Effect of Low Molecular Weight Oligopeptides Isolated from Sea Cucumber on Diabetic Wound Healing in db/db Mice
Abstract
:1. Introduction
2. Results
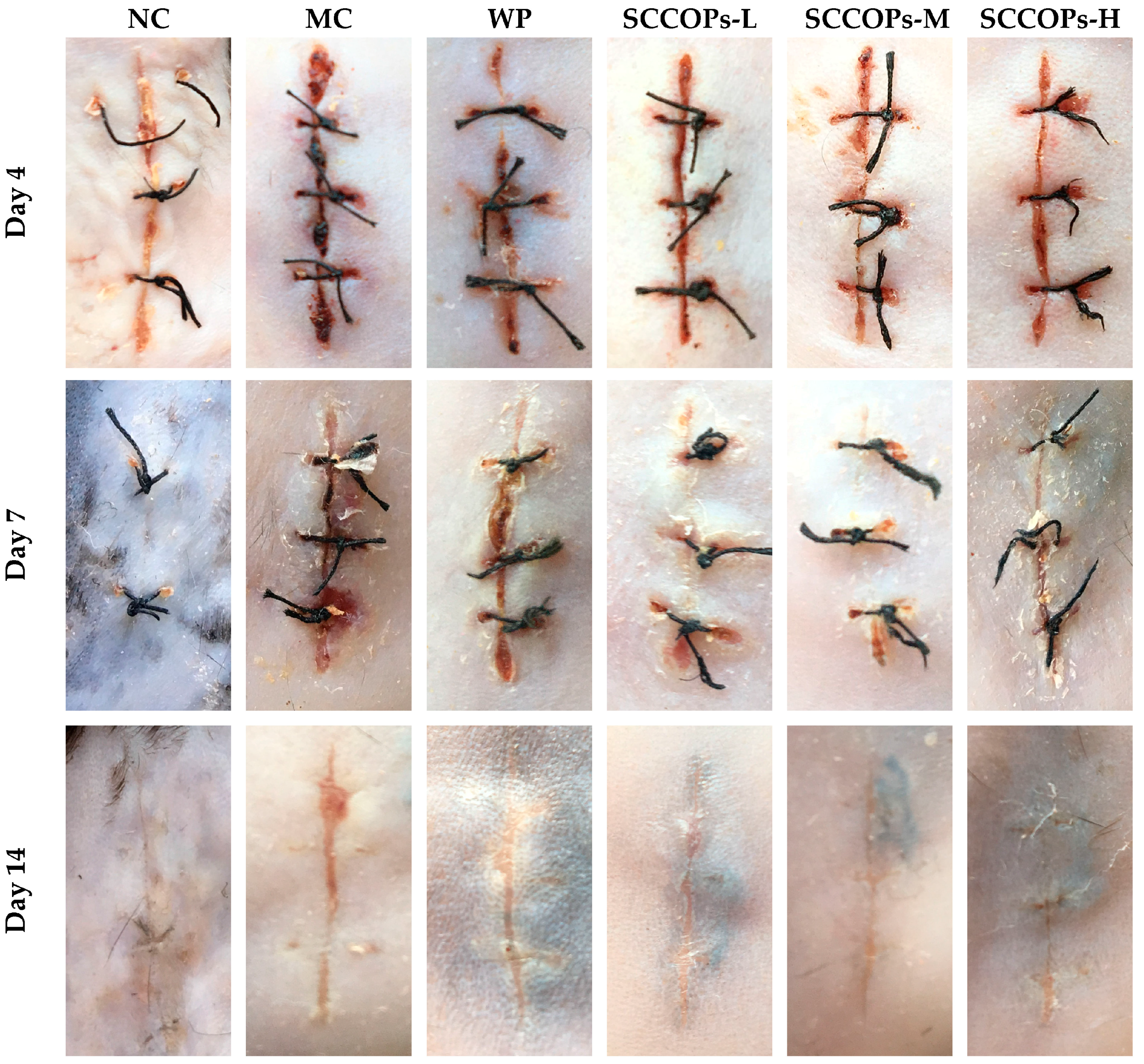
2.1. General Information
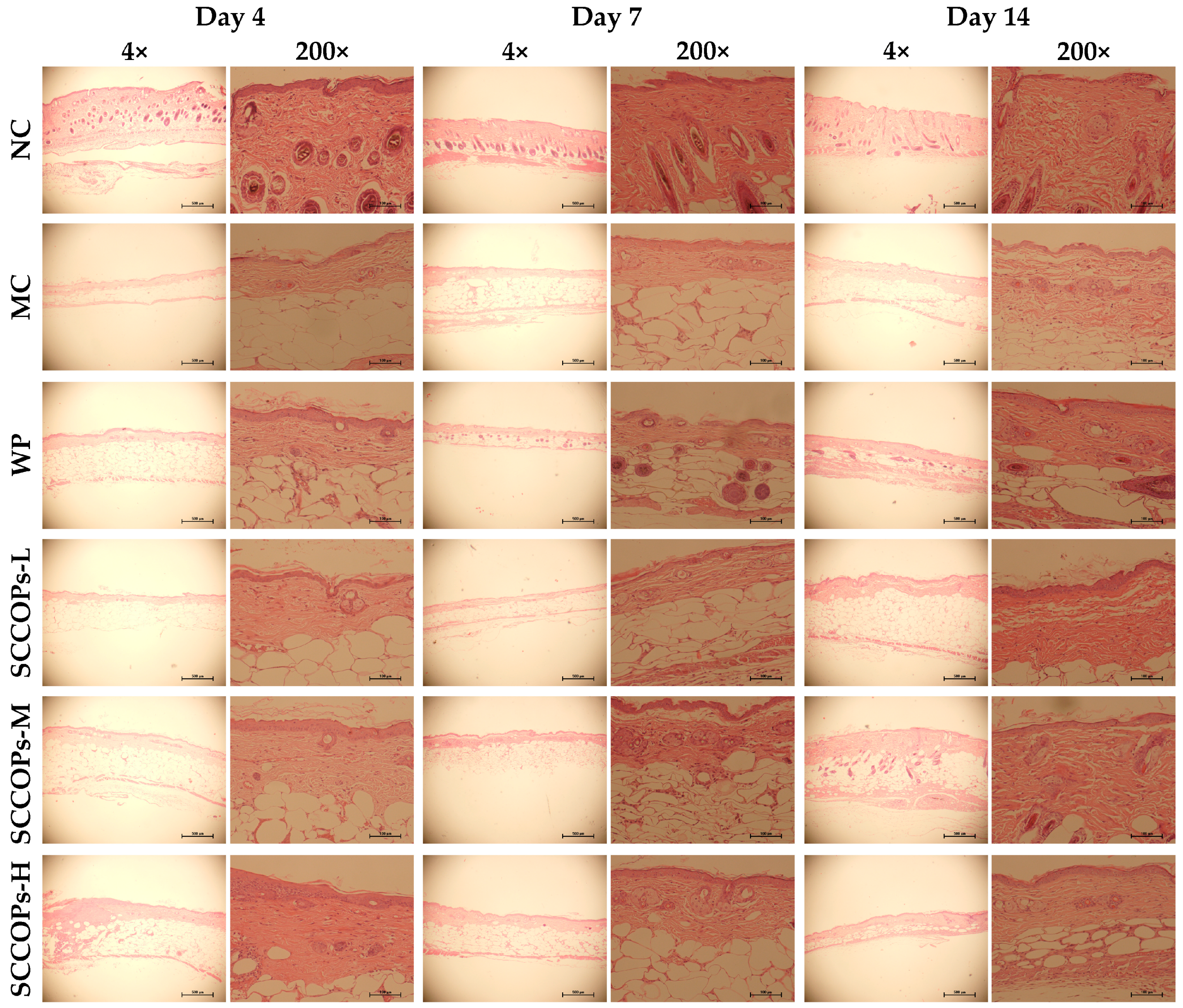
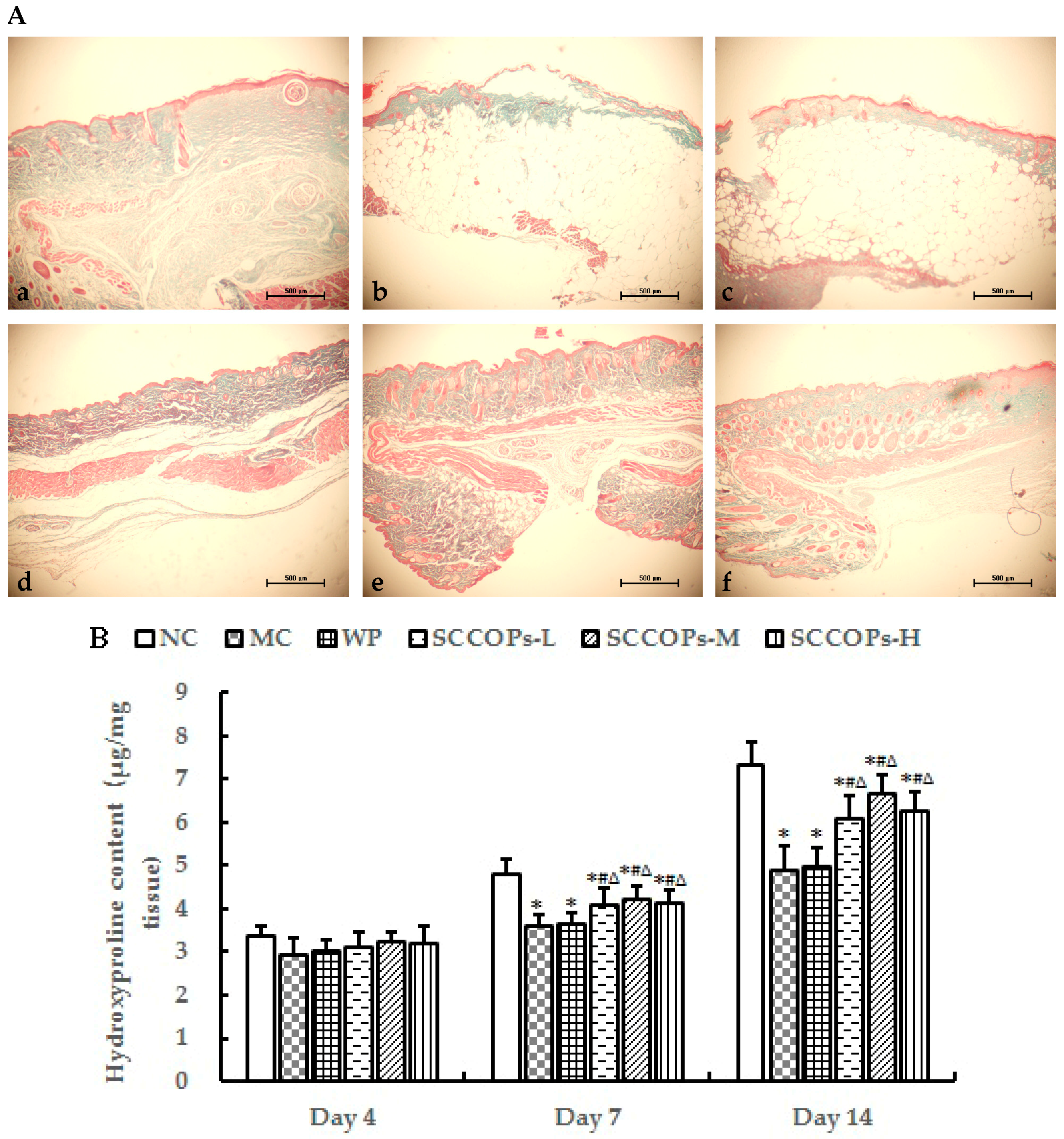
2.2. Histological Analysis
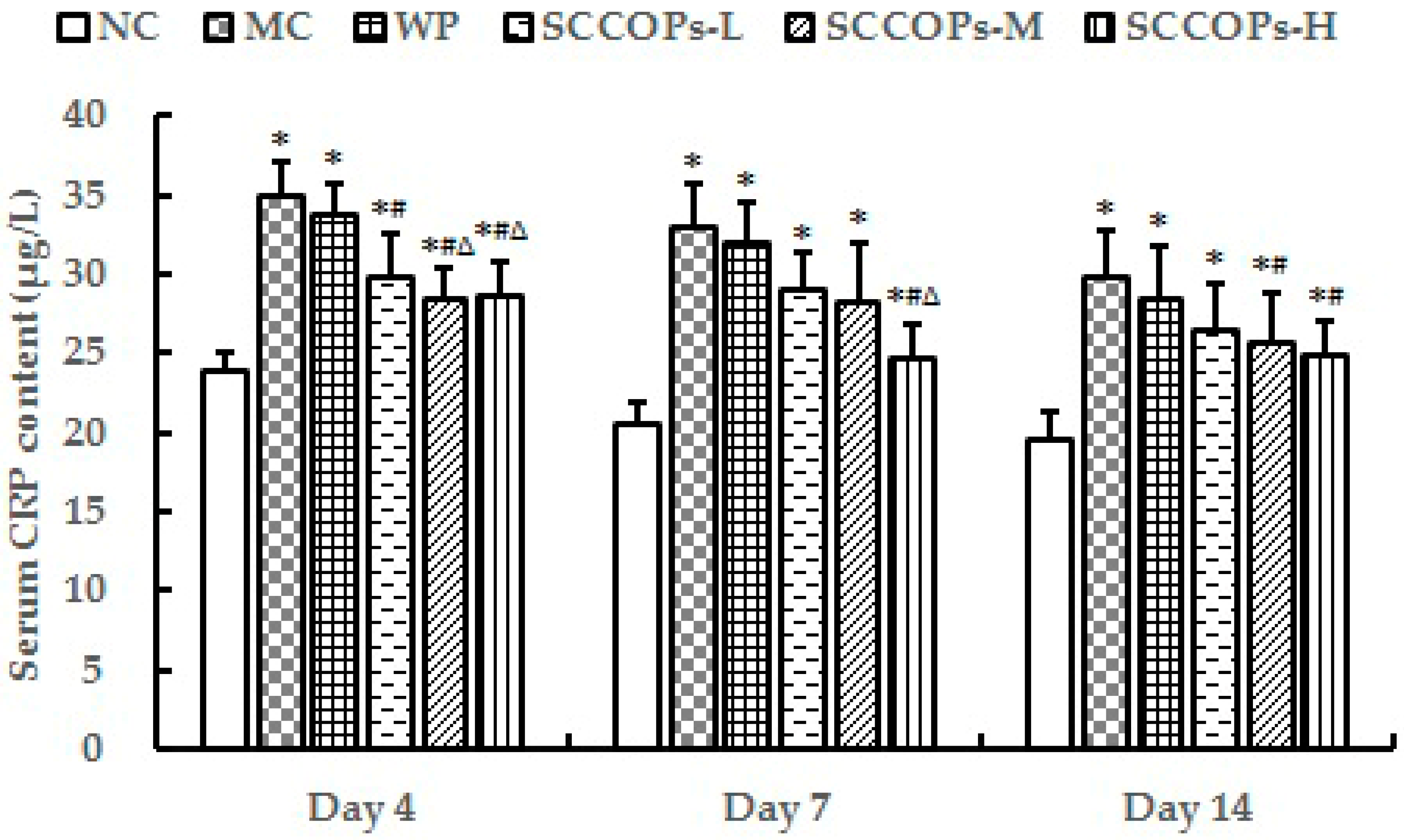
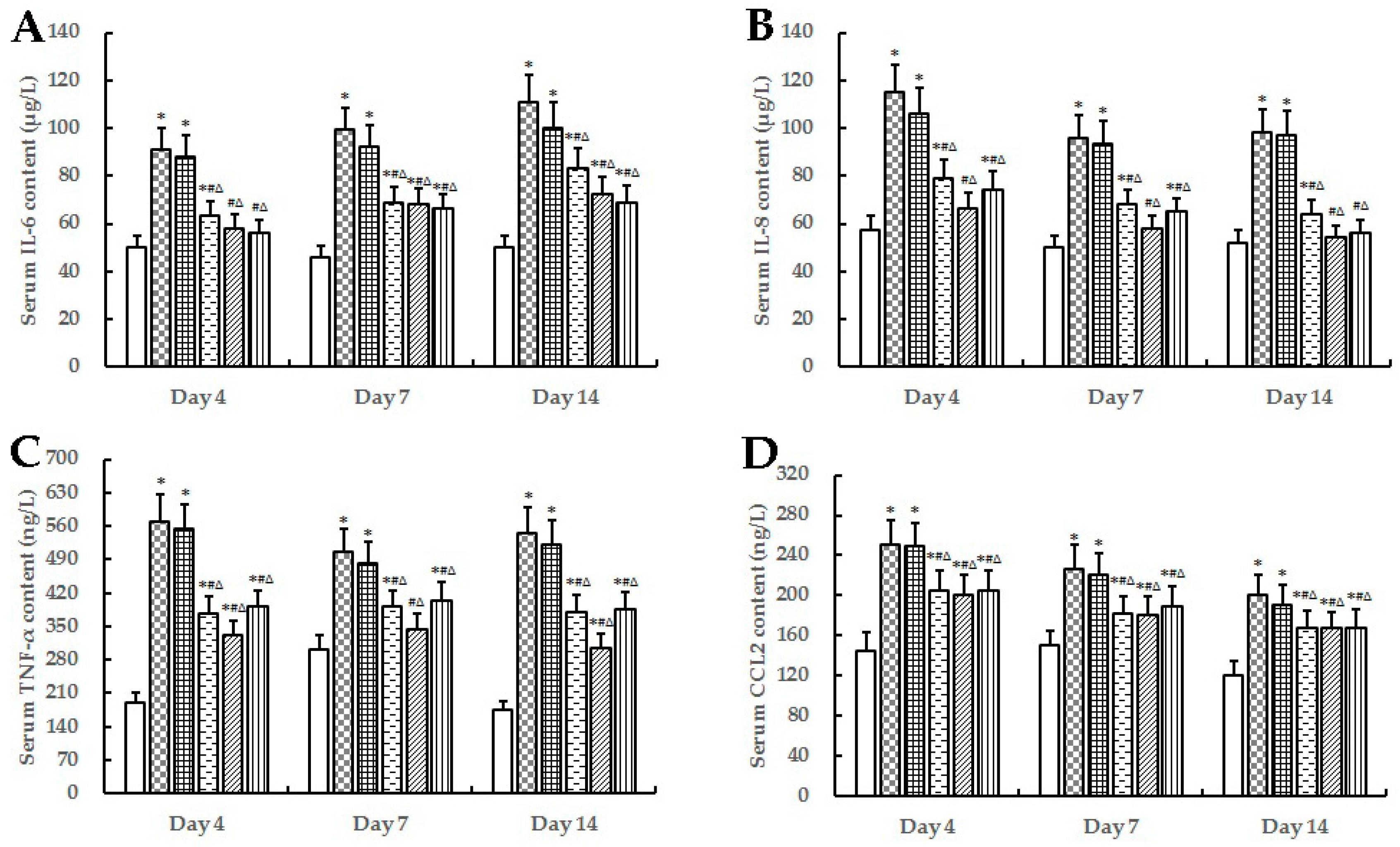
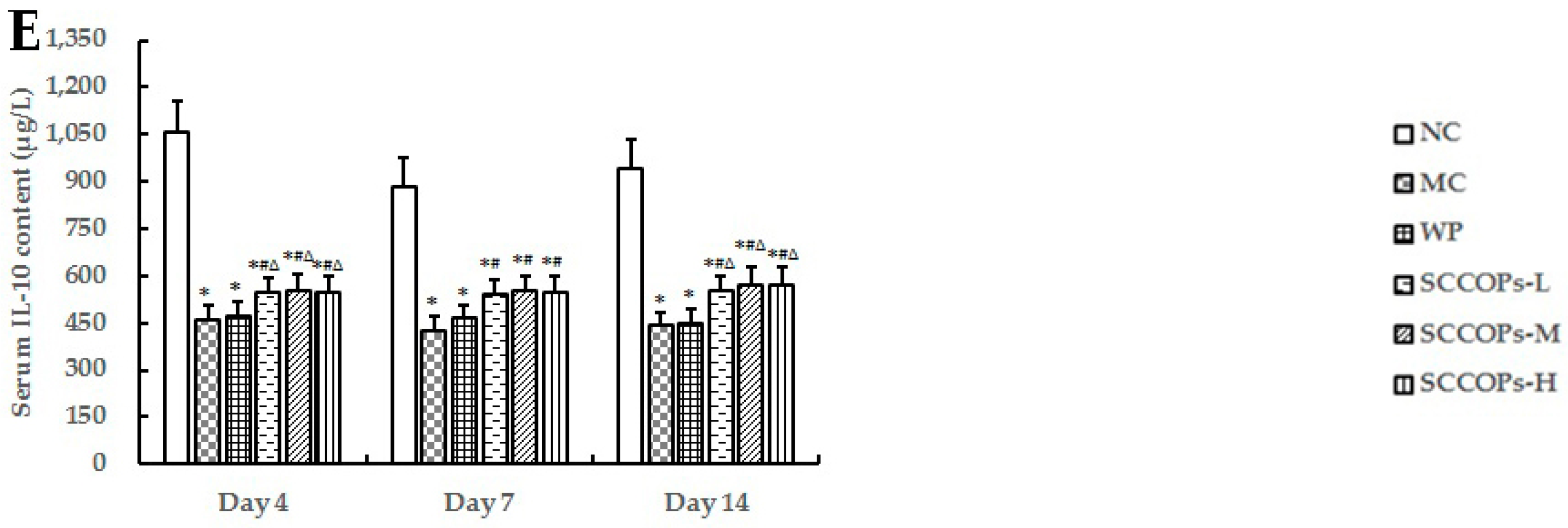
2.3. Evaluation of Inflammatory Response
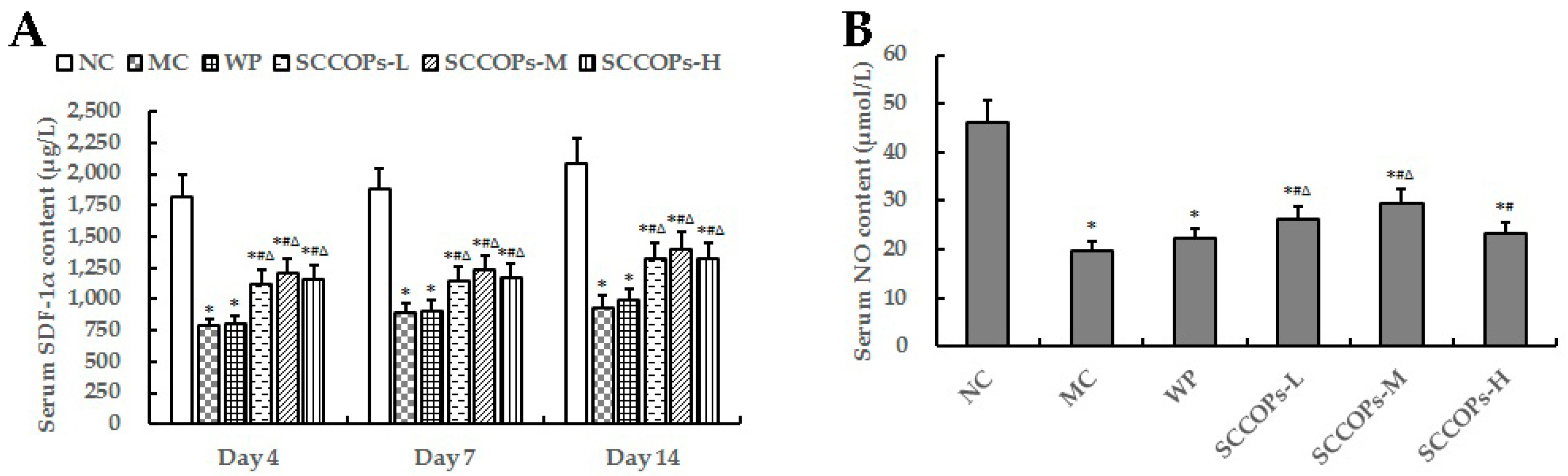
2.4. Evaluation of Stromal Cell-Derived Factor-1 Alpha (SDF-1α) and NO in Serum
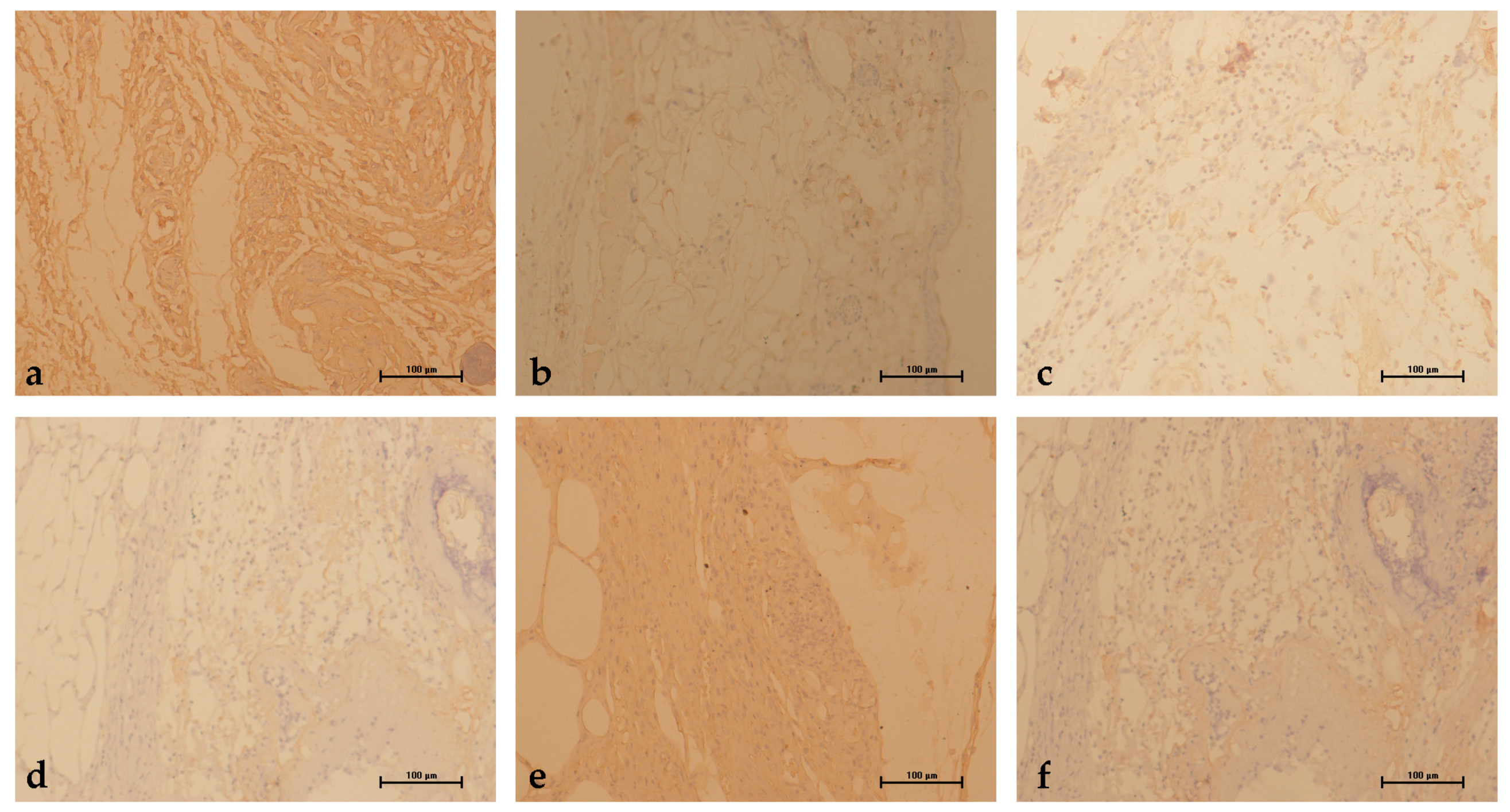
2.5. Evaluation of Immunostaining for Vascular Endothelial Growth Factor (VEGF)
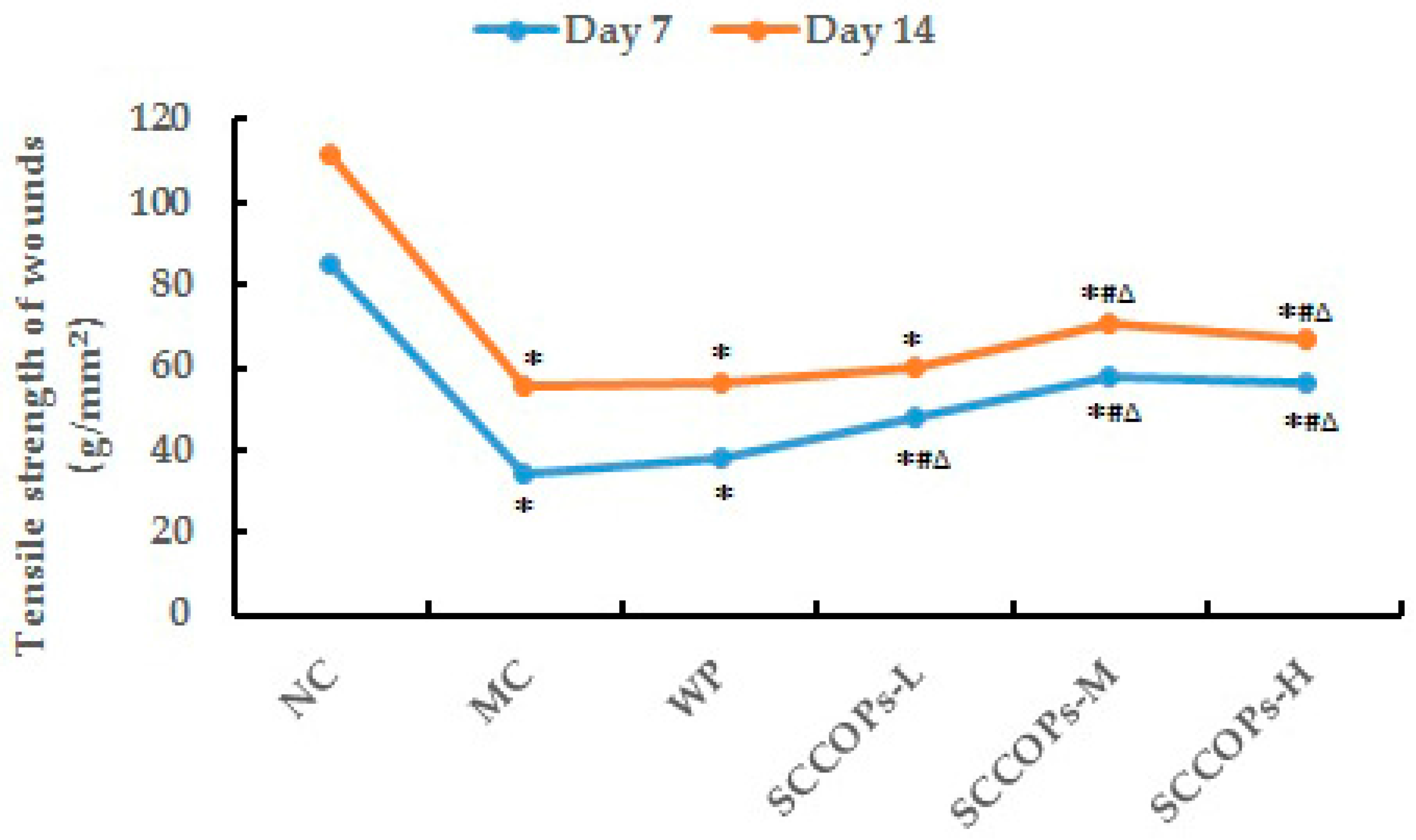
2.6. Tensile Strength
2.7. Collagen Accumulation
2.8. Evaluation of Reactive Oxygen Species (ROS) in Serum
2.9. Albumin, Prealbumin and Transferrin Concentrations in Serum
3. Discussion
4. Materials and Methods
4.1. Preparation and Identification of SCCOPs
4.2. Animals and Groups
4.3. Incision Wound Healing Model
4.4. Measurement of Tensile Strength
4.5. Biochemical Assay
4.6. Histological Observation
4.7. Immunohistochemistry
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Takahashi, P.Y.; Kiemele, L.J.; Chandra, A.; Cha, S.S.; Targonski, P.V. A retrospective cohort study of factors that affect healing in long-term care residents with chronic wounds. Ostomy Wound Manag. 2009, 55, 32–37. [Google Scholar]
- Greenhalgh, D.G. Wound healing and diabetes mellitus. Clin. Plast. Surg. 2003, 30, 37–45. [Google Scholar] [CrossRef]
- Galeano, M.; Bitto, A.; Altavilla, D.; Minutoli, L.; Polito, F.; Calo, M.; Lo, C.P.; Stagno, D.F.; Squadrito, F. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen. 2008, 16, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Castleberry, S.A.; Almquist, B.D.; Li, W.; Reis, T.; Chow, J.; Mayner, S.; Hammond, P.T. Self-Assembled Wound Dressings Silence MMP-9 and Improve Diabetic Wound Healing In Vivo. Adv. Mater. 2016, 28, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Gottrup, F.; Apelqvist, J. Present and new techniques and devices in the treatment of DFU: A critical review of evidence. Diabetes Metab. Res. Rev. 2012, 28, 64–71. [Google Scholar] [CrossRef] [PubMed]
- He, L.X.; Ren, J.W.; Liu, R.; Chen, Q.H.; Zhao, J.; Wu, X.; Zhang, Z.F.; Wang, J.B.; Pettinato, G.; Li, Y. Ginseng (Panax ginseng Meyer) oligopeptides regulate innate and adaptive immune responses in mice via increased macrophage phagocytosis capacity, NK cell activity and Th cells secretion. Food Funct. 2017, 8, 3523–3532. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J. Bioactive Egg Components and Inflammation. Nutrients 2015, 7, 7889–7913. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Y.; Zhang, H.; Hu, B.; Wang, L.; Qian, H.; Qi, X. The anti-diabetic activity of oat beta-d-glucan in streptozotocin-nicotinamide induced diabetic mice. Int. J. Biol. Macromol. 2016, 91, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.A.; FitzGerald, R.J. Angiotensin converting enzyme inhibitory peptides derived from food proteins: Biochemistry, bioactivity and production. Curr. Pharm. Des. 2007, 13, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Future Market Insights, Healthcare and Medical Devices, Peptide therapeutics market: Forecast and analysis 2015–2025. Drug Discov. Today 2015, 19, 124. [Google Scholar]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, M.; Wang, J.; Ding, Y.; Dai, X.; Li, Y. Oral administration of skin gelatin isolated from Chum salmon (Oncorhynchus keta) enhances wound healing in diabetic rats. Mar. Drugs 2011, 9, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Oliveracastillo, L.; Pérezvega, J.; Gómezruiz, J.Á.; Hernándezledesma, B. Release of bioactive peptides by simulated gastrointestinal digestion of sea cucumber protein (Isostichopus Badionotus). Ann. Nutr. Metab. 2011, 58, 121. [Google Scholar]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Vega, J.A.; Olivera-Castillo, L.; Hernández-Ledesma, B. Release of multifunctional peptides by gastrointestinal digestion of sea cucumber (Isostichopus badionotus ). J. Funct. Foods 2013, 5, 869–877. [Google Scholar] [CrossRef]
- He, L.X.; Zhang, Z.F.; Sun, B.; Chen, Q.H.; Liu, R.; Ren, J.W.; Wang, J.B.; Li, Y. Sea cucumber (Codonopsis pilosula) oligopeptides: Immunomodulatory effects based on stimulating Th cells, cytokine secretion and antibody production. Food Funct. 2016, 7, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Baum, C.L.; Arpey, C.J. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatol. Surg. 2005, 31, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, W.K.; Digenis, A.G.; Tobin, G.R. Physiology and healing dynamics of chronic cutaneous wounds. Am. J. Surg. 1998, 176, 26S–38S. [Google Scholar] [CrossRef]
- Wood, S.; Jayaraman, V.; Huelsmann, E.J.; Bonish, B.; Burgad, D.; Sivaramakrishnan, G.; Qin, S.; DiPietro, L.A.; Zloza, A.; Zhang, C.; et al. Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLoS ONE 2014, 9, e91574. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.R.; Yang, R.Y.; Zhang, Z.F.; Gao, L.F.; Wang, J.B.; Xu, Y.J.; Zhao, M.; Han, X.L.; Liu, Z.G.; Li, Y. Marine collagen peptide isolated from Chum Salmon (Oncorhynchus keta) skin facilitates learning and memory in aged C57BL/6J mice. Food Chem. 2009, 118, 333–340. [Google Scholar] [CrossRef]
- Douraiswami, B.; Dilip, P.K.; Harish, B.N.; Jagdish, M. C-reactive protein and interleukin-6 levels in the early detection of infection after open fractures. J. Orthop. Surg. 2012, 20, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.M.; Stuart, G.S.; Real, N.C.; Fleming, S.B.; Mercer, A.A. Orf virus IL-10 accelerates wound healing while limiting inflammation and scarring. Wound Repair Regen. 2014, 22, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Roy, H.; Bhardwaj, S.; Yla-Herttuala, S. Biology of vascular endothelial growth factors. FEBS Lett. 2006, 580, 2879–2887. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Nigam, Y. Naturally derived factors and their role in the promotion of angiogenesis for the healing of chronic wounds. Angiogenesis 2013, 16, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chai, L.; Chen, L.; Chen, W.; Ge, L.; Li, X.; Li, H.; Li, S.; Cao, C. Stromal cell-derived factor 1 (SDF-1) accelerated skin wound healing by promoting the migration and proliferation of epidermal stem cells. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Ara, T.; Tokoyoda, K.; Sugiyama, T.; Egawa, T.; Kawabata, K.; Nagasawa, T. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity 2003, 19, 257–267. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Lingaraj, K.; Poh, C.K.; Wang, W. Vascular endothelial growth factor (VEGF) is expressed during articular cartilage growth and re-expressed in osteoarthritis. Ann. Acad. Med. Singap. 2010, 39, 399–403. [Google Scholar] [PubMed]
- Santos, S.C.; Miguel, C.; Domingues, I.; Calado, A.; Zhu, Z.; Wu, Y.; Dias, S. VEGF and VEGFR-2 (KDR) internalization is required for endothelial recovery during wound healing. Exp. Cell Res. 2007, 313, 1561–1574. [Google Scholar] [CrossRef] [PubMed]
- Galiano, R.D.; Tepper, O.M.; Pelo, C.R.; Bhatt, K.A.; Callaghan, M.; Bastidas, N.; Bunting, S.; Steinmetz, H.G.; Gurtner, G.C. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am. J. Pathol. 2004, 164, 1935–1947. [Google Scholar] [CrossRef]
- Bitto, A.; Minutoli, L.; Galeano, M.R.; Altavilla, D.; Polito, F.; Fiumara, T.; Calo, M.; Lo, C.P.; Zentilin, L.; Giacca, M.; et al. Angiopoietin-1 gene transfer improves impaired wound healing in genetically diabetic mice without increasing VEGF expression. Clin. Sci. 2008, 114, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Witte, M.B.; Kiyama, T.; Barbul, A. Nitric oxide enhances experimental wound healing in diabetes. Br. J. Surg. 2002, 89, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Romana-Souza, B.; Nascimento, A.P.; Monte-Alto-Costa, A. Propranolol improves cutaneous wound healing in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2009, 611, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Ding, Y.; Dai, X.; Li, Y. Oral administration of marine collagen peptides from Chum Salmon skin enhances cutaneous wound healing and angiogenesis in rats. J. Sci. Food Agric. 2011, 91, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, Y.; Kubomura, D.; Sato, Y.; Sato, K. Dose-dependent changes in the levels of free and peptide forms of hydroxyproline in human plasma after collagen hydrolysate ingestion. Food Chem. 2014, 159, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Negrao, R.; Valente, I.; Castela, A.; Duarte, D.; Guardao, L.; Magalhaes, P.J.; Rodrigues, J.A.; Guimaraes, J.T.; Gomes, P.; et al. Xanthohumol modulates inflammation, oxidative stress, and angiogenesis in type 1 diabetic rat skin wound healing. J. Nat. Prod. 2013, 76, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Eo, H.; Lee, H.J.; Lim, Y. Ameliorative effect of dietary genistein on diabetes induced hyper-inflammation and oxidative stress during early stage of wound healing in alloxan induced diabetic mice. Biochem. Biophys. Res. Commun. 2016, 478, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Senel, O.; Cetinkale, O.; Ozbay, G.; Ahcioglu, F.; Bulan, R. Oxygen free radicals impair wound healing in ischemic rat skin. Ann. Plast. Surg. 1997, 39, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, Y.T.; Byun, H.G.; Nam, K.S.; Joo, D.S.; Shahidi, F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska pollack skin. J. Agric. Food Chem. 2001, 49, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Khantaphant, S.; Benjakul, S. Comparative study on the proteases from fish pyloric caeca and the use for production of gelatin hydrolysate with antioxidative activity. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 410–419. [Google Scholar] [CrossRef] [PubMed]

| Time | NC | MC | WP | SCCOPs-L | SCCOPs-M | SCCOPs-H |
|---|---|---|---|---|---|---|
| Day 0 | 29.42 ± 1.71 | 54.87 ± 3.75 * | 53.48 ± 5.24 * | 54.64 ± 4.62 * | 55.64 ± 3.42 * | 55.57 ± 5.56 * |
| Day 4 | 27.05 ± 2.04 | 49.44 ± 3.57 * | 49.23 ± 5.31 * | 49.61 ± 4.41 * | 51.03 ± 3.04 * | 50.29 ± 5.68 * |
| Day 7 | 28.21 ± 2.09 | 49.77 ± 1.54 * | 47.83 ± 4.21 * | 49.80 ± 5.56 * | 49.19 ± 3.56 * | 49.79 ± 3.46 * |
| Day 14 | 27.50 ± 1.58 | 50.94 ± 4.43 * | 50.45 ± 4.42 * | 48.03 ± 4.21 * | 51.92 ± 3.50 * | 48.40 ± 4.04 * |
| Group | ALB (μg/L) | PA (μg/mL) | TRF (nmol/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 4 | Day 7 | Day 14 | Day 4 | Day 7 | Day 14 | Day 4 | Day 7 | Day 14 | |
| NC | 567.07 ± 50.97 | 527.70 ± 32.51 | 568.88 ± 56.10 | 58.83 ± 2.08 | 49.65 ± 4.13 | 53.45 ± 3.35 | 281.55 ± 21.26 | 306.12 ± 17.73 | 296.91 ± 19.30 |
| MC | 314.97 ± 16.11 * | 212.46 ± 23.19 * | 282.29 ± 20.40 * | 29.55 ± 1.11 * | 26.99 ± 0.82 * | 28.01 ± 2.81 * | 144.00 ± 8.85 * | 138.81 ± 14.26 * | 154.14 ± 10.60 * |
| WP | 358.20 ± 5.80 * | 304.02 ± 23.91 *# | 341.04 ± 36.75 * | 35.69 ± 3.43 * | 29.97 ± 1.58 * | 33.98 ± 2.27 *# | 163.99 ± 9.49 * | 166.76 ± 12.86 *# | 170.91 ± 16.18 * |
| SCCOPs-L | 331.36 ± 39.03 * | 291.97 ± 39.64 *# | 323.75 ± 23.80 * | 34.38 ± 4.09 * | 29.42 ± 2.99 * | 33.39 ± 4.41 *# | 174.97 ± 12.00 *# | 179.90 ± 11.71 *# | 183.74 ± 17.56 *# |
| SCCOPs-M | 357.71 ± 29.58 * | 311.09 ± 31.00 *# | 351.37 ± 28.00 *# | 35.99 ± 3.91 *# | 35.27 ± 5.93 *# | 33.42 ± 5.38 *# | 177.39 ± 10.76 *# | 183.65 ± 12.56 *# | 186.01 ± 18.55 *# |
| SCCOPs-H | 366.80 ± 21.03 * | 336.59 ± 21.85 *# | 341.50 ± 23.99 *# | 37.07 ± 3.18 *# | 36.32 ± 4.22 *# | 33.63 ± 3.61 *# | 185.16 ± 18.86 *# | 189.22 ± 17.44 *# | 190.59 ± 19.43 *# |
| Amino Acid | Amino Acid Composition of SCCOPs (g/100 g) |
|---|---|
| Asp | 0.046 |
| Glu | 0.324 |
| Ser | 0.009 |
| His | 0.038 |
| Gly | 0.130 |
| Thr | 0.249 |
| Arg | 3.077 |
| Ala | 0.254 |
| Tyr | 0.779 |
| Cys | 0.033 |
| Val | 0.213 |
| Met | 0.179 |
| Phe | 0.593 |
| Ile | 0.175 |
| Leu | 0.972 |
| Lys | 0.411 |
| Pro | 0.015 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Li, L.; Xu, T.; Wang, T.; Ren, J.; Liu, X.; Li, Y. Effect of Low Molecular Weight Oligopeptides Isolated from Sea Cucumber on Diabetic Wound Healing in db/db Mice. Mar. Drugs 2018, 16, 16. https://doi.org/10.3390/md16010016
Li D, Li L, Xu T, Wang T, Ren J, Liu X, Li Y. Effect of Low Molecular Weight Oligopeptides Isolated from Sea Cucumber on Diabetic Wound Healing in db/db Mice. Marine Drugs. 2018; 16(1):16. https://doi.org/10.3390/md16010016
Chicago/Turabian StyleLi, Di, Lin Li, Teng Xu, Tianxing Wang, Jinwei Ren, Xinran Liu, and Yong Li. 2018. "Effect of Low Molecular Weight Oligopeptides Isolated from Sea Cucumber on Diabetic Wound Healing in db/db Mice" Marine Drugs 16, no. 1: 16. https://doi.org/10.3390/md16010016





