Resistance and Inactivation Kinetics of Bacterial Strains Isolated from the Non-Chlorinated and Chlorinated Effluents of a WWTP
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbiological Quality
2.1.1. Sampling
2.1.2. Mesophilic Aerobic Bacterial Counts
2.2. Isolation and Identification of Bacterial Strains
2.2.1. Isolation of Bacterial Strains
2.2.2. Preparation of Bacterial Suspensions
2.2.3. Molecular Identification of Bacterial Strains
2.3. NaClO Resistance Tests
2.4. Inactivation Kinetics of the Bacterial Strains
2.5. Statistical Analysis
3. Results and Discussion
3.1. Microbiological Quality
3.1.1. Sampling
3.1.2. Mesophilic Aerobic Bacterial Count
3.2. Isolation and Identification of Bacterial Strains
3.2.1. Isolation of Bacterial Strains
3.2.2. Molecular Identification of Bacterial Strains
| WWTP Effluent | Test | Closest taxon | Access number | % Similarity |
|---|---|---|---|---|
| Non-chlorinated | RT | Aeromonas hydrophila AN-2 | AY987736.1 | 95 |
| Enterobacter cloacae A5-B25 | AF406657.1 | 92 | ||
| Escherichia coli | CP002516.1 | 98 | ||
| Escherichia coli BL21 | AM946981.2 | 89 | ||
| Escherichia coli PD3 | FR715025.1 | 95 | ||
| Chlorinated | IK | Bacillus sp. FRC_Y9-2 | EF158823.1 | 100 |
| Citrobacter freundiia | NR_028894.1 | 96 | ||
| Citrobacter freundiib | FN997639.1 | 99 | ||
| Enterobacter sp. MS5 | FN997607.1 | 88 | ||
| Kluyvera cryocrescensa | AM933754.1 | 98 | ||
| Kluyvera cryocrescensb | AM933754.1 | 94 | ||
| Kluyvera cryocrescensc | AM933754.1 | 95 | ||
| Kluyvera intermedia | NR_028802.1 | 99 |
3.3. NaClO Resistance Tests
| Closest taxon | Log inactivation | Resistance (log10(CFU·100 mL−1)) | |||
|---|---|---|---|---|---|
| Maximum | Treatment | Minimum | Treatment | ||
| A. hydrophila | 0.84 | 10.43 | TI 30 mg·L−g/15 min | 11.27 | TII 20 mg·L−g/0 min |
| E. coli | 1.31 | 10.15 | TI 8 mg·L−g/30 min | 11.45 | TIII 8 mg·L−g/30 min |
| E. coli PD3 | 1.86 | 10.62 | TIII 30 mg·L−g/0 min | 12.48 | TI 30 mg·L−g/0 min |
| E. coli BL21 | 0.80 | 10.37 | TI 8 mg·L−g/0 min | 11.18 | TII 20 mg·L−g/0 min |
| E. cloacae | 0.81 | 10.38 | TIII 30 mg·L−g/30 min | 11.19 | TIII 8 mg·L−g/0 min |
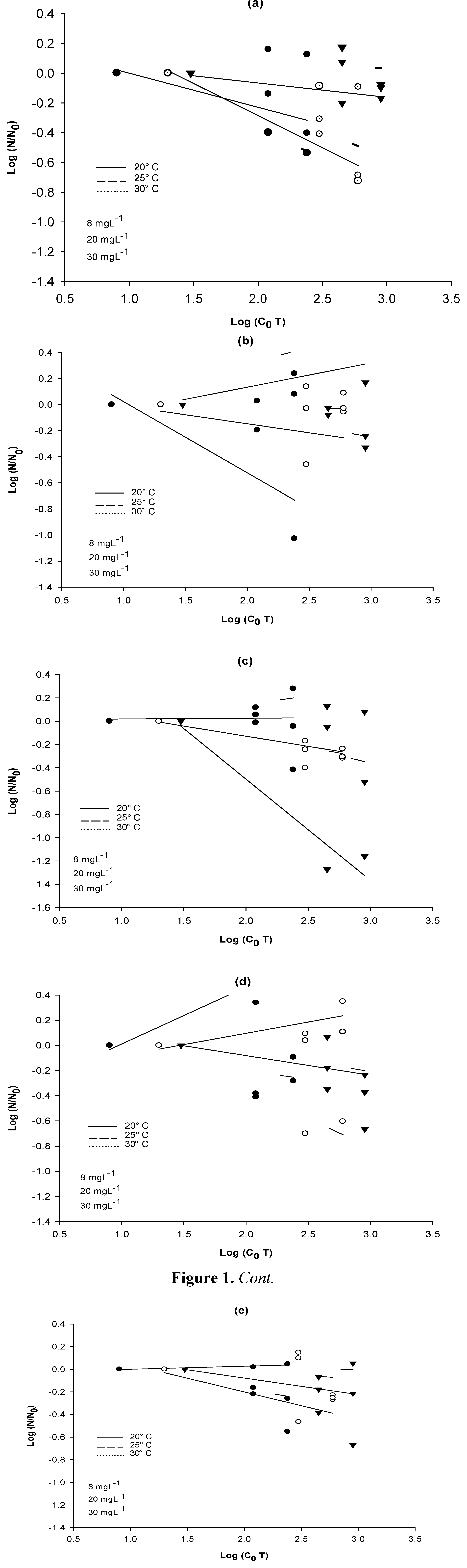
3.4. Inactivation Kinetics of the Bacterial Strains
| Closest taxon | Removal % | Inactivation | |
|---|---|---|---|
| T = 90 min | Max% | (log10(CFU·100 mL−1)) | |
| Bacillus sp. FRC_Y9-2 | 98.87 | 98.87 | 1.96 |
| Citrobacter freundiia | 92.52 | 92.52 | 1.86 |
| C. freundiib | 99.71 | 99.71 | 2.25 |
| Enterobacter sp. MS5 | 0 | 98.91 ** | 2.09 |
| Kluyvera cryocrescensa | 14.89 | 14.89 | 1.61 |
| K. cryocrescensb | 86.54 | 86.54 | 0.87 |
| K. cryocrescensc | 98.17 | 98.30 * | 1.77 |
| K. intermedia | 65.37 | 87.45 * | 0.9 |
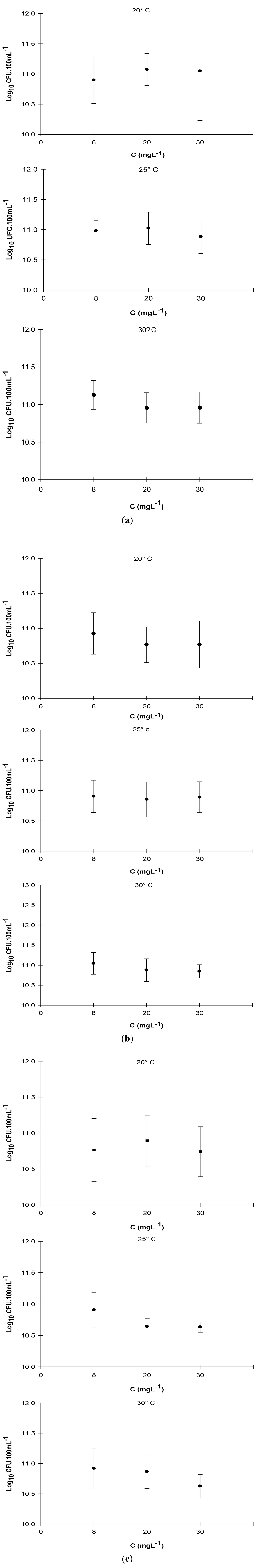
3.5. Statistical Analysis
3.5.1. NaClO Resistance Tests
| T (min) | ||||||
|---|---|---|---|---|---|---|
| 0 | 15 | 30 | ||||
| Treatment I (20 °C) | ||||||
| mg·L−1 | x | δ | x | δ | x | δ |
| 8 | 1.03E+11 | 6.99E+10 | 1.0357E+11 | 8.244E+10 | 8.244E+10 | 6.7504E+10 |
| 20 | 1.363E+11 | 7.47E+10 | 6.6674E+10 | 3.877E+10 | 9.814E+10 | 6.196E+10 |
| 30 | 6.421E+11 | 1.32E+12 | 7.3978E+10 | 5.553E+10 | 7.444E+10 | 7.5511E+10 |
| Treatment II (25 °C) | ||||||
| 8 | 1.01E+11 | 3.69E+10 | 9.4558E+10 | 6.407E+10 | 9.507E+10 | 6.2074E+10 |
| 20 | 1.205E+11 | 6.16E+10 | 8.4292E+10 | 4.971E+10 | 4.568E+10 | 1.6236E+10 |
| 30 | 8.99E+10 | 5.93E+10 | 8.8934E+10 | 5.069E+10 | 4.349E+10 | 8.326E+10 |
| Treatment III (30 °C) | ||||||
| 8 | 1.45E+11 | 6.11E+10 | 1.2841E+11 | 7.769E+10 | 1.078E+11 | 1.0039E+11 |
| 20 | 9.814E+10 | 4.39E+10 | 9.246E+10 | 7.504E+10 | 8.758E+10 | 6.5128E+10 |
| 30 | 9.838E+10 | 3.76E+10 | 7.4647E+10 | 2.888E+10 | 4.559E+10 | 1.8856E+10 |
| Source | d.f. | S | V | F | ρ |
|---|---|---|---|---|---|
| A | 2 | 0.02 | 0.01 | ||
| B | 2 | 0.08 | 0.04 | 3.67 | 4.02 |
| C | 2 | 0.19 | 0.10 | 9.24 | 12.42 |
| D | 2 | 0.10 | 0.05 | 4.88 | 5.84 |
| R | 2 | 0.13 | 0.07 | 6.23 | 7.89 |
| e1 | 16 | 0.86 | 0.05 | 5.16 | 50.23 |
| <e> | 2 | 0.02 | 0.01 | 19.60 | |
| TOTAL | 26 | 1.39 | 0.05 | 100 |
3.5.2. Inactivation Kinetics of the Bacterial Strains
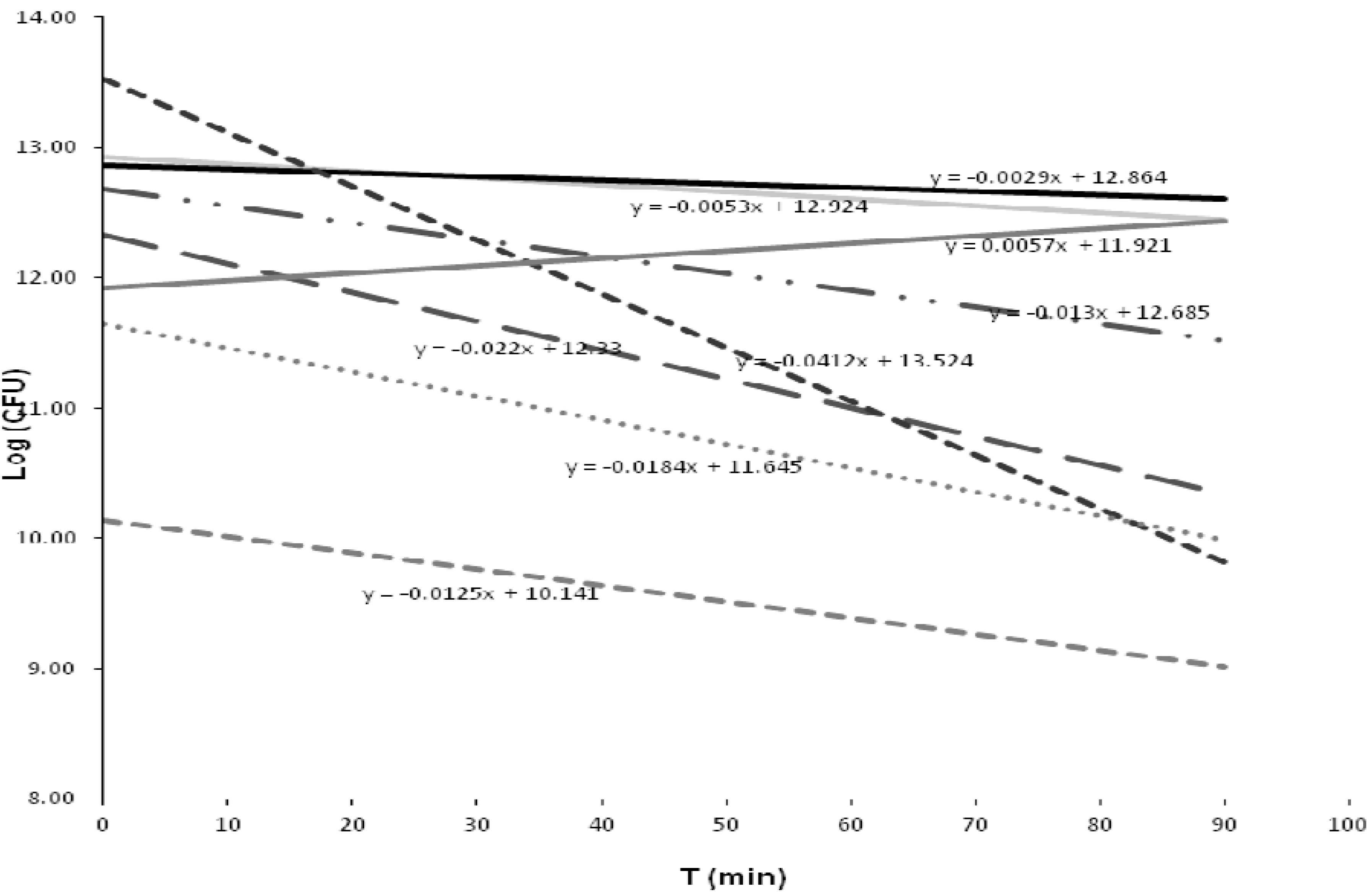
| Group | Closest taxon | Mean | Sd | T cal | T tab |
|---|---|---|---|---|---|
| A | |||||
| K. cryocrescensb | 12.64 | 0.34 | 0.68 | 2.17 | |
| K. intermedia | 12.76 | 0.28 | |||
| B | |||||
| Bacillus sp. FRC_Y9-2 | 11.56 | 0.87 | 0.15 | ||
| K. cryocrescensc | 11 | 0.79 | |||
| C | |||||
| K. cryocrescensa | 9.7 | 0.76 | 0.43 | ||
| C. freundiia | 12.23 | 0.58 | |||
| D, E | |||||
| C. freundiib | 12.53 | 0.89 | 3.34 | ||
| Enterobacter sp. MS5 | 12.11 | 0.75 | |||
| Among groups | |||||
| A–B | A | 12.7 | 0.31 | 3.9 | |
| B | 11.27 | 0.83 | |||
| C–B | |||||
| C | 10.97 | 0.67 | 2.82 | ||
| B | 11.27 | 0.83 | |||
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Helbling, E.D.; VanBriesen, M.J. Continuous monitoring of residual chlorine concentrations in response to controlled microbial intrusions in a laboratory-scale distribution system. Water Res. 2008, 42, 3162–3172. [Google Scholar] [CrossRef]
- Hassen, A.; Mehrouk, M.; Ouzari, H.; Cherif, M.; Boudabous, A.; Damelincourt, J.J. UV disinfection of treated wastewater in a large-scale pilot plant and inactivation of selected bacteria in a laboratory UV device. Bioresour. Technol. 2000, 74, 141–150. [Google Scholar] [CrossRef]
- Jeffrey, P.; Seaton, R.A.F.; Stephenson, T.; Parsons, S. Infrastructure configurations for wastewater treatment and reuse: A simulation based study of membrane bioreactors. Water Sci. Technol. 1998, 38, 105–111. [Google Scholar]
- Veschetti, E.; Cutilli, D.; Bonadonna, L.; Briancesco, R.; Martini, C.; Cecchini, G.; Anastasi, P.; Ottaviani, M. Pilot-plant comparative study of peracetic acid and sodium hypochlorite wastewater disinfection. Water Res. 2003, 37, 78–94. [Google Scholar] [CrossRef]
- Katz, A.; Narkis, N.; Orshansky, F.; Friedland, E.; Kott, Y. Disinfection of effluent by combinations of equal doses of chlorine dioxide and chlorine added simultaneously over varying contact times. Water Res. 1994, 28, 2133–2138. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering Treatment and Reuse; Metcalf and Eddy, McGraw-Hill: New York, NY, USA, 2003; pp. 1217–1330. [Google Scholar]
- Estrela, C.; Estrela, C.R.; Barbin, E.L.; Spanó, J.C.E.; Marchesan, M.A.; Pécora, J.D. Mechanism of action of sodium hypochlorite. Braz. Dent. J. 2002, 13, 113–117. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar]
- Apella, C.M.; Araujo, Z.P. Microbiología del agua. Conceptos Básicos. In Tecnologías Solares para la Desinfección y Descontaminación del Agua; Blesa, M.A., Blanco, G.J., Eds.; Solarsafewater: Buenos Aires, Argentine, 2005; pp. 33–50. [Google Scholar]
- Goel, S.; Bouwer, E.J. Factors influencing inactivation of Klebsiella pneumoniae by chlorine and chloramine. Water Res. 2004, 38, 301–308. [Google Scholar] [CrossRef]
- Wu, C.W.; Schmoller, S.K.; Shin, S.J.; Talaat, A.M. Defining the stressome of Mycobacterium avium subsp. paratuberculosis in vitro and in naturally infected cows. J. Bacteriol. 2007, 189, 7877–7886. [Google Scholar] [CrossRef]
- Berry, D.; Holder, D.; Xi, C.; Raskin, L. Comparative transcriptomics of the response of Escherichia coli to the disinfectant monochloramine and to growth conditions inducing monochloramine resistance. Water Res. 2010, 44, 4924–4931. [Google Scholar] [CrossRef]
- Winward, G.P.; Avery, L.M.; Stephenson, T.; Jefferson, B. Chlorine disinfection of grey water for reuse: Effect of organics and particles. Water Res. 2008, 42, 483–491. [Google Scholar] [CrossRef]
- Virto, R.; Mañas, P.; Álvarez, I.; Condon, S.; Raso, J. Membrane damage and microbial inactivation by chlorine in the absence and presence of a chlorine-demanding substrate. Appl. Environ. Microbiol. 2005, 71, 5022–5028. [Google Scholar]
- Djuikom, E.; Njiné, T.; Nola, M.; Kemka, N.; Zébazé Touget, S.H.; Jugnia, L.B. Significance and suitability of Aeromonas hydrophila vs. fecal coliforms in assessing microbiological water quality. World J. Microbiol. Biotechnol. 2008, 24, 2665–2670. [Google Scholar] [CrossRef]
- Salem, I.B.; Ouardani, I.; Hassine, M.; Aouni, M. Bacteriological and physico-chemical assessment of wastewater in different regions of Tunisia: Impact on human health. BMC Res. Notes 2011, 4. [Google Scholar] [CrossRef]
- Orta Ledesma, M.T.; Díaz Pérez, V.; Aparicio, G. Desinfección de Agua Potable Contaminada Con Vibrio Cholerae Adaptada Al Cloro. Consolidación Para el Desarrollo; CEPIS: Mexico City, Mexico, 1996. Available online: http://www.bvsde.paho.org/bvsaidis/caliagua/mexico/ 02392e14.pdf (accessed on 10 June 2012).
- Germer, J.; Boh, M.Y.; Schoeffler, M.; Amoah, P. Temperature and deactivation of microbial faecal indicators during small scale co-composting of faecal matter. Waste Manag. 2010, 30, 185–191. [Google Scholar] [CrossRef]
- Cho, M.; Kim, J.; Kim, J.Y.; Yeon, J.; Yoon, J.; Kim, J.H. Mechanisms of Escherichia coli inactivation by several disinfectants. Water Res. 2010, 44, 3410–3418. [Google Scholar] [CrossRef]
- Luczkiewicz, A.; Jankowska, K.; Fudala, K.S.; Olanczuc, N.K. Antimicrobial resistance of fecal indicators in municipal wastewater treatment plant. Water Res. 2010, 44, 5089–5097. [Google Scholar] [CrossRef]
- Coronel-Olivares, C.; Reyes-Gómez, L.M.; Hernández-Muñoz, A.; Martínez-Falcón, A.P.; Vázquez-Rodríguez, G.A.; Iturbe, U. Chlorine disinfection of Pseudomonas aeruginosa, total coliforms, Escherichia coli and Enterococcus faecalis: Revisiting reclaimed water regulations. Water Sci. Technol. 2011, 64, 2151–2157. [Google Scholar] [CrossRef]
- Standard Method for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 2008.
- Cavalieri, J.S. Manual de Pruebas de Susceptibilidad Antimicrobiana; American Society for Microbiology: Washington, DC, USA, 2005; pp. 39–53, 225–231. [Google Scholar]
- Fernández-No, I.C.; Böhme, K.; Gallardo, J.M.; Barros-Velázquez, J.; Cañas, B.; Calo-Mata, P. Differential characterization of biogenic amine-production bacteria involved in food poisoning using MALDI-TOF mass fingerprinting. Electrophoresis 2010, 31, 1116–1127. [Google Scholar]
- Lazarova, V.; Savoye, P.; Janex, M.L.; Blatchley, E.R.; Pommepuy, M. Advanced wastewater disinfection Technologies: State of the art and perspectives. Water Sci. Technol. 1999, 40, 203–213. [Google Scholar]
- Salgot, M.; Huertas, E.; Weber, S.; Dott, W.; Hollender, J. Wastewater reuse and risk: Definition of key objectives. Desalination 2006, 187, 29–40. [Google Scholar] [CrossRef]
- Fox, G.E.; Wisotzkey, J.D.; Jurtshuk, P. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int. J. Syst. Bacteriol. 1992, 42, 166–170. [Google Scholar] [CrossRef]
- Cabral, J.P.S. Water microbiology. Bacterial pathogens and water. Int. J. Environ. Res. Public Health 2010, 7, 3657–3703. [Google Scholar] [CrossRef]
- Picão, R.C.; Cardoso, J.P.; Campana, E.H.; Nicoletti, A.G.; Petrolini, F.V.; Assis, D.M.; Juliano, L.; Gales, A.C. The route of antimicrobial resistance from the hospital effluent to the environment: Focus on the occurrence of KPC-producing Aeromonas spp. and Enterobacteriaceae in sewage. Diagn. Microbiol. Infect. Dis. 2013, 76, 80–85. [Google Scholar] [CrossRef]
- Sarria, J.C.; Vidal, A.M.; Kimbrough, R.C., III. Infections caused by Kluyvera species in humans. Infect. Dis. Soc. Am. Clin. Infect. Dis. 2001, 33, 69–74. [Google Scholar]
- Shi, P.; Jia, S.; Zhang, X.X.; Zhang, T.; Cheng, S.; Li, A. Metagenomic insights into chlorination effects on microbial antibiotic resistance in drinking water. Water Res. 2013, 47, 111–120. [Google Scholar] [CrossRef]
- Barbeau, B.; Boulos, L.; Desjardins, R.; Coallier, J.; Prévost, M. Examining the use of aerobic spore-forming bacteria to assess the efficiency of chlorination. Water Res. 1999, 33, 2941–2948. [Google Scholar] [CrossRef]
- Dow, S.M.; Barbeau, B.; von Gunten, U.; Chandrakanth, M.; Amy, G.; Hernandez, M. The impact of selected water quality parameters on the inactivation of Bacillus subtilis spores by monochloramine and ozone. Water Res. 2006, 40, 373–382. [Google Scholar] [CrossRef]
- Figueras, M.J.; Borrego, J.J. New perspectives in monitoring drinking water microbial quality. Int. J. Environ. Res. Public Health 2010, 7, 4179–4202. [Google Scholar] [CrossRef]
- Koivunen, J.; Heinonen-Tanski, H. Inactivation of enteric microorganisms with chemical disinfectants, UV irradiation and combined chemical/UV treatments. Water Res. 2005, 39, 1519–1526. [Google Scholar] [CrossRef]
- Tree, J.A.; Adams, M.R.; Lees, D.N. Chlorination of indicator bacteria and viruses in primary sewage effluent. Appl. Environ. Microbiol. 2003, 69, 2038–2043. [Google Scholar] [CrossRef]
- King, C.H.; Shotts, E.B.; Wooley, R.E.; Porter, K.G. Survival of coliforms and bacterial pathogens within protozoa during chlorination. Appl. Environ. Microbiol. 1998, 54, 3023–3033. [Google Scholar]
- Macauley, J.J.; Qiang, Z.; Adams, D.C.; Surampalli, R.; Mormile, M.R. Disinfection of swine wastewater using chlorine, ultraviolet light and ozone. Water Res. 2006, 40, 2017–2026. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Martínez-Hernández, S.; Vázquez-Rodríguez, G.A.; Beltrán-Hernández, R.I.; Prieto-García, F.; Miranda-López, J.M.; Franco-Abuín, C.M.; Álvarez-Hernández, A.; Iturbe, U.; Coronel-Olivares, C. Resistance and Inactivation Kinetics of Bacterial Strains Isolated from the Non-Chlorinated and Chlorinated Effluents of a WWTP. Int. J. Environ. Res. Public Health 2013, 10, 3363-3383. https://doi.org/10.3390/ijerph10083363
Martínez-Hernández S, Vázquez-Rodríguez GA, Beltrán-Hernández RI, Prieto-García F, Miranda-López JM, Franco-Abuín CM, Álvarez-Hernández A, Iturbe U, Coronel-Olivares C. Resistance and Inactivation Kinetics of Bacterial Strains Isolated from the Non-Chlorinated and Chlorinated Effluents of a WWTP. International Journal of Environmental Research and Public Health. 2013; 10(8):3363-3383. https://doi.org/10.3390/ijerph10083363
Chicago/Turabian StyleMartínez-Hernández, Sylvia, Gabriela A. Vázquez-Rodríguez, Rosa I. Beltrán-Hernández, Francisco Prieto-García, José M. Miranda-López, Carlos M. Franco-Abuín, Alejandro Álvarez-Hernández, Ulises Iturbe, and Claudia Coronel-Olivares. 2013. "Resistance and Inactivation Kinetics of Bacterial Strains Isolated from the Non-Chlorinated and Chlorinated Effluents of a WWTP" International Journal of Environmental Research and Public Health 10, no. 8: 3363-3383. https://doi.org/10.3390/ijerph10083363
APA StyleMartínez-Hernández, S., Vázquez-Rodríguez, G. A., Beltrán-Hernández, R. I., Prieto-García, F., Miranda-López, J. M., Franco-Abuín, C. M., Álvarez-Hernández, A., Iturbe, U., & Coronel-Olivares, C. (2013). Resistance and Inactivation Kinetics of Bacterial Strains Isolated from the Non-Chlorinated and Chlorinated Effluents of a WWTP. International Journal of Environmental Research and Public Health, 10(8), 3363-3383. https://doi.org/10.3390/ijerph10083363








