Occurrence and Profiles of the Artificial Endocrine Disruptor Bisphenol A and Natural Endocrine Disruptor Phytoestrogens in Urine from Children in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Chemicals
2.3. Sample Pretreatment
2.4. Chemical Analysis
2.5. Quality Assurance and Quality Control
2.6. Data Analysis
3. Results
| Urinary Concentration (ng/mL) | Corrected by Urinary Creatinine (μg/g) c | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BPA | Enterolactone | Enterodiol | Daidzein | O-DMA | Genistein | Equol | BPA | Enterolactone | Enterodiol | Daidzein | O-DMA | Genistein | Equol | ||
| Children (n = 256) | |||||||||||||||
| GM a | 1.58 | 162 | 21.4 | 142 | 4.87 | 21.7 | 10.8 | 2.52 | 258 | 34.1 | 226 | 7.76 | 34.6 | 17.2 | |
| AM b | 2.47 | 407 | 120 | 435 | 33.3 | 64.4 | 44.5 | 1.93 | 948 | 379 | 1350 | 71.6 | 204 | 182 | |
| Max | 24.9 | 2910 | 4250 | 2720 | 346 | 419 | 336 | 74.3 | 258 | 34.1 | 226 | 7.76 | 34.6 | 29.6 | |
| DF% | 99.2 | 100 | 98.4 | 93.4 | 71.9 | 98.4 | 53.1 | ||||||||
| Girls (n = 124) | |||||||||||||||
| GM | 1.32 | 165 | 18.1 | 101 | 6.48 | 17.6 | 10.1 | 2.47 | 310 | 34.0 | 189 | 12.2 | 33.2 | 19.0 | |
| AM | 1.99 | 366 | 48.8 | 379 | 37.0 | 58.7 | 38.7 | 2.11 | 1010 | 251 | 1320 | 107 | 242 | 70.6 | |
| Max | 10.3 | 2910 | 595 | 2720 | 241 | 419 | 319 | 69.4 | 7090 | 4955 | 14,200 | 622 | 2180 | 308 | |
| DF% | 98.4 | 100 | 100 | 90.3 | 61.3 | 100 | 62.9 | ||||||||
| Boys (n = 132) | |||||||||||||||
| GM | 1.76 | 160 | 25.4 | 200 | 3.66 | 26.8 | 11.6 | 2.37 | 214 | 34.2 | 270 | 4.94 | 36.1 | 15.6 | |
| AM | 2.52 | 449 | 192 | 492 | 29.7 | 70.2 | 50.3 | 1.87 | 888 | 507 | 1380 | 36.2 | 166 | 241 | |
| Max | 24.9 | 2300 | 4250 | 2580 | 346 | 346 | 336 | 74.3 | 12,500 | 9680 | 22,200 | 274 | 1200 | 5770 | |
| DF% | 100 | 100 | 97.0 | 99.2 | 84.4 | 97.0 | 43.9 | ||||||||
4. Discussion
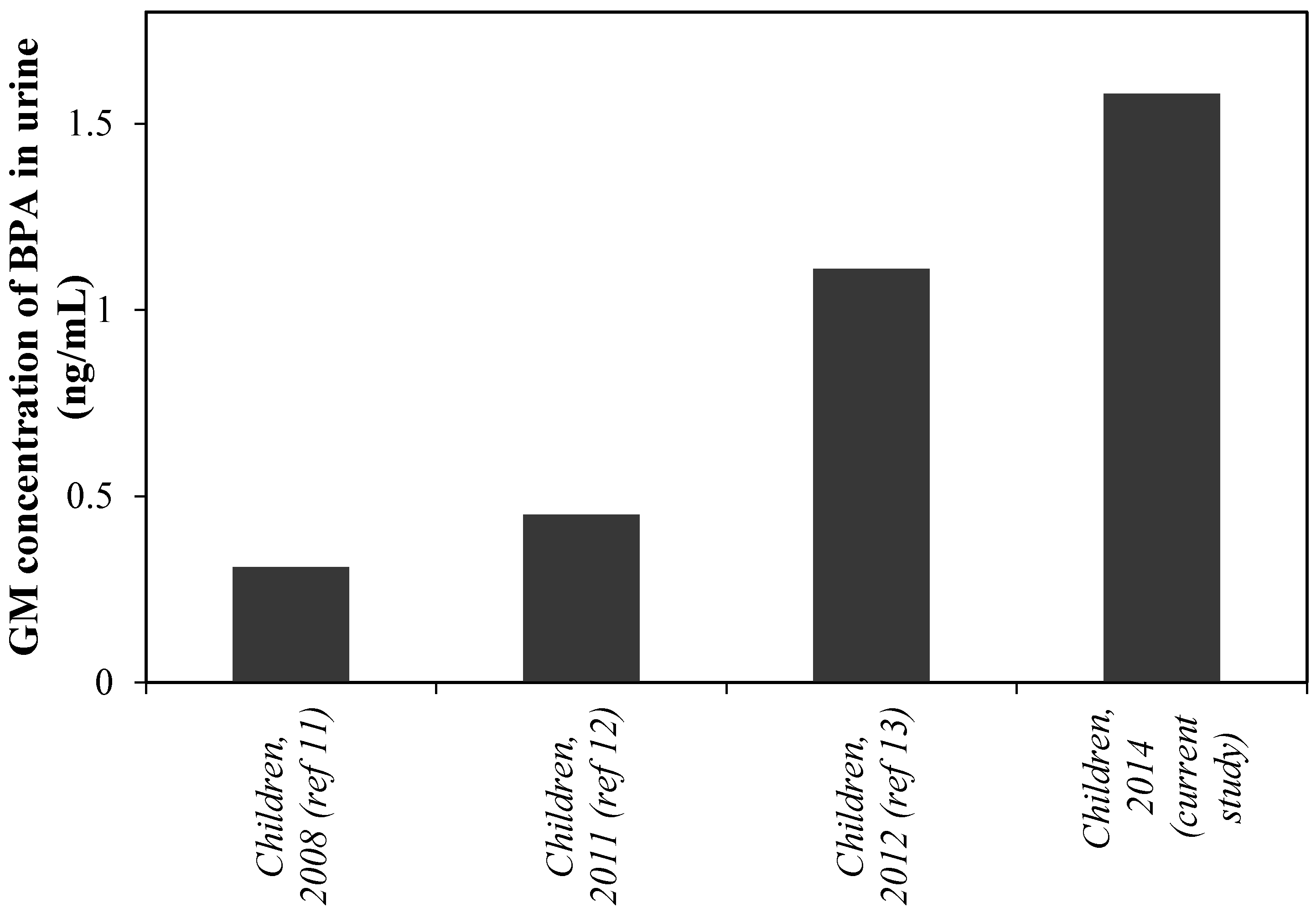
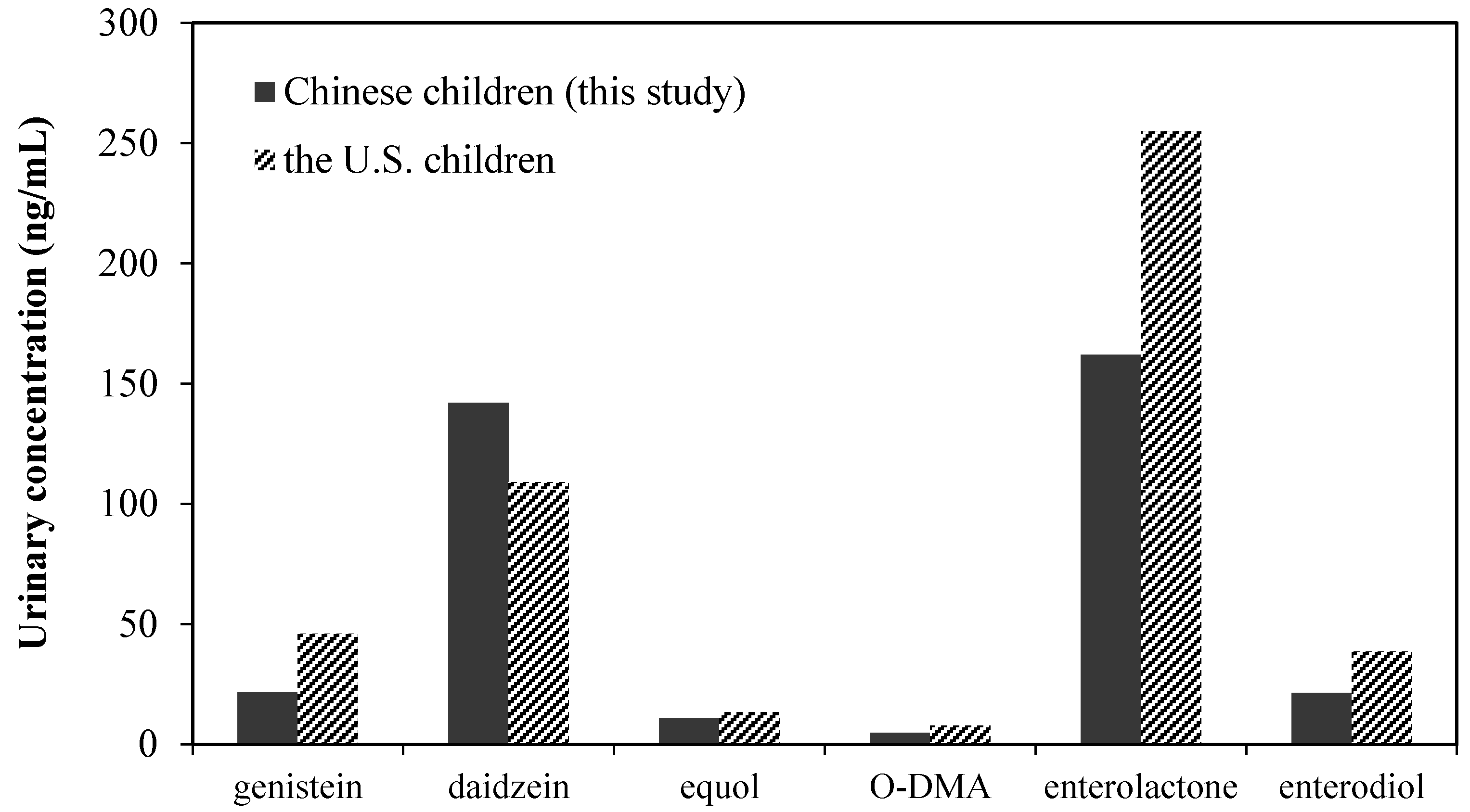
| BPA | Enterodiol | Daidzein | O-DMA | Genistein | Equol | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | R | p | R | p | R | p | |
| enterolactone | 0.224 | 0.088 | 0.530 | 0.000 | 0.097 | 0.481 | 0.230 | 0.142 | −0.010 | 0.940 | 0.462 | 0.008 |
| enterodiol | 0.073 | 0.583 | 0.324 | 0.016 | 0.326 | 0.033 | 0.251 | 0.057 | 0.142 | 0.448 | ||
| daidzein | 0.301 | 0.024 | 0.338 | 0.031 | 0.744 | 0.000 | 0.608 | 0.000 | ||||
| O-DMA | −0.037 | 0.815 | 0.095 | 0.544 | 0.222 | 0.376 | ||||||
| genistein | 0.193 | 0.143 | 0.321 | 0.079 | ||||||||
| equol | 0.407 | 0.021 | ||||||||||
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- USCDC (the U.S. Centers for Disease Control and Prevention). Fourth National Report on Human Exposure to Environmental Chemicals Updated Tables; USCDC (the U.S. Centers for Disease Control and Prevention): Clifton Road Atlanta, GA, USA, 2015. Available online: http://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015.pdf (accessed on 10 October 2015).
- Vom Saal, F.S.; Cooke, P.S.; Buchanan, D.L.; Palanza, P.; Thayer, K.A.; Nagel, S.C.; Parmigiani, S.; Welshons, W.V. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol. Ind. Health 1998, 14, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Vom Saal, F.S.; Richter, C.A.; Ruhlen, R.R.; Nagel, S.C.; Timms, B.G.; Welshons, W.V. The importance of appropriate controls, animal feed, and animal models in interpreting results from low-dose studies of bisphenol A. Birth Defects. Res. Part A 2005, 73, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Vom Saal, F.S.; Welshons, W.V. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environ. Res. 2006, 100, 50–76. [Google Scholar] [CrossRef] [PubMed]
- Hengstler, J.G.; Foth, H.; Gebel, T.; Kramer, P.J.; Lilienblum, W.; Schweinfurth, H.; Völkel, W.; Wollin, K.M.; Gundert-Remy, U. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit. Rev. Toxicol. 2011, 41, 263–291. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.E.; Witorsch, R.J.; McConnell, E.E.; Sipes, I.G.; Slayton, T.M.; Yu, C.J.; Franz, A.M.; Rhomberg, L.R. Weight-of-evidence evaluation of reproductive and developmental effects of low doses of bisphenol A. Crit. Rev. Toxicol. 2009, 39, 1–75. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A. Background Paper on BPA Biomonitoring and Biomarkerstudies; World Health Organization: Geneva, Switzerland, 2011. Available online: http://www.who.int/foodsafety/chem/chemicals/6.1_bpa_biomonitoring_and_biomarker_studies.pdf (accessed on 10 October 2015).
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.R.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef] [PubMed]
- Bushnik, T.; Haines, D.; Levallois, P.; Levesque, J.; Van Oostdam, J.; Viau, C. Lead and bisphenol A concentrations in the Canadian population. Health Rep. 2010, 21, 7–18. [Google Scholar] [PubMed]
- Kim, Y.H.; Kim, C.S.; Park, S.; Han, S.Y.; Pyo, M.Y.; Yang, M. Gender differences in the levels of bisphenol A metabolites in urine. Biochem. Biophys. Res. Commun. 2003, 312, 441–448. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Miao, M.; Herrinton, L.J.; Wu, C.; Yuan, W.; Zhou, Z.; Li, D.K. Bisphenol A levels in blood and urine in a Chinese population and the personal factors affecting the levels. Environ. Res. 2009, 109, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, Y.; Tang, C.; Wu, J.; Chen, Y.; Jiang, Q. Association between bisphenol A exposure and body mass index in Chinese school children: A cross-sectional study. Environ. Health 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, H.; Zhou, W.; He, Y.; Zhou, Y.; Chen, Y.; Jiang, Q. Exposure to bisphenol A among school children in eastern China: A multicenter cross-sectional study. J. Expo. Sci. Environ. Epidemiol. 2014, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Alomirah, H.; Cho, H.S.; Li, Y.F.; Liao, C.; Minh, T.B.; Mohd, M.A.; Nakata, H.; Ren, N.; Kannan, K. Urinary bisphenol A concentrations and their implications for human exposure in several Asian countries. Environ. Sci. Technol. 2011, 45, 7044–7050. [Google Scholar] [CrossRef] [PubMed]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Ryder, J.J.; Kurzer, M.S.; Lampe, J.W.; Messina, M.J.; Phipps, W.R.; Cassidy, A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: A systematic review and meta-analysis. Hum. Reprod. Update 2009, 15, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Patisaul, H.B.; Luskin, J.R.; Wilson, M.E. A soy supplement and tamoxifen inhibit sexual behavior in female rats. Hormones Behav. 2004, 45, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Asimakopoulos, A.G.; Kannan, K. Accumulation of 19 environmental phenolic and xenobiotic heterocyclic aromatic compounds in human adipose tissue. Environ. Int. 2015, 78, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Kunisue, T.; Tanabe, S.; Isobe, T.; Aldous, K.M.; Kannan, K. Profiles of phytoestrogens in human urine from several Asian countries. J. Agric. Food Chem. 2010, 58, 9838–9846. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Y.; Zhang, W.; Kannan, K. Widespread occurrence and distribution of bisphenol A diglycidyl ether (BADGE) and its derivatives in human urine from the United States and China. Environ. Sci. Technol. 2012, 46, 12968–12976. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Wong, C.K.; Zheng, J.S.; Bouwman, H.; Barra, R.; Wahlström, B.; Neretin, L.; Wong, M.H. Bisphenol A (BPA) in China: A review of sources, environmental levels, and potential human health impacts. Environ. Int. 2002, 42, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Tang, R. Association of Prenatal Phytoestrogen Exposures and Maternal Estrogen Metabolite Levels with Birth Outcomes. Master thesis, Nanjing Medical University, Nanjing, China, 2013. [Google Scholar]
- Xia, Y.K.; Chen, M.J.; Zhu, P.F.; Lu, C.C.; Fu, G.B.; Zhou, X.J.; Chen, D.Z.; Wang, H.H.; Hang, B.; Wang, S.L.; et al. Urinary phytoestrogen levels related to idiopathic male infertility in Chinese men. Environ. Int. 2013, 59, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Cai, H.; Gao, Y.T.; Dai, Q.; Li, H.L.; Cai, Q.Y.; Yang, G.; Franke, A.A.; Zheng, W.; Shu, X.O. Correlations of urinary phytoestrogen excretion with lifestyle factors and dietary intakes among middle-aged and elderly Chinese women. Int. J. Mol. Epidemiol. Genet. 2012, 3, 18–29. [Google Scholar] [PubMed]
- Cederroth, C.R.; Zimmermann, C.; Nef, S. Soy, phytoestrogens and their impact on reproductive health. Mol. Cell. Endocrinol. 2012, 355, 192–200. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Duan, Z.; Wu, Y.; Liu, Z.; Li, K.; Wang, L. Occurrence and Profiles of the Artificial Endocrine Disruptor Bisphenol A and Natural Endocrine Disruptor Phytoestrogens in Urine from Children in China. Int. J. Environ. Res. Public Health 2015, 12, 15110-15117. https://doi.org/10.3390/ijerph121214964
Zhang M, Duan Z, Wu Y, Liu Z, Li K, Wang L. Occurrence and Profiles of the Artificial Endocrine Disruptor Bisphenol A and Natural Endocrine Disruptor Phytoestrogens in Urine from Children in China. International Journal of Environmental Research and Public Health. 2015; 12(12):15110-15117. https://doi.org/10.3390/ijerph121214964
Chicago/Turabian StyleZhang, Mingyue, Zhenghua Duan, Yinghong Wu, Zhen Liu, Ke Li, and Lei Wang. 2015. "Occurrence and Profiles of the Artificial Endocrine Disruptor Bisphenol A and Natural Endocrine Disruptor Phytoestrogens in Urine from Children in China" International Journal of Environmental Research and Public Health 12, no. 12: 15110-15117. https://doi.org/10.3390/ijerph121214964






