgE-mediated late-phase asthmatic response and airway hyperresponsiveness via neutrophilic inflammation in mice. J. Immunol. 2012, 188, 5694–5705. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.; Parsons, P.J.; Lawrence, D.A. Lead differentially modifies cytokine production in vitro and in vivo. Toxicol. Appl. Pharmacol. 1996, 138, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Lutz, P.M.; Wilson, T.J.; Ireland, J.; Jones, A.L.; Gorman, J.S.; Gale, N.L.; Johnson, J.C.; Hewett, J.E. Elevated immunoglobulin E (IgE) levels in children with exposure to environmental lead. Toxicology 1999, 134, 63–78. [Google Scholar] [CrossRef]
- Sarasua, S.M.; Vogt, R.F.; Henderson, L.O.; Jones, P.A.; Lybarger, J.A. Serum immunoglobulins and lymphocyte subset distributions in children and adults living in communities assessed for lead and cadmium exposure. J. Toxicol. Environ. Health 2000, 60, 1–15. [Google Scholar]
- Yu, M.; Tsai, M.; Tam, S.-Y.; Jones, C.; Zehnder, J.; Galli, S.J. Mast cells can promote the development of multiple features of chronic asthma in mice. J. Clin. Investig. 2006, 116, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. Mast cells in allergy and infection: Versatile effector and regulatory cells in innate and adaptive immunity. Eur. J. Immunol. 2010, 40, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Gilfillan, A.M.; Austin, S.J.; Metcalfe, D.D. Mast cell biology: Introduction and overview. Adv. Exp. Med. Biol. 2011, 716, 2–12. [Google Scholar] [PubMed]
- Hofmann, A.M.; Abraham, S.N. New roles for mast cells in pathogen defense and allergic disease. Discov. Med. 2010, 9, 79–83. [Google Scholar] [PubMed]
- Moon, T.C.; St Laurent, C.D.; Morris, K.E.; Marcet, C.; Yoshimura, T.; Sekar, Y.; Befus, A.D. Advances in mast cell biology: New understanding of heterogeneity and function. Mucosal. Immunol. 2010, 3, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Pala, G.; Pignatti, P.; Moscato, G. The use of fractional exhaled nitric oxide in investigation of work-related cough in a hairdresser. Am. J. Ind. Med. 2011, 54, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Díaz Angulo, S.; Szram, J.; Welch, J.; Cannon, J.; Cullinan, P. Occupational asthma in antibiotic manufacturing workers: Case reports and systematic review. J. Allergy 2011. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, A.; Folletti, I.; Moscato, G. Non-IgE-mediated and irritant-induced work-related rhinitis. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Pralong, J.A.; Cartier, A.; Vandenplas, O.; Labrecque, M. Occupational asthma: New low-molecular-weight causal agents, 2000–2010. J. Allergy 2012. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kurai, J.; Igishi, T.; Yamasaki, A.; Burioka, N.; Takeuchi, H.; Sako, T.; Touge, H.; Nakamoto, M.; Hasegawa, Y.; et al. Influence of Asian desert dust on lower respiratory tract symptoms in patients with asthma over 4 years. Yonago Acta Med. 2012, 55, 41–48. [Google Scholar] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.T.; Higashi, T.; Kambayashi, Y.; Anyenda, E.O.; Michigami, Y.; Hara, J.; Fujimura, M.; Tsujiguchi, H.; Kitaoka, M.; Asakura, H.; et al. A Longitudinal Study of Association between Heavy Metals and Itchy Eyes, Coughing in Chronic Cough Patients: Related with Non-Immunoglobulin E Mediated Mechanism. Int. J. Environ. Res. Public Health 2016, 13, 110. https://doi.org/10.3390/ijerph13010110
Nguyen TTT, Higashi T, Kambayashi Y, Anyenda EO, Michigami Y, Hara J, Fujimura M, Tsujiguchi H, Kitaoka M, Asakura H, et al. A Longitudinal Study of Association between Heavy Metals and Itchy Eyes, Coughing in Chronic Cough Patients: Related with Non-Immunoglobulin E Mediated Mechanism. International Journal of Environmental Research and Public Health. 2016; 13(1):110. https://doi.org/10.3390/ijerph13010110
Chicago/Turabian StyleNguyen, Thao Thi Thu, Tomomi Higashi, Yasuhiro Kambayashi, Enoch Olando Anyenda, Yoshimasa Michigami, Johsuke Hara, Masaki Fujimura, Hiromasa Tsujiguchi, Masami Kitaoka, Hiroki Asakura, and et al. 2016. "A Longitudinal Study of Association between Heavy Metals and Itchy Eyes, Coughing in Chronic Cough Patients: Related with Non-Immunoglobulin E Mediated Mechanism" International Journal of Environmental Research and Public Health 13, no. 1: 110. https://doi.org/10.3390/ijerph13010110





