Anxiolytic-Like Effect of a Salmon Phospholipopeptidic Complex Composed of Polyunsaturated Fatty Acids and Bioactive Peptides
Abstract
:1. Introduction
2. Results
2.1. Phospholipopeptidic Complex
2.1.1. Characterization of Lipids
| Minerals | Fatty acids | Amino acids | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (μg/g) | (%) | |||||||||
| Component | PLPC | Component | PLPC | Fatty acids | Percentage | Amino acids | PLPC | Salmon marine collagen peptide * | ||
| Arsenic | <1.1 | Rubidium | 0.547 ± 0.05 | C14:0 | 3.54 ± 0.01 | Asparagine | 606.49 | 11.44 | 80.12 | - |
| Barium | <2 | Antimony | <0.1 | C15:0 | 0.24 ± 0.02 | Glutamic acid | 713.83 | 13.47 | 105.02 | 12.22 |
| Beryllium | <0.45 | Samarium | <0.01 | C15:1 | 0.27 ± 0.01 | Serine | 290.26 | 5.48 | 30.50 | 4.23 |
| Bismuth | <0.09 | Tin | <0.3 | C16:0 | 17.04 ± 0.04 | Glycine | 822.50 | 15.52 | 61.74 | 23.77 |
| Cadmium | <0.1 | Strontium | 34.21 ± 3.42 | C16:1 n-7 | 0.31 ± 0.01 | Histidine | nq | nq | nq | 1.61 |
| Cerium | <0.12 | Tantalum | <0.01 | C16:1 n-9 | 4.10 ± 0.05 | Arginine | 369.15 | 6.96 | 64.30 | 6.08 |
| Cobalt | <0.2 | Terbium | <0.005 | C16:3 n-3 | 0.42 ± 0.02 | Threonine | 324.20 | 6.12 | 38.61 | 2.53 |
| Chromium | <3 | Thorium | <0.03 | C16:4 n-3 | 0.33 ± 0.01 | Alanine | 578.68 | 10.92 | 51.55 | 6.59 |
| Caesium | <0.05 | Thulium | <0.001 | C18:0 | 3.96 ± 0.01 | Proline | 367.39 | 6.93 | 42.29 | 9.79 |
| Copper | 11.7 ± 1.17 | Uranium | <0.04 | C18:1 n-9 | 16.49 ± 0.02 | Tyrosine | 92.43 | 1.74 | 16.74 | 0.03 |
| Dysprosium | <0.009 | Vanadium | <0.75 | C18:1 n-7 | 2.66 ± 0.06 | Valine | 175.77 | 3.32 | 20.59 | 2.94 |
| Erbium | <0.005 | Tungsten | <0.1 | C18:2 n-6 | 4.39 ± 0.01 | Methionine | 106.07 | 2.00 | 15.82 | 0.03 |
| Europium | <0.004 | Yttrium | <0.15 | C18:3 n-4 | 2.77 ± 0.01 | Isoleucine | 162.29 | 3.06 | 21.28 | 2.57 |
| Gallium | <0.16 | Ytterbium | <0.004 | C18:3 n-3 | 1.45 ± 0.01 | Leucine | 280.66 | 5.29 | 36.81 | 4.64 |
| Gadolinium | <0.01 | Zinc | 266.3 ± 26.63 | C20:1 n-9 | 0.93 ± 0.01 | Phenylalanine | 144.35 | 2.72 | 23.84 | 2.51 |
| Germanium | <0.1 | Zirconium | <1 | C20:1 n-7 | 0.69 ± 0.01 | Lysine | 266.45 | 5.03 | 38.95 | 5.66 |
| Hafnium | <0.026 | Silicon | <0.02 | C20:2 n-6 | 2.11 ± 0.10 | Hydroxyproline | - | - | - | 7.51 |
| Holmium | <0.002 | Aluminium | <0.02 | C20:4 n-6 | 1.50 ± 0.01 | Aspartic acid | - | - | - | 7.29 |
| Indium | <0.07 | Iron | 0.0021 ± 0.0003 | C20:3 n-3 | 0.96 ± 0.01 | |||||
| Lanthanum | <0.06 | Manganese | 0.00007 ± 0.000007 | C20:5 n-3 EPA | 10.53 ± 0.02 | |||||
| Lutetium | <0.002 | Magnesium | 0.0092 ± 0.0009 | C21:5 n-3 | 1.06 ± 0.01 | |||||
| Molybdenum | <0.3 | Calcium | 0.047 ± 0.007 | C22:4 n-6 | 0.30 ± 0.01 | |||||
| Niobium | <0.06 | Sodium | 0.077 ± 0.011 | C22:5 n-6 | 0.33 ± 0.01 | |||||
| Neodymium | <0.04 | Potassium | 0.025 ± 0.0042 | C22:5 n-3 | 3.10 ± 0.01 | |||||
| Nickel | <4 | Thallium | <0.03 | C22:6 n-3 DHA | 20.34 ± 0.01 | |||||
| Lead | <0.6 | Phosphorus | 0.132 ± 0.013 | |||||||
| Praseodymium | <0.01 | - | - | |||||||
2.1.2. Characterization of Proteins
| Protein groups | Parent protein | Score (%) | m/z |
|---|---|---|---|
| 1 | Collagen 1a1 (Oncorhynchusmykiss) | 99.21 | 137,117 |
| 2 | Fast myotomal muscle actin (Salmosalar) | 99.17 | 41,932 |
| 3 | Collagen alpha-2(I) chain precursor (Oncorhynchusmykiss) | 99.14 | 126,985 |
| 4 | Type I collagen alpha 2 chain (Oncorhynchusketa) | 99.13 | 126,443 |
| 5 | Myosin heavy chain (Oncorhynchusketa) | 99.05 | 222,153 |
| 6 | Creatine kinase-2 (Salmosalar) | 98.87 | 39,470 |
| 7 | Collagen a3(I) (Oncorhynchusmykiss) | 98.84 | 137,758 |
| 8 | Collagen 1a (Salmosalar) | 98.69 | 35,016 |
| 9 | PREDICTED: Sarcoplasmic/Endoplasmic reticulum calcium ATPase 1-like isoform 2 (Ailuropodamelanoleuca) | 98.47 | 109,208 |
| 10 | Glyceraldehyde phosphate dehydrogenase (Oncorhynchusmykiss) | 97.89 | 7,035,481 |
| 11 | Myosin regulatory light chain 2 (Salmosalar) | 97.79 | 18,996 |
| 12 | Glycogen phosphorylase muscle form (Salmosalar) | 97.67 | 97,485 |
| 13 | Nucleoside diphosphate kinase A (Salmosalar) | 84.25 | 17,092 |
| 14 | Troponin C skeletal muscle (Salmosalar) | 81.75 | 18,239 |
| 15 | Enolase 3-1 (Salmosalar) | 81.6 | 47,246 |
| 16 | Hemoglobin subunit beta (Salmosalar) | 70.85 | 16,124 |
| 17 | Parvalbumin beta-1 (Salmosalar) | 66.48 | 11,901 |
| 18 | Unnamed protein product (Oikopleuradioica) | 65.23 | 140,061 |
| 19 | Histone cluster 2 H2ab (Daniorerio) | 59.52 | 13,631 |
| 20 | Hemoglobin subunit beta-1 (Salmosalar) | 61.73 | 16,016 |
| 21 | Unnamed protein product (Tetraodonnigroviridis) | 61.63 | 351,956 |
| 22 | Adenylate kinase (Salmosalar) | 61.61 | 21,288 |
| 23 | Myosin light polypeptide 3-1 (Salmosalar) | 58.05 | 21,001 |
| 24 | Alpha-globin (Salmosalar) | 55.94 | 15,142 |
| 25 | Collagen type I alpha 3 chain (Dicentrarchuslabrax) | 51.66 | 137,004 |
| 26 | l-Lactate dehydrogenase A chain (Salmosalar) | 50.9 | 36,327 |
2.1.3. Minerals and Element Trace Composition
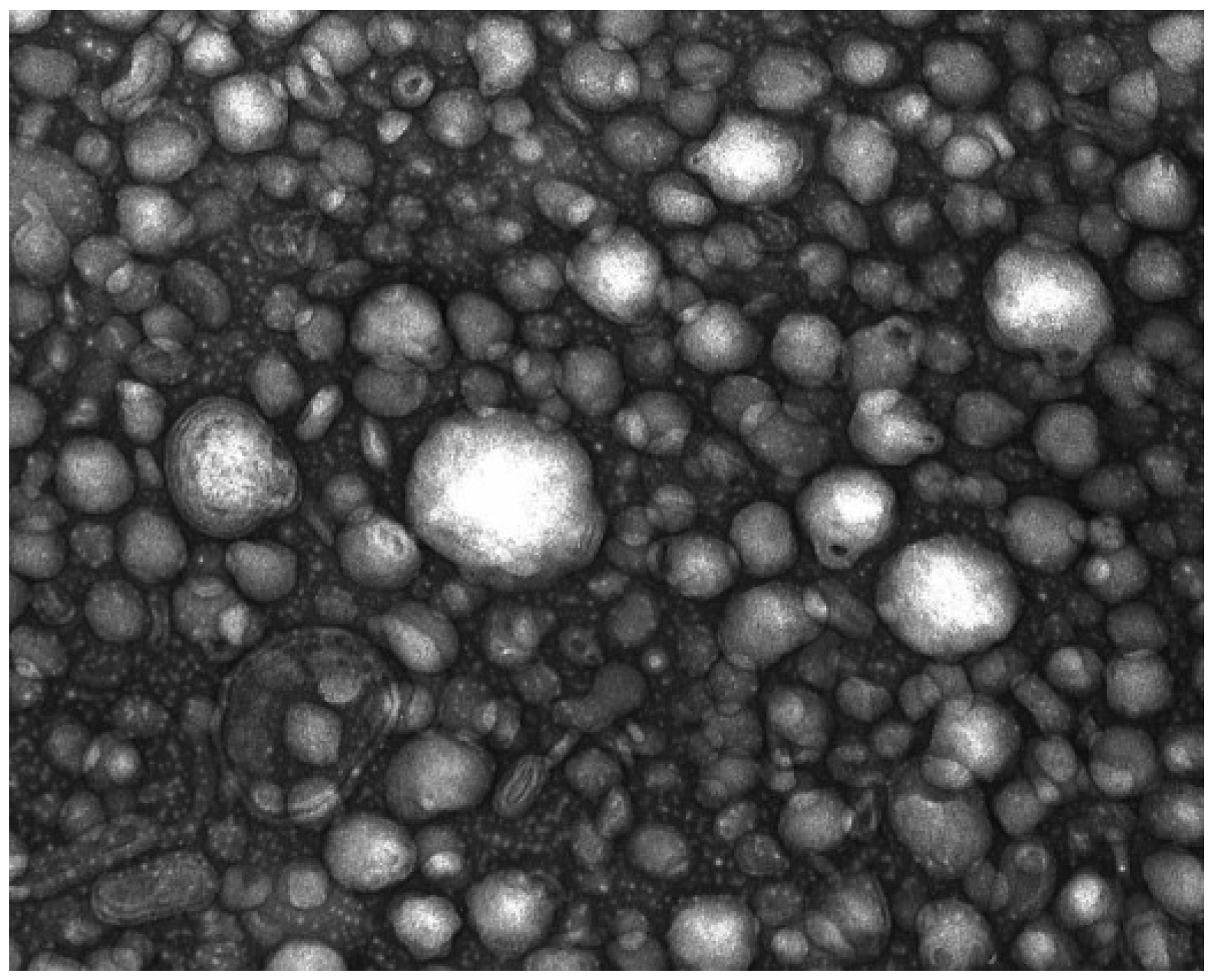
2.1.4. Treatment Characterization
2.2. In Vivo Test
2.2.1. Body Weight
2.2.2. Behavioral Test
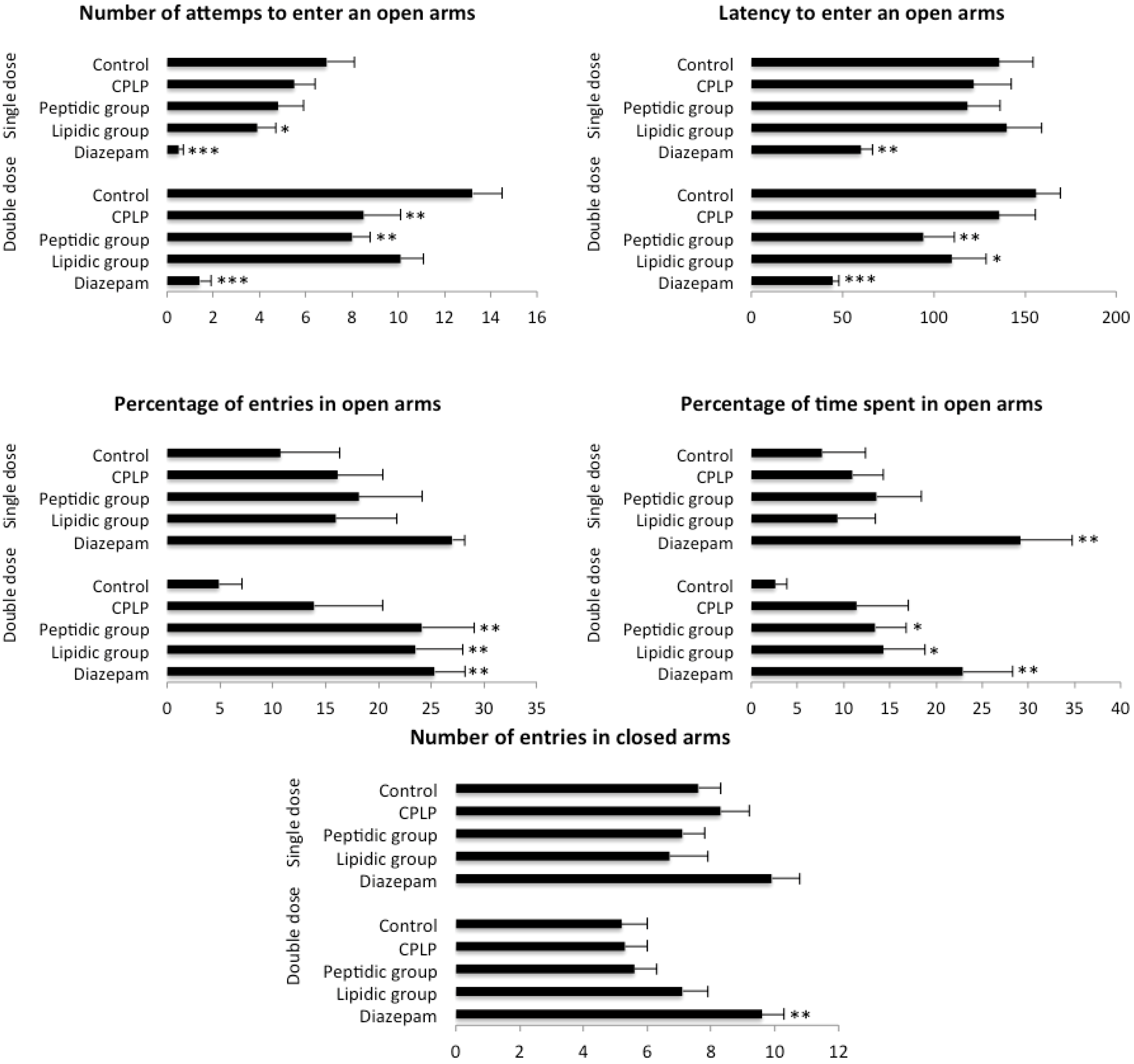
2.2.2.1. Elevated plus Maze (EPM)
2.2.2.2. Hole-Board Test

2.2.2.3. Light-Dark Box Test

2.2.2.4. Open Field Test
2.3. ROS Evaluation in Neural Cells
| Mean fluorescent intensity (arbitrary units) | ANOVA | |||||
|---|---|---|---|---|---|---|
| Dose | Control (n = 8) | PLPC (n = 8) | Peptidic fraction (n = 8) | Lipidic fraction (n = 8) | F(3,28) | p |
| SD | 4.64 ± 0.68 | 3.72 ± 0.51 | 3.67 ± 0.30 | 4.76 ± 0.61 | 1.13 | Not significant |
| DD | 4.09 ± 0.41 | 3.43 ± 0.13 (t) | 3.14 ± 0.11 ** | 3.48 ± 0.14 (t) | 2.97 | 0.048 |
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Phospholipopeptidic Complex Characterization
4.2.1. Minerals and Element Trace Composition
4.2.2. Lipid Classes
4.2.3. Fatty Acid Composition
4.2.4. Determination of Amino Acid Composition
4.2.5. Proteomics
4.2.6. Treatments and Doses
| Treatment | Single dose (SD) | Double dose (DD) |
|---|---|---|
| Phospholipopeptidic complex | 123 mg/kg/10 mL | 246 mg/kg/10 mL |
| Lipidic fraction of the PLPC (30%) | 36.9 mg/kg/10 mL | 73.8 mg/kg/10 mL |
| Peptidic fraction of the PLPC (70%) | 86.1 mg/kg/10 mL | 172.2 mg/kg/10 mL |
4.2.6.1. Static Light Scattering
4.2.6.2. Transmission Electronmicroscopy (TEM)
4.2.6.3. Particle Size, Electrophoretic Mobility and Polydispersity Index of Nanoparticles
4.3. In Vivo Study
4.3.1. Animals
4.3.2. Study Design
4.3.3. Behavioral Tests
4.3.3.1. Elevated Plus Maze (EPM)
4.3.3.2. Hole Board Test (HBT)
4.3.3.3. Light-Dark Box Test (LDBT)
4.3.3.4. Open Field Test (OF)
4.3.4. ROS Evaluation in Neural Cells
4.3.5. Data Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Öhman, A. Anxiety. In Encyclopedia of Stress; Fink, G., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 226–231. [Google Scholar]
- Graziani, P. Anxiété et Troubles Anxieux; Armand, C., Ed.; Nathan VUEF: Paris, France, 2005. [Google Scholar]
- Emilien, G. L’anxiété Sociale; Hayen: Sprimont, Belgium, 2003. [Google Scholar]
- Weinberger, D.R. Anxiety at the frontier of molecular medicine. N. Engl. J. Med. 2001, 344, 1247–1249. [Google Scholar] [CrossRef]
- Gross, C.; Hen, R. The developmental origins of anxiety. Nat. Rev. Neurosci. 2004, 5, 545–552. [Google Scholar] [CrossRef]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef]
- Lieb, R.; Becker, E.; Altamura, C. The epidemiology of generalized anxiety disorder in Europe. Eur. Neuropsychopharmacol. 2005, 15, 445–452. [Google Scholar] [CrossRef]
- Kuloglu, M.; Atmaca, M.; Tezcan, E.; Gecici, O.; Tunckol, H.; Ustundag, B. Antioxidant enzyme activities and malondialdehyde levels in patients with obsessive-compulsive disorder. Neuropsychobiology 2002, 46, 27–32. [Google Scholar] [CrossRef]
- Kuloglu, M.; Atmaca, M.; Tezcan, E.; Ustundag, B.; Bulut, S. Antioxidant enzyme and malondialdehyde levels in patients with panic disorder. Neuropsychobiology 2002, 46, 186–189. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Soulimani, R. Oxidative stress and anxiety relationship and cellular pathways. Oxid. Med. Cell. Longev. 2009, 2, 63–67. [Google Scholar] [CrossRef]
- Hovatta, I.; Juhila, J.; Donner, J. Oxidative stress in anxiety and comorbid disorders. Neurosci. Res. 2010, 68, 261–275. [Google Scholar] [CrossRef]
- Rammal, H.; Bouayed, J.; Falla, J.; Boujedaini, N.; Soulimani, R. The impact of high anxiety level on cellular and humoral immunity in mice. Neuroimmunomodulation 2010, 17, 1–8. [Google Scholar] [CrossRef]
- Rammal, H.; Bouayed, J.; Younos, C.; Soulimani, R. Evidence that oxidative stress is linked to anxiety-related behaviour in mice. Brain Behav. Immun. 2008, 22, 1156–1159. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Clarendon Press: Oxford, UK, 1999. [Google Scholar]
- Ng, F.; Berk, M.; Dean, O.; Bush, A.I. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008, 11, 851–876. [Google Scholar]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Breggin, P.R. Analysis of adverse behavioral effects of benzodiazepines with a discussion on drawing scientific conclusions from the FDA’s spontaneous reporting system. J. Mind Behav. 1998, 19, 21–49. [Google Scholar]
- Longo, L.P.; Johnson, B. Addiction: Part I. Benzodiazepines—Side effects, abuse risk and alternatives. Am. Family Phys. 2000, 61, 2121–2128. [Google Scholar]
- Owen, R.T.; Tyrer, P. Benzodiazepine dependence. A review of the evidence. Drugs 1983, 25, 385–398. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A.S. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef]
- Kim, S.-K.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Rai, A.K.; Jini, R.; Swapna, H.C.; Sachindra, N.M.; Bhaskar, N.; Baskaran, V. Application of Native Lactic Acid Bacteria (LAB) for fermentative recovery of lipids and proteins from fish processing wastes: Bioactivities of fermentation products. J. Aquat. Food Prod. Technol. 2011, 20, 32–44. [Google Scholar] [CrossRef]
- Nguyen, H.T.M.; Sylla, K.S.B.; Randriamahatody, Z.; Donnay-Moreno, C.; Moreau, J.; Tran, L.T.; Berge, J.P. Enzymatic hydrolysis of yellowfin tuna (Thunnus albacares) by-products using protamex protease. Food Technol. Biotechnol. 2011, 49, 48–55. [Google Scholar]
- Je, J.Y.; Park, P.J.; Kim, S.K. Antioxidant activity of a peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res. Int. 2005, 38, 45–50. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Yang, L.; Zu, Y.; Qu, S.; Zhang, Y. Oxidative stability of fish oil supplemented with carnosic acid compared with synthetic antioxidants during long-term storage. Food Chem. 2011, 128, 93–99. [Google Scholar] [CrossRef]
- Khoddami, A.; Ariffin, A.A.; Bakar, J.; Ghazali, H.M. Fatty acid profile of the oil extracted from fish waste (Head, Intestine and Liver) (Sardinella lemuru). World Appl. Sci. J. 2009, 7, 127–131. [Google Scholar]
- Rubio-Rodríguez, N.; de Diego, S.M.; Beltrán, S.; Jaime, I.; Sanz, M.T.; Rovira, J. Supercritical fluid extraction of fish oil from fish by-products: A comparison with other extraction methods. J. Food Eng. 2012, 109, 238–248. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Kidd, P.M. Omega-3 DHA and EPA for cognition, behavior, and mood: Clinical findings and structural-functional synergies with cell membrane phospholipids. Altern. Med. Rev. J. Clin. Ther. 2007, 12, 207–227. [Google Scholar]
- Buydens-Branchey, L.; Branchey, M. N-3 polyunsaturated fatty acids decrease anxiety feelings in a population of substance abusers. J. Clin. Psychopharmacol. 2006, 26, 661–665. [Google Scholar] [CrossRef]
- Lu, F.S.; Nielsen, N.S.; Baron, C.P.; Jacobsen, C. Oxidative degradation and non-enzymatic browning due to the interaction between oxidised lipids and primary amine groups in different marine PL emulsions. Food Chem. 2012, 135, 2887–2896. [Google Scholar] [CrossRef]
- Gbogouri, G.A.; Linder, M.; Fanni, J.; Parmentier, M. Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. J. Food Sci. 2004, 69, 615–622. [Google Scholar] [CrossRef]
- He, H.L.; Chen, X.L.; Wu, H.; Sun, C.Y.; Zhang, Y.Z.; Zhou, B.C. High throughput and rapid screening of marine protein hydrolysates enriched in peptides with angiotensin-I-converting enzyme inhibitory activity by capillary electrophoresis. Bioresour. Technol. 2007, 98, 3499–3505. [Google Scholar] [CrossRef]
- Salampessy, J.; Phillips, M.; Seneweera, S.; Kailasapathy, K. Release of antimicrobial peptides through bromelain hydrolysis of leatherjacket (Meuchenia sp.) insoluble proteins. Food Chem. 2010, 120, 556–560. [Google Scholar] [CrossRef]
- Ono, S.; Hosokawa, M.; Miyashita, K.; Takahashi, K. Inhibition properties of dipeptides from salmon muscle hydrolysate on angiotensin I-converting enzyme. Int. J. Food Sci. Technol. 2006, 41, 383–386. [Google Scholar] [CrossRef]
- Miller, M.R.; Nichols, P.D.; Barnes, J.; Davies, N.W.; Peacock, E.J.; Carter, C.G. Regiospecificity profiles of storage and membrane lipids from the gill and muscle tissue of Atlantic salmon (Salmo salar L.) grown at elevated temperature. Lipids 2006, 41, 865–876. [Google Scholar] [CrossRef]
- Lu, F.S.H.; Nielsen, N.S.; Baron, C.P.; Jensen, L.H.S.; Jacobsen, C. Physico-Chemical properties of marine phospholipid emulsions. J. Am. Oil Chem. Soc. 2012, 89, 2011–2024. [Google Scholar] [CrossRef]
- Pei, X.; Yang, R.; Zhang, Z.; Gao, L.; Wang, J.; Xu, Y.; Zhao, M.; Han, X.; Liu, Z.; Li, Y. Marine collagen peptide isolated from Chum Salmon (Oncorhynchus keta) skin facilitates learning and memory in aged C57BL/6J mice. Food Chem. 2010, 118, 333–340. [Google Scholar] [CrossRef]
- Martínez-Alvarez, O.; Montero, P.; Gómez-Guillén, C. Evidence of an active laccase-like enzyme in deepwater pink shrimp (Parapenaeus longirostris). Food Chem. 2008, 108, 624–632. [Google Scholar] [CrossRef]
- Pena-Ramos, E.A.; Xiong, Y.L.; Arteaga, G.E. Fractionation and characterisation for antioxidant activity of hydrolysed whey protein. J. Sci. Food Agric. 2004, 84, 1908–1918. [Google Scholar] [CrossRef]
- Li-Chan, E.C.Y.; Hunag, S.-L.; Jao, C.-L.; Ho, K.-P.; Hsu, K.-C. Peptides derived from atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J. Agric. Food Chem. 2012, 60, 973–978. [Google Scholar] [CrossRef]
- Liaset, B.; Espe, M. Nutritional composition of soluble and insoluble fractions obtained by enzymatic hydrolysis of fish-raw materials. Process Biochem. 2008, 43, 42–48. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Losada, V.; Prego, R. Distribution of lipids and trace minerals in different muscle sites of farmed and wild turbot (Psetta maxima). Int. J. Food Sci. Technol. 2007, 42, 1456–1464. [Google Scholar] [CrossRef] [Green Version]
- Klang, V.; Matsko, N.B.; Valenta, C.; Hofer, F. Electron microscopy of nanoemulsions: An essential tool for characterisation and stability assessment. Micron 2012, 43, 85–103. [Google Scholar]
- Belhaj, N.; Arab-Tehrany, E.; Linder, M. Oxidative kinetics of salmon oil in bulk and in nanoemulsion stabilized by marine lecithin. Process Biochem. 2010, 45, 187–195. [Google Scholar]
- Chalon, S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 259–269. [Google Scholar] [CrossRef]
- Ross, B.M. Omega-3 polyunsaturated fatty acids and anxiety disorders. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 309–312. [Google Scholar] [CrossRef]
- Carrie, I.; Clement, M.; de Javel, D.; Frances, H.; Bourre, J.M. Phospholipid supplementation reverses behavioral and biochemical alterations induced by n-3 polyunsaturated fatty acid deficiency in mice. J. Lipid Res. 2000, 41, 473–480. [Google Scholar]
- Takeuchi, T.; Iwanaga, M.; Harada, E. Possible regulatory mechanism of DHA-induced anti-stress reaction in rats. Brain Res. 2003, 964, 136–143. [Google Scholar] [CrossRef]
- Fedorova, I.; Salem, N., Jr. Omega-3 fatty acids and rodent behavior. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 271–289. [Google Scholar] [CrossRef]
- Harauma, A.; Moriguchi, T. Dietary n-3 fatty acid deficiency in mice enhances anxiety induced by chronic mild stress. Lipids 2011, 46, 409–416. [Google Scholar] [CrossRef]
- Ferraz, A.C.; Delattre, A.M.; Almendra, R.G.; Sonagli, M.; Borges, C.; Araujo, P.; Andersen, M.L.; Tufik, S.; Lima, M.M. Chronic omega-3 fatty acids supplementation promotes beneficial effects on anxiety, cognitive and depressive-like behaviors in rats subjected to a restraint stress protocol. Behav. Brain Res. 2011, 219, 116–122. [Google Scholar] [CrossRef]
- Vinot, N.; Jouin, M.; Lhomme-Duchadeuil, A.; Guesnet, P.; Alessandri, J.-M.; Aujard, F.; Pifferi, F. Omega-3 fatty acids from fish oil lower anxiety, improve cognitive functions and reduce spontaneous locomotor activity in a non-human primate. PLoS One 2011, 6, e20491. [Google Scholar]
- Hiratsuka, S.; Ishihara, K.; Kitagawa, T.; Wada, S.; Yokogoshi, H. Effect of dietary docosahexaenoic acid connecting phospholipids on the lipid peroxidation of the brain in mice. J. Nutr. Sci. Vitaminol. 2008, 54, 501–506. [Google Scholar] [CrossRef]
- Singh, M. Essential fatty acids, DHA and human brain. Indian J. Pediatr. 2005, 72, 239–242. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 19, 1699–1707. [Google Scholar] [CrossRef]
- Matsui, T.; Matsumoto, K. Antihypertensive peptides from natural resources. Adv. Phytomed. 2006, 2, 255–271. [Google Scholar] [CrossRef]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef]
- Guérard, F.; Decourcelle, N.; Sabourin, C.; Floch-Laizet, C.; le Grel, L.; le Floch, P.; Gourlay, F.; le Delezir, R.; Jaouen, P.; Bourseau, P. Recent developments of marine ingredients for food and nutraceutical applications: A review. J. Sci. Halieut. Aquat. 2010, 2, 21–27. [Google Scholar]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Kim, S.K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Miclo, L.; Perrin, E.; Driou, A.; Papadopoulos, V.; Boujrad, N.; Vanderesse, R.; Boudier, J.F.; Desor, D.; Linden, G.; Gaillard, J.L. Characterization of alpha-casozepine, a tryptic peptide from bovine alpha(s1)-casein with benzodiazepine-like activity. FASEB J. 2001, 15, 1780–1782. [Google Scholar]
- Chalamaiah, M.; Dinesh Kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar]
- Erdmann, K.; Cheung, B.W.; Schroder, H. The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J. Nutr. Biochem. 2008, 19, 643–654. [Google Scholar] [CrossRef]
- Linder, M.; Fanni, J.; Parmentier, M. A process for the valorisation of marine by-products in mild conditions. Mar. Biotechnol. 2005, 15, 70–76. [Google Scholar] [CrossRef]
- Carignan, J.; Hild, P.; Mevelle, G.; Morel, J.; Yeghicheyan, D. Routine analyses of trace elements in geological samples using flow injection and low pressure on-line liquid chromatography coupled to ICP-MS: A study of geochemical reference materials BR, DR-N, UB-N, AN-G and GH. Geostand. Newslett. 2001, 25, 187–198. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride‚ Äìmethanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar]
- Bockisch, M. Fish oil—From the bad and the ugly to the precious and good. Eur. J. Lipid Sci. Technol. 2010, 112, 948–960. [Google Scholar] [CrossRef]
- Schlienger, J.L. Acides gras alimentaires: Les lignes bougent! Med. Mal. Metab. 2010, 4, 473–474. [Google Scholar]
- Food and Drug Administration (FDA), Guidance for Industry, Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; Center for Drug Evaluation and Research, U.S. Department of Health and Human Services: Washington, WA, USA, 2005.
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Belhaj, N.; Dupuis, F.; Arab-Tehrany, E.; Denis, F.M.; Paris, C.; Lartaud, I.; Linder, M. Formulation, characterization and pharmacokinetic studies of coenzyme Q10 PUFA’s nanoemulsions. Eur. J. Pharm. Sci. 2012, 47, 305–312. [Google Scholar] [CrossRef]
- Colas, J.-C.; Shi, W.; Rao, V.S.N.M.; Omri, A.; Mozafari, M.R.; Singh, H. Microscopical investigations of nisin-loaded nanoliposomes prepared by Mozafari method and their bacterial targeting. Micron 2007, 38, 841–847. [Google Scholar]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes; European Union: Luxembourg, Luxembourg, 2010.
- Belzung, C. Measuring Rodent Exploratory Behavior. In Handbook of Molecular Genetic Techniques for Brain and Behaviour Research; Crusio, W., Gerlai, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 738–749. [Google Scholar]
- Bourin, M.; Hascoet, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Lister, R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 1987, 92, 180–185. [Google Scholar]
- Van Gaalen, M.M.; Steckler, T. Behavioural analysis of four mouse strains in an anxiety test battery. Behav. Brain Res. 2000, 115, 95–106. [Google Scholar] [CrossRef]
- Bilkei-Gorzo, A.; Gyertyan, I. Some doubts about the basic concept of hole-board test. Neurobiology (Bp.) 1996, 4, 405–415. [Google Scholar]
- Joanes, D.N.; Gill, C.A. Comparing measures of sample skewness and kurtosis. J. R. Stat. Soc. Ser. D 1998, 47, 183–189. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Belhaj, N.; Desor, F.; Gleizes, C.; Denis, F.M.; Arab-Tehrany, E.; Soulimani, R.; Linder, M. Anxiolytic-Like Effect of a Salmon Phospholipopeptidic Complex Composed of Polyunsaturated Fatty Acids and Bioactive Peptides. Mar. Drugs 2013, 11, 4294-4317. https://doi.org/10.3390/md11114294
Belhaj N, Desor F, Gleizes C, Denis FM, Arab-Tehrany E, Soulimani R, Linder M. Anxiolytic-Like Effect of a Salmon Phospholipopeptidic Complex Composed of Polyunsaturated Fatty Acids and Bioactive Peptides. Marine Drugs. 2013; 11(11):4294-4317. https://doi.org/10.3390/md11114294
Chicago/Turabian StyleBelhaj, Nabila, Frédéric Desor, Céline Gleizes, Frédéric M. Denis, Elmira Arab-Tehrany, Rachid Soulimani, and Michel Linder. 2013. "Anxiolytic-Like Effect of a Salmon Phospholipopeptidic Complex Composed of Polyunsaturated Fatty Acids and Bioactive Peptides" Marine Drugs 11, no. 11: 4294-4317. https://doi.org/10.3390/md11114294