Isolation and Characterization of Collagen and Antioxidant Collagen Peptides from Scales of Croceine Croaker (Pseudosciaena crocea)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Collagen
2.1.1. Proximate Analysis
2.1.2. Amino Acid Composition
| Amino Acid | ASC-C | CSC | Amino Acid | ASC-C | CSC |
|---|---|---|---|---|---|
| Hyp | 79.4 | 95.1 | Leu | 23.9 | 23.4 |
| Asp | 40.7 | 45.7 | Tyr | 4.1 | 3.7 |
| Thr | 24.2 | 18.4 | Phe | 15.2 | 3.3 |
| Ser | 30.5 | 33.2 | Hyl | 5.5 | 7.7 |
| Glu | 64.7 | 75.9 | Lys | 24.8 | 26.5 |
| Pro | 110.0 | 121.5 | His | 8.2 | 5.3 |
| Gly | 347.1 | 330.6 | Arg | 44.9 | 51.0 |
| Ala | 124.5 | 119.7 | Met | 13.2 | 6.1 |
| Cys | 3.2 | 0.0 | Trp | 0.0 | 0.0 |
| Val | 24.5 | 21.5 | Imino acid | 189.4 | 216.6 |
| Ile | 11.4 | 11.4 | Total | 1000.0 | 1000.0 |
2.1.3. Electrophoretic Pattern of ASC-C
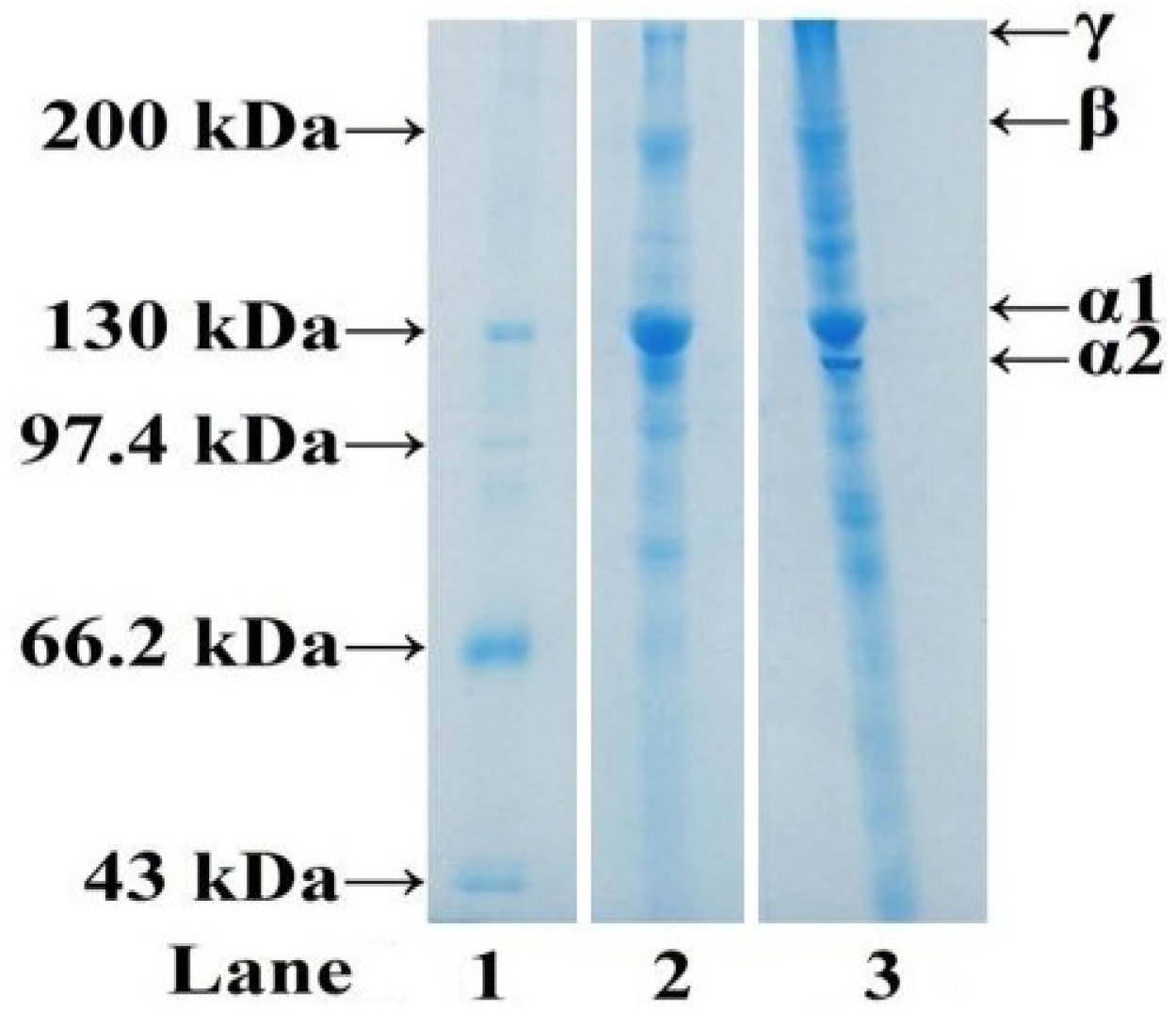
2.2. Isolation of Antioxidant Peptides from ASC-C
2.2.1. Double-Enzyme Hydrolysis and Preparation of Hydrolysate of ASC-C (ACH)
| Hydrolysates | DH (%) | Hydroxyl Radical Scavenging Activity (%)(c = 10 mg/mL) |
|---|---|---|
| Trypsin | 17.47 ± 0.63 | 53.11 ± 0.97 |
| Pepsin | 13.73 ± 0.83 | 44.96 ± 1.97 |
| DE-1 | 20.28 ± 1.05 | 62.33 ± 3.01 |
| DE-2 | 19.67 ± 0.68 | 60.24 ± 3.38 |
| DE-3 | 25.11 ± 0.67 | 85.92 ± 3.84 |
| DE-4 | 22.61 ± 0.74 | 65.46 ± 2.56 |
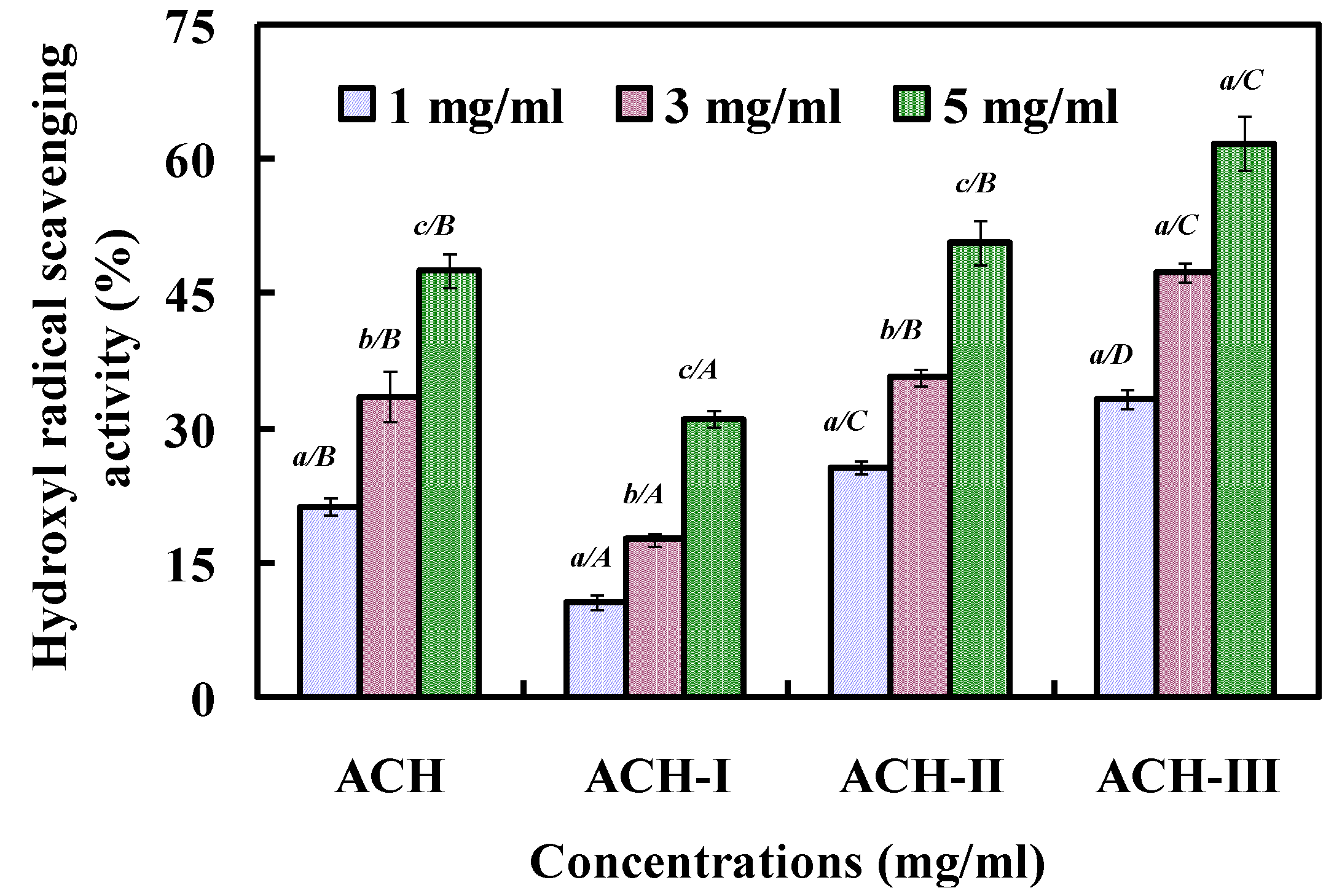
2.2.2. Fractionation of ACH by Ultrafiltration
2.2.3. Gel Filtration Chromatography of ACH-III
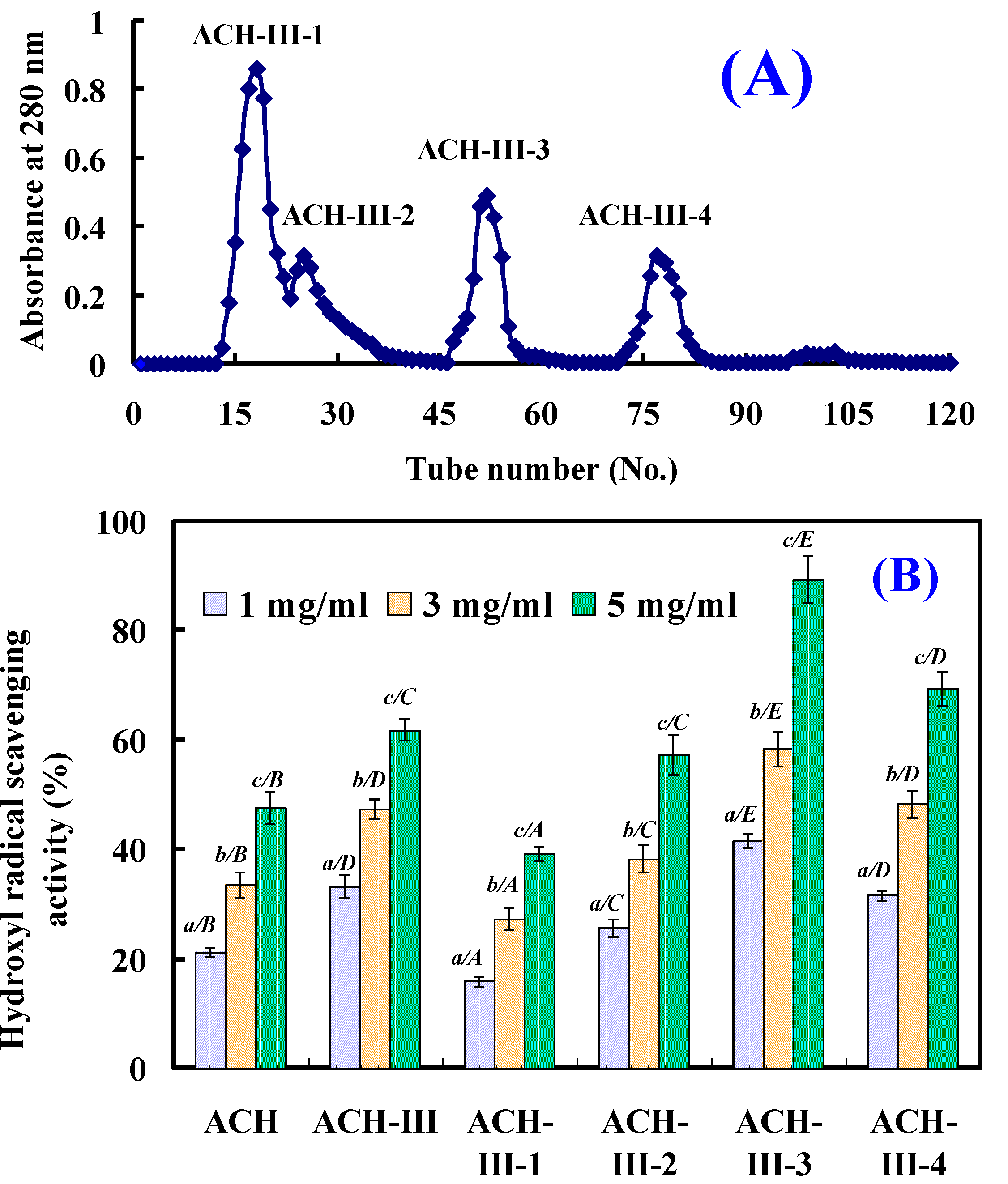
2.2.4. Isolation Peptides from ACH-III-3 by RP-HPLC

2.3. Amino Acid Sequence Analysis and Molecular Mass Determination
2.4. Antioxidative Activity of ACH-P1, ACH-P2, and ACH-P3
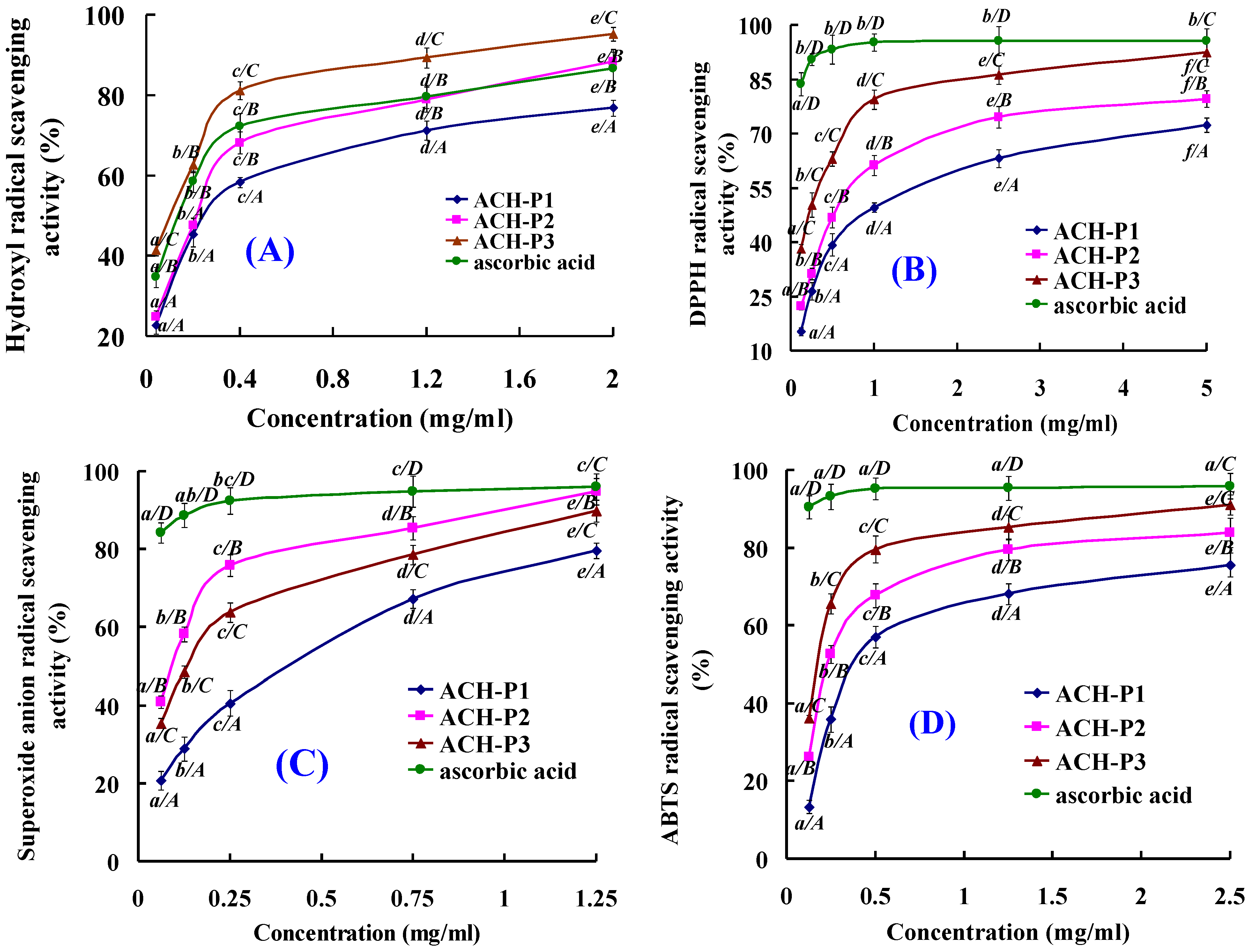
2.4.1. Hydroxyl Radical Scavenging Activity
2.4.2. DPPH Radical Scavenging Activity
2.4.3. Superoxide Anion Radical Scavenging Activity
2.4.4. ABTS Radical Scavenging Activity
2.4.5. Lipid Peroxidation Inhibition Assay

2.4.6. Relationship between Antioxidant Activities and Amino Acid Compositions of Peptides
3. Experimental Section
3.1. Materials
3.2. Preparation of Collagen from Scales
3.3. Characterization of Collagen
3.3.1. Proximate Analysis
3.3.2. Determination of Amino Acid Composition
3.3.3. SDS-polyacrylamide Gel Electrophoresis (SDS-PAGE) of ASC-C
3.4. Isolation of Antioxidant Peptides from ASC-C
3.4.1. Double-Enzyme Hydrolysis and Preparation of Hydrolysate of ASC-C (ACH)
3.4.2. Fractionation of ACH by Ultrafiltration
3.4.3. Gel Filtration Chromatography of ACH-III
3.4.4. Isolation Peptides from ACH-III-3 by RP-HPLC
3.5. Characterization of Collagen Peptides
3.5.1. Degree of Hydrolysis (DH)
3.5.2. Amino Acid Sequence Analysis and Molecular Mass Determination
3.5.3. Antioxidative Activity
3.5.3.1. Hydroxyl Radical Scavenging Activity
3.5.3.2. DPPH Radical Scavenging Activity
3.5.3.3. Superoxide Anion Radical Scavenging Activity
3.5.3.4. ABTS Radical Scavenging Activity
3.5.3.5. Lipid Peroxidation Inhibition Assay
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zhang, T.; Li, Y.; Miao, M.; Jiang, B. Purification and characterisation of a new antioxidant peptide from chickpea (Cicer arietium L.) protein hydrolysates. Food Chem. 2011, 128, 28–33. [Google Scholar] [CrossRef]
- Sampath Kumar, N.S.; Nazeer, R.A.; Jaiganesh, R. Purification and identification of antioxidant peptides from the skin protein hydrolysate of two marine fishes, horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber). Amino Acids 2012, 42, 1641–1649. [Google Scholar] [CrossRef]
- Esmaeili, M.A.; Sonboli, A. Antioxidant, free radical scavenging activities of Salvia brachyantha and its protective effect against oxidative cardiac cell injury. Food Chem. Toxicol. 2010, 48, 846–853. [Google Scholar] [CrossRef]
- Lee, W.S.; Jeon, J.K.; Byun, H.G. Characterization of a novel antioxidative peptide from the sand eel Hypoptychus dybowskii. Proc. Biochem. 2011, 46, 1207–1211. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X.; Zhuang, Y. Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides 2012, 38, 13–21. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Chen, H.M.; Muramoto, K.; Yamauchi, F.; Nokihara, K. Antioxidant Activity of Designed Peptides Based on the Antioxidative Peptide Isolated from Digests of a Soybean Protein. J. Agric. Food Chem. 1996, 44, 2619–2623. [Google Scholar] [CrossRef]
- Wang, B.; Li, L.; Chi, C.F.; Ma, J.H.; Luo, H.Y.; Xu, Y.F. Purification and characterisation of a novel antioxidant peptide derived from blue mussel (Mytilus edulis) protein hydrolysate. Food Chem. 2013, 138, 1713–1719. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 85, 81–89. [Google Scholar] [CrossRef]
- Liu, D.S.; Liang, L.; Regenstein, J.M.; Zhou, P. Extraction and characterisation of pepsin-solubilised collagen from Fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis). Food Chem. 2012, 133, 1441–1448. [Google Scholar]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Nalinanon, S. Compositional and physicochemical characteristics of acid solubilized collagen extracted from the skin of unicorn leatherjacket (Aluterus monoceros). Food Hydrocoll. 2010, 24, 588–594. [Google Scholar] [CrossRef]
- Balti, R.; Jridi, M.; Sila, A.; Souissi, N.; Nedjar-Arroume, N.; Guillochon, D.; Nasri, M. Extraction and functional properties of gelatin from the skin of cuttlefish (Sepia officinalis) using smooth hound crude acid protease-aided process. Food Hydrocoll. 2011, 25, 943–950. [Google Scholar] [CrossRef]
- Huang, Y.R.; Shiau, C.Y.; Chen, H.H.; Huang, B.C. Isolation and characterization of acid and pepsin-solubilized collagens from the skin of balloon fish (Diodon holocanthus). Food Hydrocoll. 2011, 25, 1507–1513. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Comparative study on characteristics of gelatin from the skins of brownbanded bamboo shark and blacktip shark as affected by extraction conditions. Food Hydrocoll. 2010, 24, 164–171. [Google Scholar] [CrossRef]
- Wang, L.; Zou, Y.; Jiang, S.; Xu, J.; Jiang, S.; Hu, Q. Chromatographic separation and physicochemical properties of collagen species in the skin of deep-sea redfish (Sebastes mentella). Food Hydrocoll. 2011, 25, 1134–1138. [Google Scholar] [CrossRef]
- Żelechowska, E.; Sadowska, M.; Turk, M. Isolation and some properties of collagen from the backbone of Baltic cod (Gadus morhua). Food Hydrocoll. 2010, 24, 325–329. [Google Scholar] [CrossRef]
- Zhu, B.; Dong, X.; Zhou, D.; Gao, Y.; Yang, J.; Li, D.; Zhao, X.; Ren, T.; Ye, W.; Tan, H.; et al. Physicochemical properties and radical scavenging capacities of pepsin-solubilized collagen from sea cucumber Stichopus japonicus. Food Hydrocoll. 2012, 28, 182–188. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, Y.T.; Byun, H.G.; Nam, K.S.; Joo, D.S.; Shahidi, F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska pollack skin. J. Agric. Food Chem. 2001, 49, 1984–1989. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Kim, S.K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef]
- Sankar, S.; Sekar, S.; Mohan, R.; Rani, S.; Sundaraseelan, J.; Sastry, T.P. Preparation and partial characterization of collagen sheet from fish (Lates calcarifer) scales. Int. J. Biol. Macromol. 2008, 42, 6–9. [Google Scholar]
- Krishnan, S.; Sekar, S.; Katheem, M.F.; Krishnakumar, S.; Sastry, T.P. Fish scale collagen—a novel material for corneal tissue engineering. Artif. Organs 2012, 36, 829–835. [Google Scholar] [CrossRef]
- Duan, R.; Zhang, J.; Du, X.; Yao, X.; Konno, K. Properties of collagen from skin, scale and bone of carp (Cyprinus carpio). Food Chem. 2009, 112, 702–706. [Google Scholar] [CrossRef]
- Nagai, T.; Izumi, M.; Ishii, M. Fish scale collagen. Preparation and partial characterization. Int. J. Food Sci. Technol. 2004, 39, 239–244. [Google Scholar] [CrossRef]
- Liu, W.; Li, G.; Miao, Y.; Wu, X. Preparation and characterization of pepsin solubilized type I collagen from the scales of snakehead (Ophiocephalus Argus). J. Food Biochem. 2009, 33, 20–37. [Google Scholar] [CrossRef]
- Matmaroh, K.; Benjakul, S.; Prodpran, T.; Encarnacion, A.B.; Kishimura, H. Characteristics of acid soluble collagen and pepsin soluble collagen from scale of spotted golden goatfish (Parupeneus heptacanthus). Food Chem. 2011, 129, 1179–1186. [Google Scholar] [CrossRef]
- Ogawa, M.; Portier, R.J.; Moody, M.W.; Bell, J.; Schexnayder, M.A.; Losso, J.N. Biochemical properties of bone and scale collagens isolated from the subtropical fish black drum (Pogonia Cromis) and sheepshead seabream (Archosargus Probatocephalus). Food Chem. 2004, 88, 495–501. [Google Scholar] [CrossRef]
- Ikoma, T.; Kobayashi, H.; Tanaka, J.; Walsh, D.; Mann, S. Physical properties of type I collagen extracted from fish scales of Pagrus major and Oreochromis niloticas. Int. J. Biol. Macromol. 2003, 32, 199–204. [Google Scholar] [CrossRef]
- Zhang, J.J.; Duan, R.; Ye, C.; Konno, K. Isolation and characterization of collagens from scale of silver carp (Hypophthalmichthys molitrix). J. Food Biochem. 2010, 34, 1343–1354. [Google Scholar] [CrossRef]
- Wang, L.; An, X.; Yang, F.; Xin, Z.; Zhao, L.; Hu, Q. Isolation and characterization of collagens from the skin, scale and bone of deep-sea redfish (Sebastes mentella). Food Chem. 2008, 108, 616–623. [Google Scholar] [CrossRef]
- Pati, F.; Adhikari, B.; Dhara, S. Isolation and characterization of fish scale collagen of higher thermal stability. Bioresour. Technol. 2010, 101, 3737–3742. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Chi, C.; Gong, Y.; Luo, H.; Ding, G. Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from Sphyrna lewini, Dasyatis akjei and Raja porosa. Food Res. Int. 2013, 51, 283–293. [Google Scholar] [CrossRef]
- Thiansilakul, Y.; Benjakul, S.; Shahidi, F. Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decapterus maruadsi). Food Chem. 2007, 103, 1385–1394. [Google Scholar] [CrossRef]
- Ranathunga, S.; Rajapakse, N.; Kim, S.K. Purification and characterization of antioxidative peptide derived from muscle of conger eel (Conger myriaster). Eur. Food Res. Technol. 2006, 222, 310–315. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Purification and identification of antioxidative peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Res. Int. 2010, 43, 1167–1173. [Google Scholar]
- Wang, B.; Li, Z.; Chi, C.; Zhang, Q.; Luo, H. Preparation and evaluation of antioxidant peptides from ethanol-soluble proteins hydrolysate of Sphyrna lewini muscle. Peptides 2012, 36, 240–250. [Google Scholar] [CrossRef]
- Rajapakse, N.; Mendis, E.; Byun, H.G.; Kim, S.K. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J. Nutr. Biochem. 2005, 16, 562–569. [Google Scholar] [CrossRef]
- Qian, Z.J.; Jung, W.K.; Byun, H.G.; Kim, S.K. Protective effect of an antioxidative peptide purified from gastrointestinal digests of oyster, Crassostrea gigas against free radical induced DNA damage. Bioresour. Technol. 2008, 99, 3365–3371. [Google Scholar] [CrossRef]
- Kim, E.K.; Lee, S.J.; Jeon, B.T.; Moon, S.H.; Kim, B.K.; Park, T.K.; Hand, J.S.; Park, P.J. Purification and characterisation of antioxidative peptides from enzymatic hydrolysates of venison protein. Food Chem. 2009, 114, 1365–1370. [Google Scholar] [CrossRef]
- Li, Y.H.; Jiang, B.; Zhang, T.; Mu, W.M.; Liu, J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Suetsuna, K.; Ukeda, H.; Ochi, H. Isolation and characterization of free radical scavenging activities peptides derived from casein. J. Nutr. Biochem. 2000, 11, 128–131. [Google Scholar] [CrossRef]
- Sheih, I.C.; Wu, T.K.; Fang, T.J. Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Bioresour. Technol. 2009, 100, 3419–3425. [Google Scholar] [CrossRef]
- Tsuge, N.; Eikawa, Y.; Namura, Y.; Yamamoto, M.; Sugisawa, K. Antioxidative activity of peptides prepared by enzymic hydrolysis of egg white albumin. Nippon Nogeik. Kaishi 1991, 65, 1635–1641. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.J.; Zhao, M.Y.; Lv, L. Purification and identification of antioxidant peptides from egg white protein hydrolysate. Amino Acids 2012, 43, 457–466. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Wang, L.; Guo, X.; Wang, X.; Yao, H. Antioxidant activities of the rice endosperm protein hydrolysate: identification of the active peptide. Eur. Food Res. Technol. 2009, 229, 709–719. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, J.; Tang, X.; Xiong, Y.L. Reducing, radical scavenging and chelation properties of in vitro digests of alcalase treated zein hydrolysate. J. Agric. Food Chem. 2008, 56, 2714–2721. [Google Scholar] [CrossRef]
- Li, Z.R.; Wang, B.; Chi, C.F.; Zhang, Q.H.; Gong, Y.D.; Tang, J.J.; Luo, H.Y.; Ding, G.F. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius). Food Hydrocoll. 2013, 31, 103–113. [Google Scholar] [CrossRef]
- Lapsongphon, N.; Yongsawatdigul, J. Production and purification of antioxidant peptides from a mungbean meal hydrolysate by Virgibacillus sp. SK37 proteinase. Food Chem. 2013, 141, 992–999. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, B.; Wang, Y.-M.; Chi, C.-F.; Luo, H.-Y.; Deng, S.-G.; Ma, J.-Y. Isolation and Characterization of Collagen and Antioxidant Collagen Peptides from Scales of Croceine Croaker (Pseudosciaena crocea). Mar. Drugs 2013, 11, 4641-4661. https://doi.org/10.3390/md11114641
Wang B, Wang Y-M, Chi C-F, Luo H-Y, Deng S-G, Ma J-Y. Isolation and Characterization of Collagen and Antioxidant Collagen Peptides from Scales of Croceine Croaker (Pseudosciaena crocea). Marine Drugs. 2013; 11(11):4641-4661. https://doi.org/10.3390/md11114641
Chicago/Turabian StyleWang, Bin, Yu-Mei Wang, Chang-Feng Chi, Hong-Yu Luo, Shang-Gui Deng, and Jian-Yin Ma. 2013. "Isolation and Characterization of Collagen and Antioxidant Collagen Peptides from Scales of Croceine Croaker (Pseudosciaena crocea)" Marine Drugs 11, no. 11: 4641-4661. https://doi.org/10.3390/md11114641





