The Jellyfish Rhizostoma pulmo (Cnidaria): Biochemical Composition of Ovaries and Antibacterial Lysozyme-like Activity of the Oocyte Lysate
Abstract
:1. Introduction
2. Results
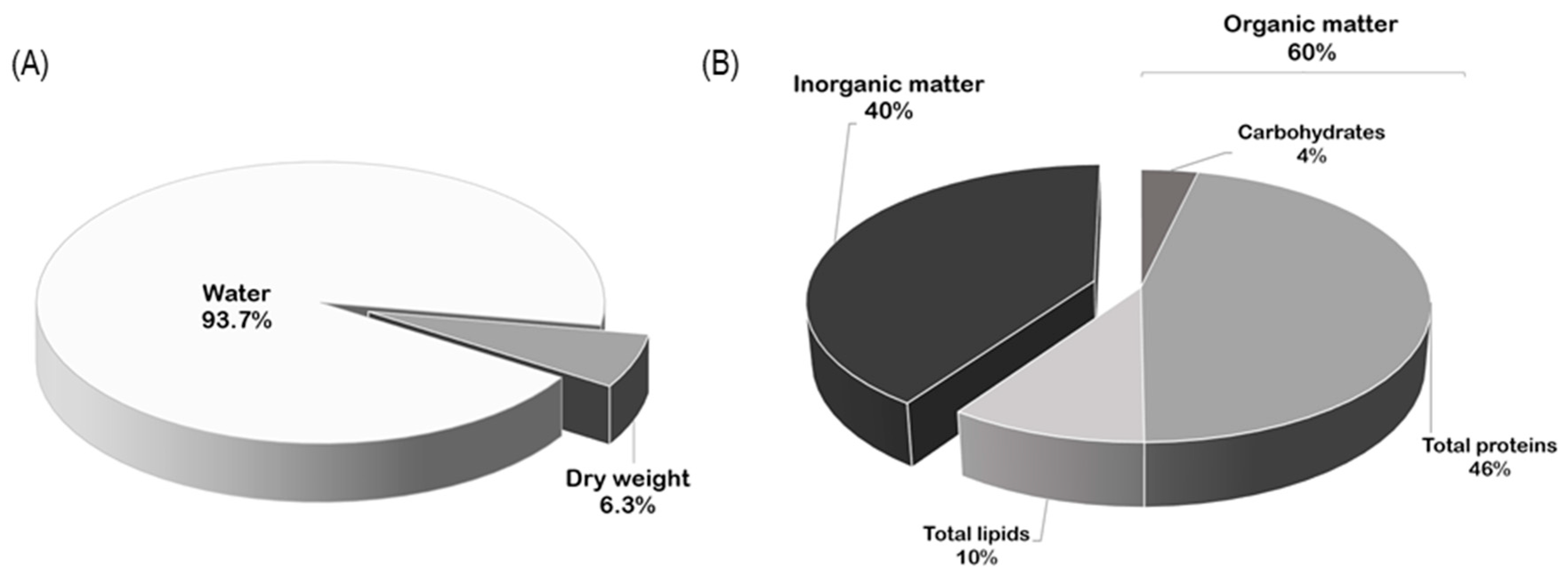
2.1. Biochemical Composition of Ovaries
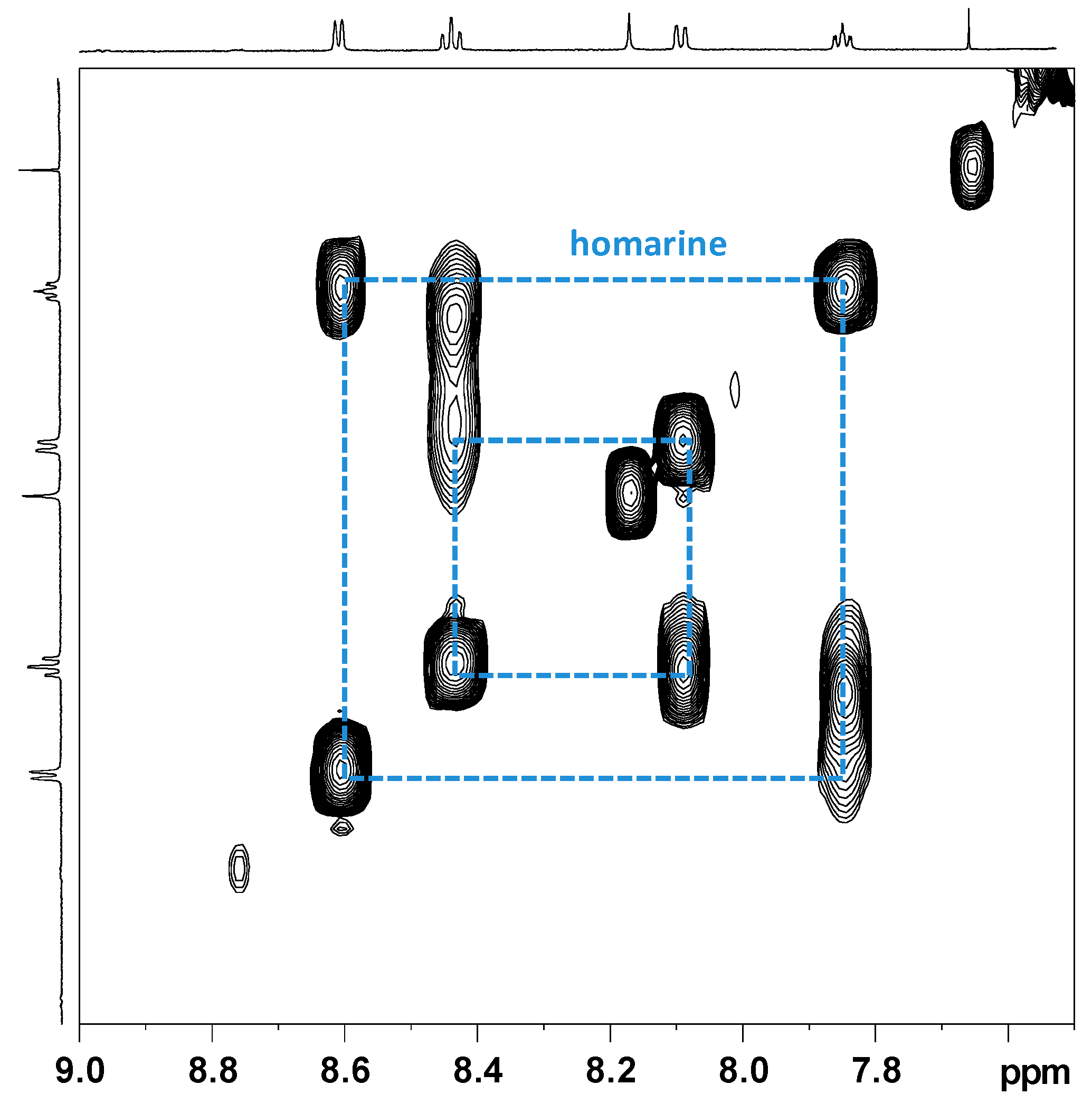
2.2. NMR Spectroscopy
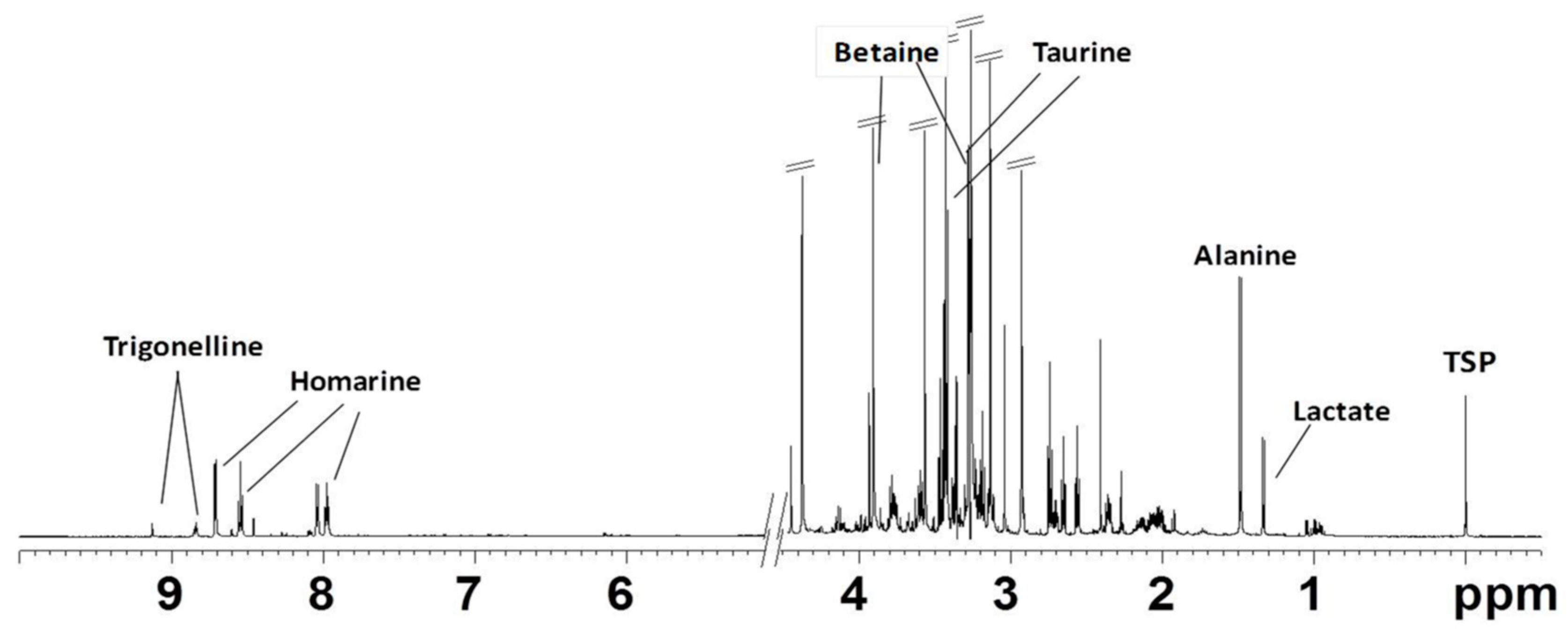
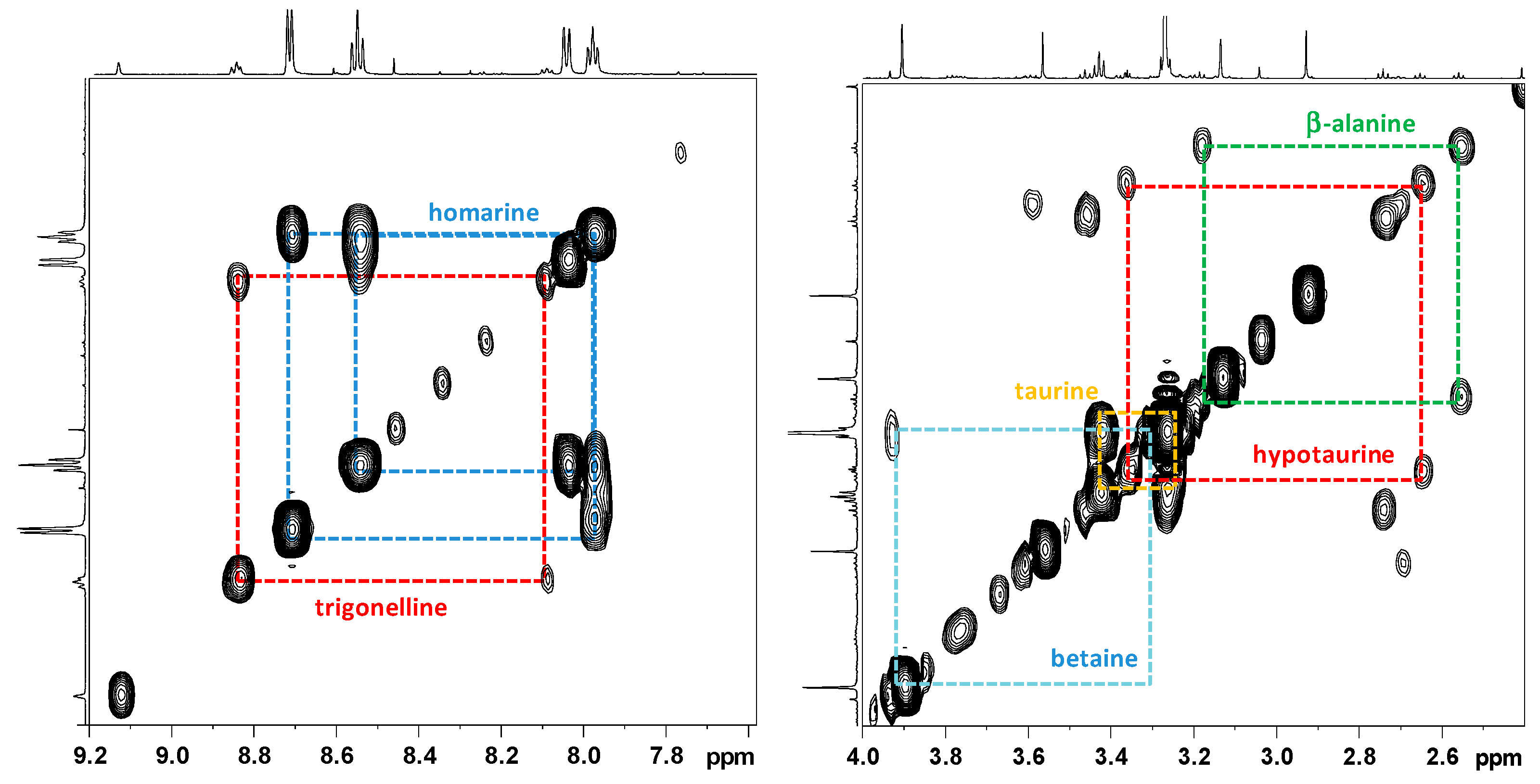
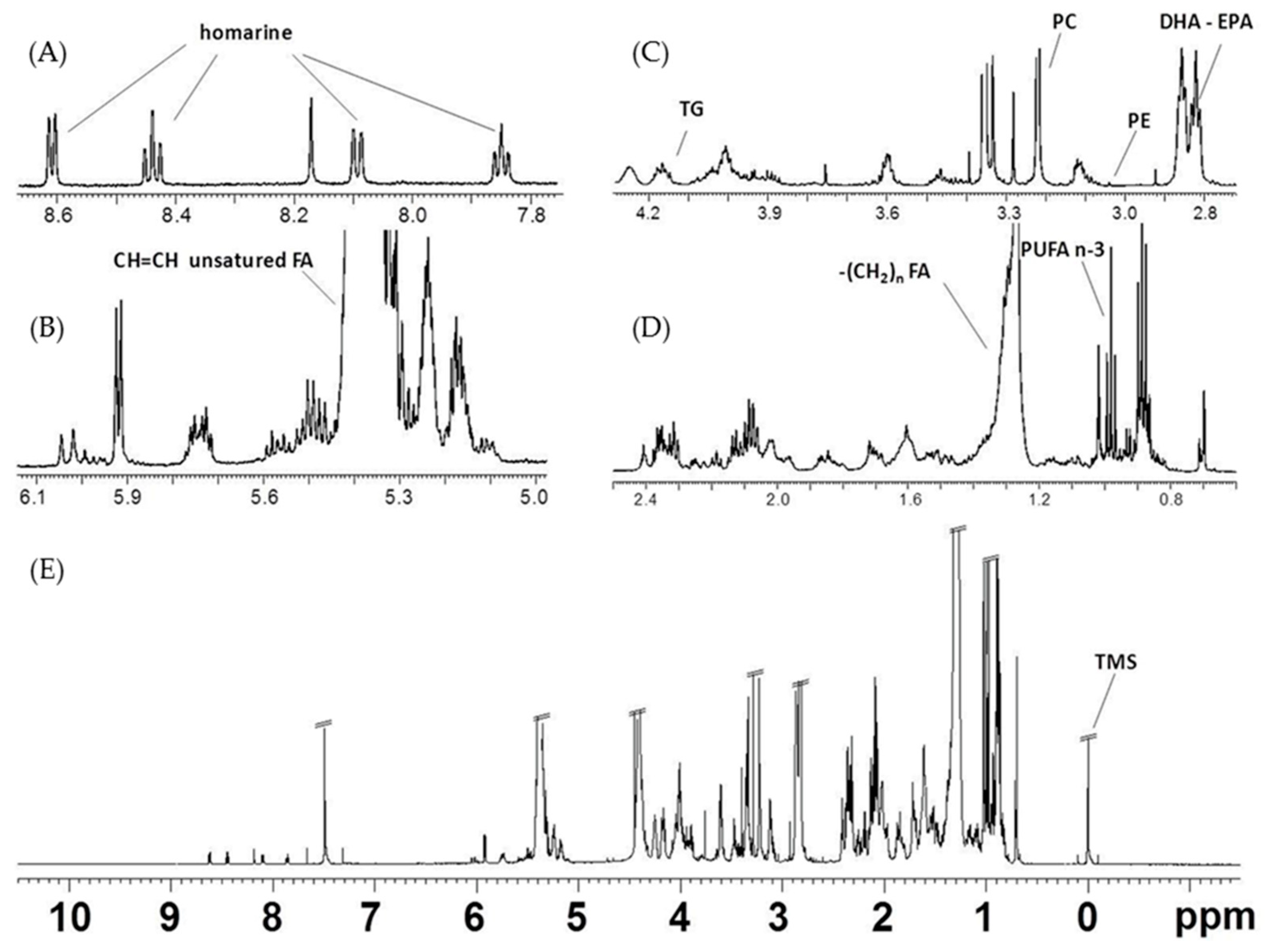
2.2.1. NMR Analysis of Female Gonads Aqueous Extracts
2.2.2. NMR Analysis of Female Gonads Lipid Extracts
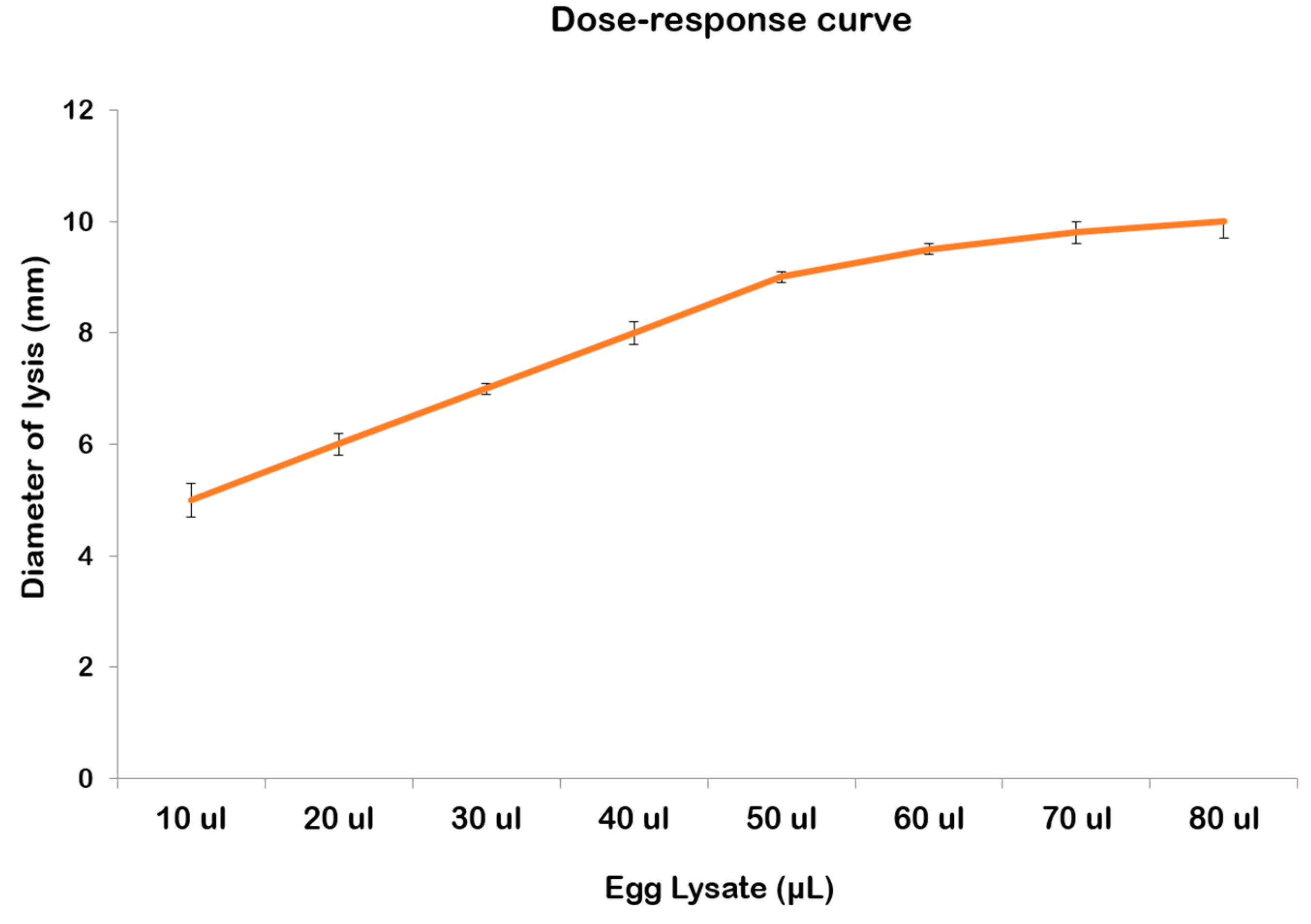
2.3. Lysozyme-like Activity in R. pulmo Oocyte Lysate
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Preparation
4.2. Biochemical Analysis
4.3. NMR Analysis
NMR Spectroscopy and Data Processing
4.4. Lysozyme-Like Activity
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hamner, W.M.; Dawson, M.N. A review and synthesis on the systematics and evolution of jellyfish blooms: Advantageous aggregations and adaptive assemblages. Hydrobiologia 2009, 616, 161–191. [Google Scholar] [CrossRef]
- Falkenhaug, T. Review of Jellyfish Blooms in the Mediterranean and Black Sea. Mar. Biol. Res. 2014, 10, 1038–1039. [Google Scholar] [CrossRef] [Green Version]
- Benedetti-Cecchi, L.; Canepa, A.; Fuentes, V.; Tamburello, L.; Purcell, J.E.; Piraino, S.; Roberts, J.; Boero, F.; Halpin, P. Deterministic factors overwhelm stochastic environmental fluctuations as drivers of jellyfish outbreaks. PLoS ONE 2015, 10, e0141060. [Google Scholar] [CrossRef] [PubMed]
- Boero, F.; Brotz, L.; Gibbons, M.J.; Piraino, S.; Zampardi, S. Impacts and effects of ocean warming on jellyfish. In Explaining Ocean Warming: Causes, Scale, Effects and Consequences; Full Report; IUCN: Gland, Switzerland, 2016; pp. 213–237. [Google Scholar]
- Milisenda, G.; Martinez-Quintana, A.; Fuentes, V.L.; Bosch-Belmar, M.; Aglieri, G.; Boero, R.R.; Piraino, S. Reproductive and bloom patterns of Pelagia noctiluca in the Strait of Messina, Italy. Estuar. Coast. Shelf Sci. 2018, 201, 29–39. [Google Scholar] [CrossRef]
- Bosch, T.C.G. The Path Less Explored: Innate Immune Reactions in Cnidarians. In Innate Immunity of Plants, Animals, and Humans; Springer: Berlin/Heidelberg, Germany, 2008; pp. 27–42. [Google Scholar]
- Mariottini, G.L.; Grice, I.D. Antimicrobials from cnidarians. A new perspective for anti-infective therapy? Mar. Drugs 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Dingley, A.J.; Augustin, R.; Anton-Erxleben, F.; Stanisak, M.; Gelhaus, C.; Gutsmann, T.; Hammer, M.U.; Podschun, R.; Bonvin, A.M.J.J.; et al. Hydramacin-1, structure and antibacterial activity of a protein from the basal metazoan hydra. J. Biol. Chem. 2009, 284, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Rumrill, S.S. Natural mortality of marine invertebrate larvae. Ophelia 1990, 32, 163–198. [Google Scholar] [CrossRef]
- Fuda, H.; Hara, A.; Yamazaki, F.; Kobayashi, K. A peculiar immunoglobulin M (IgM) identified in eggs of chum salmon (Oncorhynchus keta). Dev. Comp. Immunol. 1992, 16, 415–423. [Google Scholar] [CrossRef]
- Ozaki, H.; Ohwaki, M.; Fukada, T. Studies on lectins of amago (Oncorhyncus rhodurus) I. Amago ova lectin and its receptor on homologous macrophages. Dev. Comp. Immunol. 1983, 7, 77–87. [Google Scholar] [CrossRef]
- Kudo, S.; Teshima, C. Enzyme activities and antifungal action of fertilization envelope extract from fish eggs. J. Exp. Zool. 1991, 259, 392–398. [Google Scholar] [CrossRef]
- Ovchinnikova, T.V.; Balandin, S.V.; Aleshina, G.M.; Tagaev, A.A.; Leonova, Y.F.; Krasnodembsky, E.D.; Men’shenin, A.V.; Kokryakov, V.N. Aurelin, a novel antimicrobial peptide from jellyfish Aurelia aurita with structural features of defensins and channel-blocking toxins. Biochem. Biophys. Res. Commun. 2006, 348, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Scott, M.G. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 2000, 97, 8856–8861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, J.; Peixe, L.; Gomes, N.C.M.; Calado, R. Cnidarians as a source of new marine bioactive compounds—An overview of the last decade and future steps for bioprospecting. Mar. Drugs 2011, 9, 1860–1886. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Licciano, M.; Pagliara, P. Evidence of antibacterial and lysozyme-like activity in different planktonic larval stages of Paracentrotus lividus. Mar. Biol. 1994, 119. [Google Scholar] [CrossRef]
- Shugar, D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim. Biophys. Acta 1952, 8, 302–309. [Google Scholar] [CrossRef]
- Canicatti, C.; Roch, P. Studies on Holothuria polii (Echinodermata) antibacterial proteins. I. Evidence for and activity of a coelomocyte lysozyme. Experientia 1989, 45, 756–759. [Google Scholar] [CrossRef]
- Canicatti, C. Protease activity in holothuria polh coelomic fluid and coelomocyte lysate. Comp. Biochem. Physiol. 1990, 95B, 145–148. [Google Scholar] [CrossRef]
- Stabili, L.; Schirosi, R.; Parisi, M.G.; Piraino, S.; Cammarata, M. The mucus of Actinia equina (Anthozoa, Cnidaria): An unexplored resource for potential applicative purposes. Mar. Drugs 2015, 13, 5276–5296. [Google Scholar] [CrossRef]
- Leone, A.; Lecci, R.M.; Durante, M.; Meli, F.; Piraino, S. The bright side of gelatinous blooms: Nutraceutical value and antioxidant properties of three Mediterranean jellyfish (Scyphozoa). Mar. Drugs 2015, 13, 4654–4681. [Google Scholar] [CrossRef]
- Zotti, M.; De Pascali, S.A.; Del Coco, L.; Migoni, D.; Carrozzo, L.; Mancinelli, G.; Fanizzi, F.P. 1H NMR metabolomic profiling of the blue crab (Callinectes sapidus) from the Adriatic Sea (SE Italy): A comparison with warty crab (Eriphia verrucosa), and edible crab (Cancer pagurus). Food Chem. 2016, 196, 601–609. [Google Scholar] [CrossRef]
- Tikunov, A.P.; Johnson, C.B.; Lee, H.; Stoskopf, M.K.; Macdonald, J.M. Metabolomic investigations of American oysters using 1H-NMR spectroscopy. Mar. Drugs 2010, 8, 2578–2596. [Google Scholar] [CrossRef] [PubMed]
- De Pascali, S.A.; Del Coco, L.; Felline, S.; Mollo, E.; Terlizzi, A.; Fanizzi, F.P. 1H NMR spectroscopy and MVA analysis of Diplodus sargus eating the exotic pest Caulerpa cylindracea. Mar. Drugs 2015, 13, 3550–3566. [Google Scholar] [CrossRef] [PubMed]
- Girelli, C.R.; De Pascali, S.A.; Del Coco, L.; Fanizzi, F.P. Metabolic profile comparison of fruit juice from certified sweet cherry trees (Prunus avium L.) of Ferrovia and Giorgia cultivars: A preliminary study. Food Res. Int. 2016, 90, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Aursand, M.; Hirata, Y.; Gribbestad, I.S.; Wada, S.; Nanaka, M. Nondestructive quantitative determination of docosahexaenoic acid and n-3 fatty acids in fish oils by high-resolution 1H Nuclear Magnetic Resonance Spectroscopy. J. Am. Oil Chem. Soc. 2000, 77, 737–748. [Google Scholar] [CrossRef]
- Sacchi, R.; Savarese, M.; Falcigno, L.; Giudicianni, I.; Paolillo, L. Proton NMR of fish oils and lipids. In Modern Magnetic Resonance; Springer International Publishing: Cham, Switzerland, 2008; pp. 919–923. [Google Scholar]
- Shumilina, E.; Ciampa, A.; Capozzi, F.; Rustad, T.; Dikiy, A. NMR approach for monitoring post-mortem changes in Atlantic salmon fillets stored at 0 and 4 °C. Food Chem. 2015, 184, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Nestor, G.; Bankefors, J.; Schlechtriem, C.; BräNnäS, E.; Pickova, J.; Sandström, C. High-resolution 1H magic angle spinning nmr spectroscopy of intact arctic char (Salvelinus alpinus) muscle. quantitative analysis of n-3 fatty acids, EPA and DHA. J. Agric. Food Chem. 2010, 58, 10799–10803. [Google Scholar] [CrossRef] [PubMed]
- Brotz, L.; Cheung, W.W.L.; Kleisner, K.; Pakhomov, E.; Pauly, D. Increasing jellyfish populations: Trends in Large Marine Ecosystems. Hydrobiologia 2012, 690, 3–20. [Google Scholar] [CrossRef]
- Leone, A.; Lecci, R.M.; Durante, M.; Piraino, S. Extract from the zooxanthellate jellyfish Cotylorhiza tuberculata modulates gap junction intercellular communication in human cell cultures. Mar. Drugs 2013, 11, 1728–1762. [Google Scholar] [CrossRef]
- Doyle, T.K.; Houghton, J.D.R.; McDevitt, R.; Davenport, J.; Hays, G.C. The energy density of jellyfish: Estimates from bomb-calorimetry and proximate-composition. J. Exp. Mar. Biol. Ecol. 2007, 343, 239–252. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Foxall, P.J.D.; Spraul, M.; Farrant, R.D.; Lindon, J.C. 750 MHz 1H and 1H-13C NMR Spectroscopy of Human Blood Plasma. Anal. Chem. 1995, 67, 793–811. [Google Scholar] [CrossRef]
- Yu, H.; Li, R.; Liu, S.; Xing, R.E.; Chen, X.; Li, P. Amino acid composition and nutritional quality of gonad from jellyfish Rhopilema esculentum. Biomed. Prev. Nutr. 2014, 4, 399–402. [Google Scholar] [CrossRef]
- Khong, N.M.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Nishikawa, J. Nutritional composition and total collagen content of three commercially important edible jellyfish. Food Chem. 2016, 196, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Imura, K.; Okada, A. Amino acid metabolism in pediatric patients. Nutrition 1998, 14, 143–148. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Zotti, G. Open-source virtual archaeoastronomy. Mediterr. Archaeol. Archaeom. 2016, 16, 17–24. [Google Scholar] [CrossRef]
- Mannina, L.; Sobolev, A.P.; Capitani, D.; Iaffaldano, N.; Rosato, M.P.; Ragni, P.; Reale, A.; Sorrentino, E.; D’Amico, I.; Coppola, R. NMR metabolic profiling of organic and aqueous sea bass extracts: Implications in the discrimination of wild and cultured sea bass. Talanta 2008, 77, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Berking, S. 3 Hydrozoa Metamorphosis and Pattern Formation. Curr. Top. Dev. Biol. 1997, 38, 81–131. [Google Scholar] [CrossRef]
- Siefker, B.; Kroiler, M.; Berking, S. Induction of metamorphosis from the larval to the polyp stage is similar in Hydrozoa and a subgroup of Scyphozoa (Cnidaria, Semaeostomeae). Helgol. Mar. Res. 2000, 54, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef] [Green Version]
- Seibel, B.A.; Walsh, P.J. Trimethylamine oxide accumulation in marine animals: Relationship to acylglycerol storage. J. Exp. Biol. 2002, 205, 297–306. [Google Scholar] [CrossRef]
- Abdullah, A.; Nurjanah, N.; Hidayat, T.; Aji, D.U. Fatty Acid Profile of Jellyfish (Aurelia aurita) as a Source Raw Material of Aquatic Result Rich Beneft. Int. J. Chem. Biol. Sci. 2015, 1, 12–16. [Google Scholar]
- Besnard, J.Y. Etude des Constituents Lipidiques Dans la Gonade Femelle et les Larves de Pecten maximus L. (Mollusque Lamellibranche). Ph.D. Dissertation, Université de Caen, Caen, France, 1988; p. 154. [Google Scholar]
- Izquierdo, M.S.; Fernández-Palacios, H.; Tacon, A.G.J. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 2001, 197, 25–42. [Google Scholar] [CrossRef]
- Tulli, F.; Tibaldi, E. Changes in amino acids and essential fatty acids during early larval rearing of dentex. Aquac. Int. 1997, 5, 229–236. [Google Scholar] [CrossRef]
- Miliou, H.; Fintikaki, M.; Tzitzinakis, M.; Kountouris, T.; Verriopoulos, G. Fatty acid composition of the common octopus, Octopus vulgaris, in relation to rearing temperature and body weight. Aquaculture 2006, 256, 311–322. [Google Scholar] [CrossRef]
- Xu, Y.; Cleary, L.J.; Byrne, J.H. Identification and characterization of pleural neurons that inhibit tail sensory neurons and motor neurons in Aplysia: Correlation with FMRFamide immunoreactivity. J. Neurosci. 1994, 14, 3565–3577. [Google Scholar] [CrossRef] [PubMed]
- Stanley-Samuelson, D.W. Physiological roles of prostaglandins and other eicosanoids in invertebrates. Biol. Bull. 1987, 173, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Nichols, P.D.; Danaher, K.T.; Koslow, J.A. Occurrence of High Levels of Tetracosahexaenoic Acid in the Jellyfish Aurelia sp. Lipids 2003, 38, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhao, S. Omega-3 fatty acid eicosapentaenoic acid. A new treatment for psychiatric and neurodegenerative diseases: A review of clinical investigations. Expert Opin. Investig. Drugs 2007, 16, 1627–1638. [Google Scholar] [CrossRef]
- Song, C.; Phillips, A.G.; Leonard, B.E. Interleukin-1β enhances conditioned fear memory but impairs spatial learning in rats: Possible involvement of glucocorticoids and monoamine function in the amygdala and hippocampus. Eur. J. Neurosci. 2003, 18, 1739–1743. [Google Scholar] [CrossRef]
- Marquis, C.P.; Baird, A.H.; De Nys, R.; Holmström, C.; Koziumi, N. An evaluation of the antimicrobial properties of the eggs of 11 species of scleractinian corals. Coral Reefs 2005, 24, 248–253. [Google Scholar] [CrossRef]
- Kelman, D.; Kushmaro, A.; Loya, Y.; Kashman, Y.; Benayahu, Y. Antimicrobial activity of a Red Sea soft coral, Parerythropodium fulvum fulvum: Reproductive and developmental considerations. Mar. Ecol. Prog. Ser. 1998, 169, 87–95. [Google Scholar] [CrossRef]
- Gunthorpe, L.; Cameron, A.M. Widespread but variable toxicity in scleractinian corals. Toxicon 1990, 28, 1199–1219. [Google Scholar] [CrossRef]
- Gunthorpe, L.; Cameron, A.M. Intracolonial variation in toxicity in scleractinian corals. Toxicon 1990, 28, 1221–1227. [Google Scholar] [CrossRef]
- Bowden, B.; Coll, J.; Willis, R. Some chemical aspects of spawning in alcyonacean corals. In Proceedings of the Fifth International Coral Reef Congress, Tahiti, French Polynesia, 27 May–1 June 1985; Volume 4, pp. 325–329. [Google Scholar]
- Baird, A.H.; Pratchett, M.S.; Gibson, D.J.; Koziumi, N.; Marquis, C.P. Variable palatability of coral eggs to a planktivorous fish. Mar. Freshw. Res. 2001, 52, 865–868. [Google Scholar] [CrossRef]
- Fusetani, N.; Toyoda, T.; Asai, N.; Matsunaga, S.; Maruyama, T. Montiporic acids A and B, cytotoxic and antimicrobial polyacetylene carboxylic acids from eggs of the scleractinian coral Montipora digitata. J. Nat. Prod. 1996, 59, 796–797. [Google Scholar] [CrossRef] [PubMed]
- Lesser, M.P.; Stochaj, W.R.; Tapley, D.W.; Shick, J.M. Bleaching in coral reef anthozoans: Effects of irradiance, ultraviolet radiation, and temperature on the activities of protective enzymes against active oxygen. Coral Reefs 1995, 8, 225–232. [Google Scholar] [CrossRef]
- Leclerc, M. Humoral factors in marine invertebrates. In Molecular and Subcellular Biology: Invertebrate Immunology; Rinkevich, B., Muller, W.E.G., Eds.; Springer: Berlin, Germany, 1996; pp. 1–9. [Google Scholar]
- Stryer, L. Biochemie; Spektrum Akademischer Verlag: Heidelberg/Berlin, Germany, 2013; New York, korr. Nachdruck 1991 der völlig neubearb. Aufl. 1990; pp. 1–1024. [Google Scholar]
- Jielian, W.; Baoqing, H.; Chungen, W.; Peipei, Y. Characterization and roles of lysozyme in molluscs. ISJ 2017, 14, 432–442. [Google Scholar]
- Chang, K.Y.; Carr, C.W. Studies on the structure and function of lysozyme: I. The effect of pH and cation concentration on lysozyme activity. Biochim. Biophys. Acta 1917, 229, 496–503. [Google Scholar] [CrossRef]
- Davies, R.C.; Neuberger, A.; Wilson, B.M. The dependence of lysozyme activity on pH and ionic strength. Biochim. Biophys. Acta 1969, 178, 294–305. [Google Scholar] [CrossRef]
- Labro, M.T. The prohost effect of antimicrobial agents as a predictor of clinical outcome. J. Chemother. 1997, 9, 100–108. [Google Scholar]
- SeGall, G.K.; Lennarz, W.J. Chemical characterization of the component of the jelly coat from sea urchin eggs responsible for induction of the acrosome reaction. Dev. Biol. 1979, 71, 33–48. [Google Scholar] [CrossRef]
- Slattery, M.; McClintock, J.B. Population structure and feeding deterrence in three shallow-water antarctic soft corals. Mar. Biol. 1995, 122, 461–470. [Google Scholar] [CrossRef]
- Dubois, M.; Gille, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent [Folin-Ciocalteu reagent]. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.; Blackstock, J. Estimation of lipids in marine animals and tissues: Detailed investigation of the sulphophosphovanilun method for “total” lipids. J. Exp. Mar. Bio. Ecol. 1973, 12, 103–118. [Google Scholar] [CrossRef]
- Jacobs, D.S.; Kasten, B.L.; Demott, W.R. Laboratory Test Handbook, 2nd ed. J. Intraven. Nurs. 1991, 14, 354. [Google Scholar]
- Bucolo, G.; David, H. Quantitative determination of serum triglycerides by the use of enzymes. Clin. Chem. 1973, 19, 476–482. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Wu, H.; Southam, A.D.; Hines, A.; Viant, M.R. High-throughput tissue extraction protocol for NMR- and MS-based metabolomics. Anal. Biochem. 2008, 372, 204–212. [Google Scholar] [CrossRef]
- Moreira-Ferro, C.K.; Daffre, S.; James, A.A.; Marinotti, O. A lysozyme in the salivary glands of the malaria vector Anopheles darlingi. Insect Mol. Biol. 1998, 7, 257–264. [Google Scholar] [CrossRef]
- Daffre, S.; Kylsten, P.; Samakovlis, C.; Hultmark, D. The lysozyme locus in Drosophila melanogaster: An expanded gene family adapted for expression in the digestive tract. Mol. Gen. Genet. 1994, 242, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.C.; Arizza, V.; Barela Hudgell, M.A.; Barone, G.; Bodnar, A.G.; Buckley, K.M.; Cunsolo, V.; Dheilly, N.M.; Franchi, N.; Fugmann, S.D.; et al. Echinodermata: The Complex Immune System in Echinoderms. In Advances in Comparative Immunology; Cooper, E.L., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 409–501. ISBN 978-3-319-76768-0. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Anderson, M.; Braak, C. Ter Permutation tests for multi-factorial analysis of variance. J. Stat. Comput. Simul. 2003, 73, 85–113. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
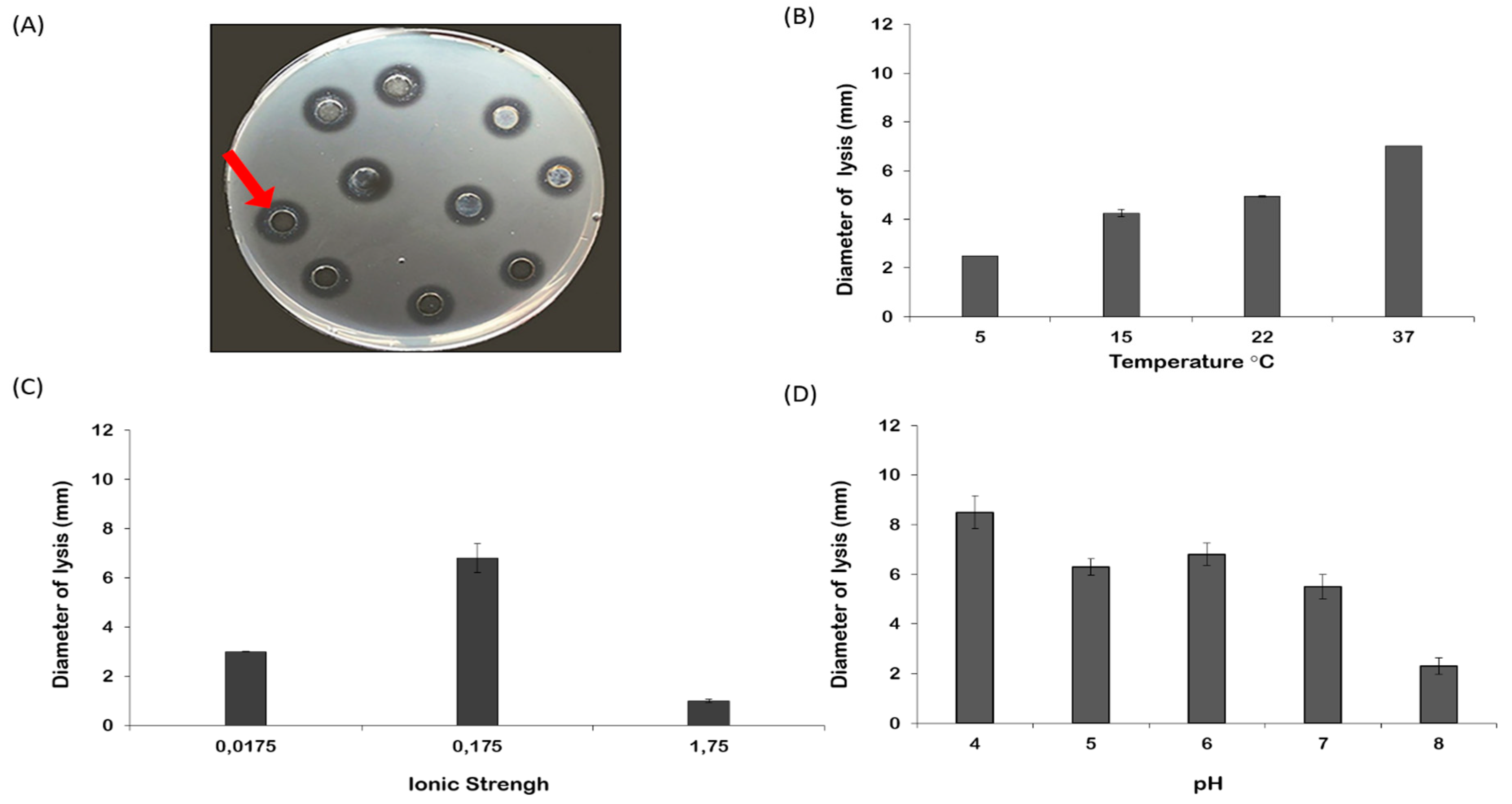
| df | MS | F | p | |
|---|---|---|---|---|
| Temperature Residual Total | 3 8 11 | 10.416 0.0163 | 640.72 | *** |
| Ionic Strength Residual Total | 2 6 8 | 27.417 0.33799 | 81.117 | ** |
| pH Residual Total | 4 10 14 | 21.35 0.58333 | 36.6 | *** |
| Temperature | T | P(MC) | Ionic Strength | T | P(MC) | pH | T | P(MC) |
|---|---|---|---|---|---|---|---|---|
| 5 vs. 15 | 12.12 | *** | 0.0175 vs. 0.175 | 6.88 | 0.0029 | 4 vs. 5 | 4.025 | * |
| 5 vs. 22 | 84.87 | *** | 0.0175 vs. 1.75 | 28.73 | 0.0001 | 4 vs. 6 | 2.646 | ns |
| 5 vs. 37 | 1561 | *** | 0.175 vs. 1.75 | 10.21 | 0.0006 | 4 vs. 7 | 5.277 | ** |
| 15 vs. 22 | 4.756 | ** | 4 vs. 8 | 11 | *** | |||
| 15 vs. 37 | 19.08 | *** | 5 vs. 6 | 1 | ns | |||
| 22 vs. 37 | 70.83 | *** | 5 vs. 7 | 1.89 | ns | |||
| 5 vs. 8 | 13 | *** | ||||||
| 6 vs. 7 | 2.324 | ns | ||||||
| 6 vs. 8 | 8.66 | ** | ||||||
| 7 vs. 8 | 12.12 | *** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stabili, L.; Rizzo, L.; Fanizzi, F.P.; Angilè, F.; Del Coco, L.; Girelli, C.R.; Lomartire, S.; Piraino, S.; Basso, L. The Jellyfish Rhizostoma pulmo (Cnidaria): Biochemical Composition of Ovaries and Antibacterial Lysozyme-like Activity of the Oocyte Lysate. Mar. Drugs 2019, 17, 17. https://doi.org/10.3390/md17010017
Stabili L, Rizzo L, Fanizzi FP, Angilè F, Del Coco L, Girelli CR, Lomartire S, Piraino S, Basso L. The Jellyfish Rhizostoma pulmo (Cnidaria): Biochemical Composition of Ovaries and Antibacterial Lysozyme-like Activity of the Oocyte Lysate. Marine Drugs. 2019; 17(1):17. https://doi.org/10.3390/md17010017
Chicago/Turabian StyleStabili, Loredana, Lucia Rizzo, Francesco Paolo Fanizzi, Federica Angilè, Laura Del Coco, Chiara Roberta Girelli, Silvia Lomartire, Stefano Piraino, and Lorena Basso. 2019. "The Jellyfish Rhizostoma pulmo (Cnidaria): Biochemical Composition of Ovaries and Antibacterial Lysozyme-like Activity of the Oocyte Lysate" Marine Drugs 17, no. 1: 17. https://doi.org/10.3390/md17010017






