Integrated Diagnostic Approach Using Basophil Activation Test and IgE Assays for Shrimp and Prawn Allergy †
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Recruitment and Allergic Testing
2.2. Measurement of Total IgE and sIgE
2.3. Basophil Activation Test
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics and Classifications of Participants
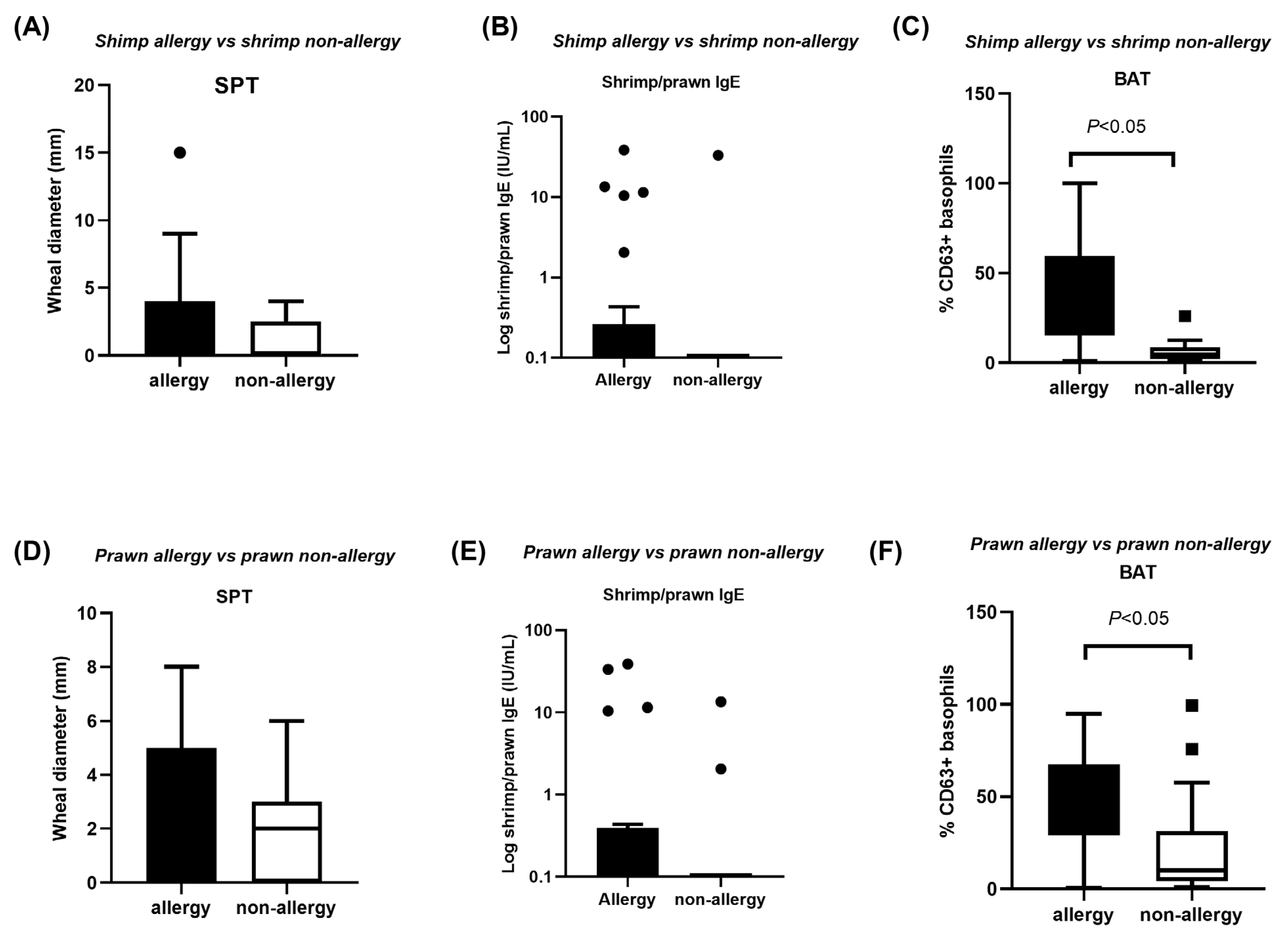
3.2. SPT, sIgE and BAT Indices in Shellfish Allergy Versus Non-Allergy
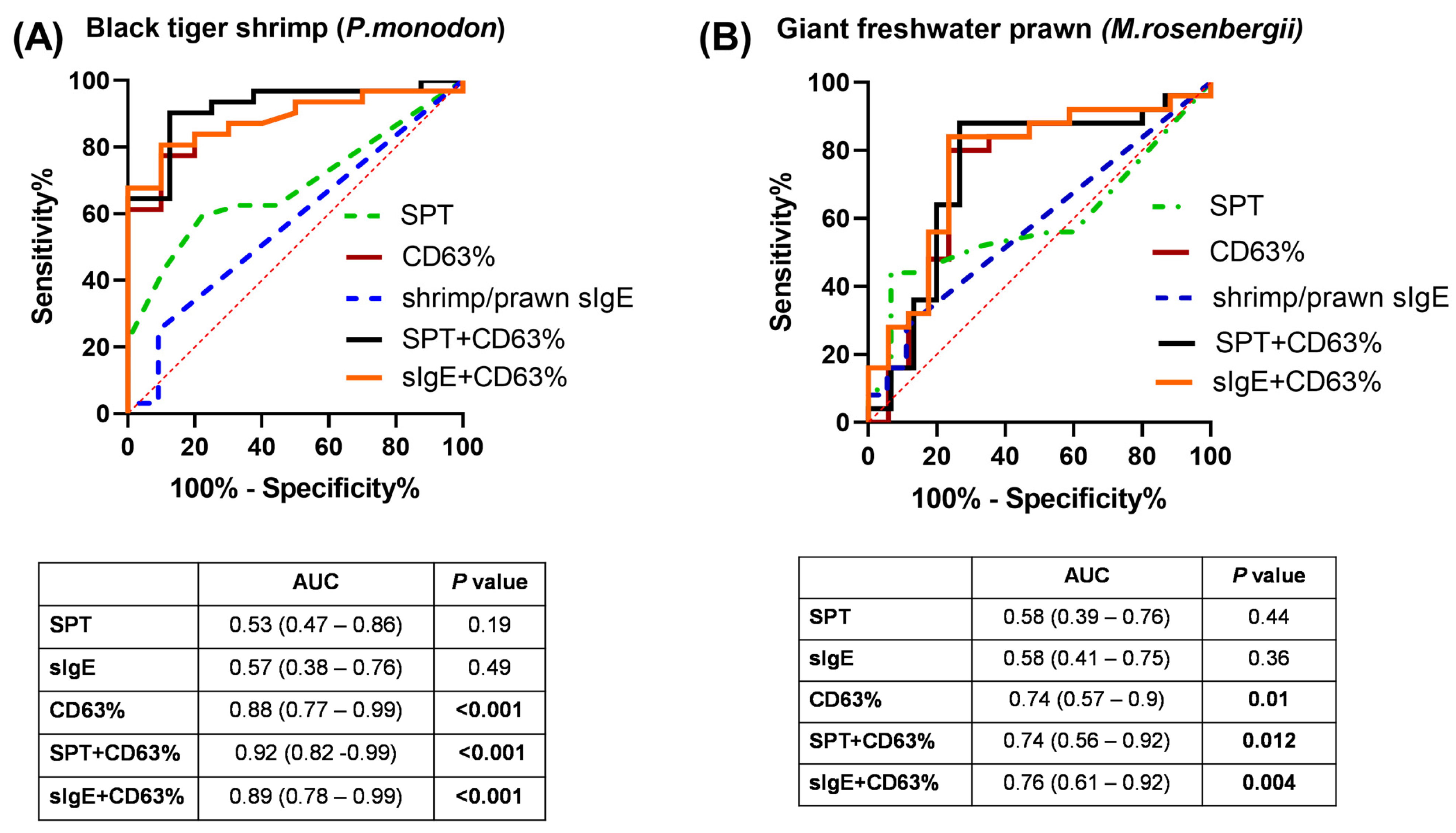
3.3. Diagnostic Values of BAT in Discriminating Shellfish-Allergic from Non-Allergic Subjects
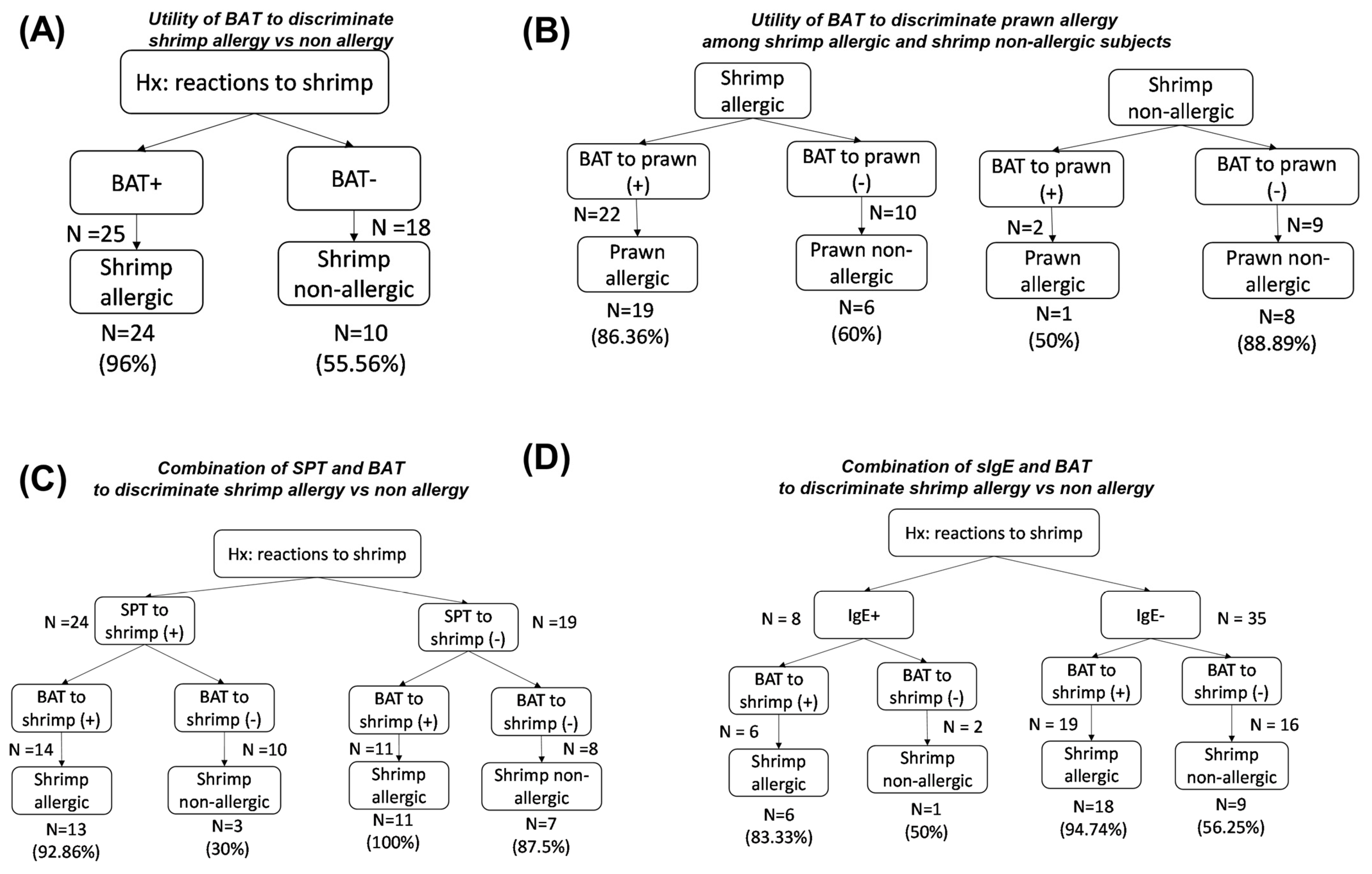
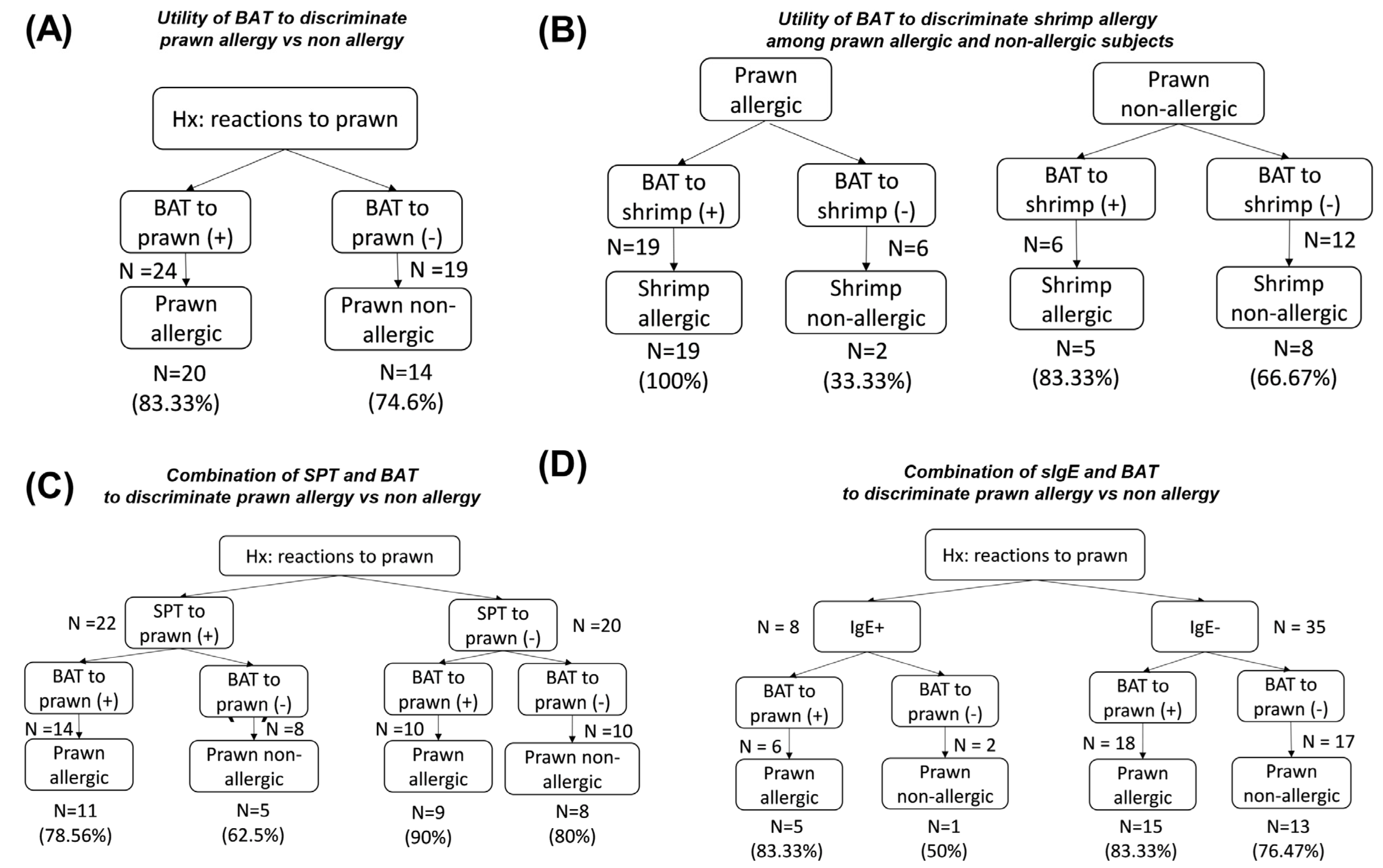
3.4. Combination of BAT and SPT, Shrimp/Prawn sIgE in Discriminating Shellfish-Allergic from Non-Allergic Subjects
3.5. Values of BAT and Allergic Testing in Predicting Reactivity to Shrimp or Prawn
- BAT as the first and only diagnostic test to diagnose shrimp or prawn allergy, as well as to predict reactions to other species;
3.5.1. Shrimp Allergy
3.5.2. Prawn Allergy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAT | Basophil activation test |
| IgE | Immunoglobulin E |
| Prawn | Giant tiger prawn |
| Shrimp | Black tiger shrimp |
| SPT | Skin prick test |
| sIgE | Specific IgE |
References
- Tong, W.S.; Yuen, A.W.; Wai, C.Y.; Leung, N.Y.; Chu, K.H.; Leung, P.S. Diagnosis of fish and shellfish allergies. J. Asthma Allergy 2018, 11, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Pawankar, R.; Canonica, G.W.; Holgate, S.T.; Lockey, R.F.; Blaiss, M.S. WAO white book on allergy. Milwaukee WI World Allergy Organ. 2011, 3, 156–157. [Google Scholar]
- Giovannini, M.; Beken, B.; Buyuktiryaki, B.; Barni, S.; Liccioli, G.; Sarti, L.; Lodi, L.; Pontone, M.; Bartha, I.; Mori, F. IgE-mediated shellfish allergy in children. Nutrients 2023, 15, 2714. [Google Scholar] [CrossRef]
- Wang, H.T.; Warren, C.M.; Gupta, R.S.; Davis, C.M. Prevalence and characteristics of shellfish allergy in the pediatric population of the United States. J. Allergy Clin. Immunol. Pract. 2020, 8, 1359–1370.e2. [Google Scholar] [CrossRef]
- Hajeb, P.; Selamat, J. A contemporary review of seafood allergy. Clin. Rev. Allergy Immunol. 2012, 42, 365–385. [Google Scholar] [CrossRef]
- Lee, A.J.; Thalayasingam, M.; Lee, B.W. Food allergy in Asia: How does it compare? Asia Pac. Allergy 2013, 3, 3–14. [Google Scholar] [CrossRef]
- Le, T.T.; Tran, T.T.; Ho, H.T.; Vu, A.T.; McBryde, E.; Lopata, A.L. The predominance of seafood allergy in Vietnamese adults: Results from the first population-based questionnaire survey. World Allergy Organ. J. 2020, 13, 100102. [Google Scholar] [CrossRef]
- Thalayasingam, M.; Gerez, I.; Yap, G.C.; Llanora, G.; Chia, I.; Chua, L.; Lee, C.; Ta, L.; Cheng, Y.; Thong, B. Clinical and immunochemical profiles of food challenge proven or anaphylactic shrimp allergy in tropical Singapore. Clin. Exp. Allergy 2015, 45, 687–697. [Google Scholar] [CrossRef]
- Wai, C.Y.; Leung, N.Y.; Leung, A.S.; Shum, Y.; Leung, P.S.; Chu, K.H.; Kwan, Y.W.; Lee, Q.U.; Wong, J.S.; Lam, I.C. Cell-based functional IgE assays are superior to conventional allergy tests for shrimp allergy diagnosis. J. Allergy Clin. Immunol. Pract. 2021, 9, 236–244.9. [Google Scholar] [CrossRef]
- Tsedendorj, O.; Chinuki, Y.; Ueda, K.; Kohno, K.; Adachi, A.; Morita, E. Tropomyosin is a minor but distinct allergen in patients with shrimp allergies in Japan. J. Cutan. Immunol. Allergy 2018, 1, 100–108. [Google Scholar] [CrossRef]
- McGowan, E.C.; Saini, S. Update on the performance and application of basophil activation tests. Curr. Allergy Asthma Rep. 2013, 13, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.; Santos, A.; Mayorga, C.; Nopp, A.; Eberlein, B.; Ferrer, M.; Rouzaire, P.; Ebo, D.; Sabato, V.; Sanz, M. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy 2015, 70, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Keswani, T.; Patil, S.U. Basophil activation test in food allergy: Is it ready for real-time? Curr. Opin. Allergy Clin. Immunol. 2021, 21, 442–447. [Google Scholar] [CrossRef]
- Ocmant, A.; Mulier, S.; Hanssens, L.; Goldman, M.; Casimir, G.; Mascart, F.; Schandené, L. Basophil activation tests for the diagnosis of food allergy in children. Clin. Exp. Allergy 2009, 39, 1234–1245. [Google Scholar] [CrossRef]
- Marcelino, J.; Baumann, K.; Skov, P.S.; Pereira Santos, M.C.; Wyroslak, I.; Scheffel, J.; Altrichter, S.; Woetmann, A.; Pereira-Barbosa, M.; Costa, C. What basophil testing tells us about CSU patients–results of the CORSA study. Front. Immunol. 2021, 12, 742470. [Google Scholar] [CrossRef]
- Ruethers, T.; Johnston, E.B.; Karnaneedi, S.; Nie, S.; Nugraha, R.; Taki, A.C.; Kamath, S.D.; Williamson, N.A.; Mehr, S.S.; Campbell, D.E. Commercial shellfish skin prick test extracts show critical variability in allergen repertoire. Allergy 2023, 78, 3261. [Google Scholar] [CrossRef]
- Dorney, R.D.; Johnston, E.B.; Karnaneedi, S.; Ruethers, T.; Kamath, S.D.; Gopi, K.; Mazumder, D.; Sammut, J.; Jerry, D.; Williamson, N.A. Variation in Shrimp Allergens: Place of Origin Effects on Food Safety Assessment. Int. J. Mol. Sci. 2024, 25, 4531. [Google Scholar] [CrossRef]
- Elizur, A.; Goldberg, M.R. Pro: Skin prick testing with fresh foods. Ann. Allergy Asthma Immunol. 2020, 124, 441–442. [Google Scholar] [CrossRef]
- Valenta, R.; Karaulov, A.; Niederberger, V.; Zhernov, Y.; Elisyutina, O.; Campana, R.; Focke-Tejkl, M.; Curin, M.; Namazova-Baranova, L.; Wang, J.-Y. Allergen extracts for in vivo diagnosis and treatment of allergy: Is there a future? J. Allergy Clin. Immunol. Pract. 2018, 6, 1845–1855.e2. [Google Scholar] [CrossRef]
- Trinh, T.; Duong, C.; Pham, T.; Au, T.H.; Tran, L.; Nguyen, C.; Nguyen, H.; Tran, N.; Phan, Q.; Le, T. Risk Factors for Severe Seafood Allergy in an Urban City in Vietnam. J. Allergy Clin. Immunol. 2023, 151, AB176. [Google Scholar] [CrossRef]
- Piboonpocanun, S.; Ittiporn, S.; Ubonsri, P.; Wangtan, A.; Pacharn, P.; Visitsunthorn, N.; Jirapongsananuruk, O. Defining Biomarkers to Predict Natural Resolution in Shrimp Allergy. Allergy Asthma Immunol. Res. 2022, 14, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Bird, J.A.; Leonard, S.; Groetch, M.; Assa’ad, A.; Cianferoni, A.; Clark, A.; Crain, M.; Fausnight, T.; Fleischer, D.; Green, T. Conducting an oral food challenge: An update to the 2009 adverse reactions to foods committee work group report. J. Allergy Clin. Immunol. Pract. 2020, 8, 75–90.e17. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Van Wijk, R.G.; Bindslev-Jensen, C.; Sicherer, S.; Teuber, S.S.; Burks, A.W.; Dubois, A.E.; Beyer, K.; Eigenmann, P.A.; Spergel, J.M. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology–European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J. Allergy Clin. Immunol. 2012, 130, 1260–1274. [Google Scholar] [PubMed]
- Van Kampen, V.; De Blay, F.; Folletti, I.; Kobierski, P.; Moscato, G.; Olivieri, M.; Quirce, S.; Sastre, J.; Walusiak-Skorupa, J.; Raulf-Heimsoth, M. EAACI position paper: Skin prick testing in the diagnosis of occupational type I allergies. Allergy 2013, 68, 580–584. [Google Scholar] [CrossRef]
- Trinh, H.K.T.; Le, K.M. Comparison of Conventional IgE Assay and Measurement of Specific IgE to Haemocyanin for the Diagnosis of Adult Crab Allergy. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2024, 55, 347–349. [Google Scholar] [CrossRef]
- Nguyen, N.Q.N.; Le, K.-M.; Nguyen, H.T.; Duong, B.T.; Pham, L.D.; Trinh, H.K.T. The Application of Basophil Activation Test in Seafood Allergy Diagnosis: The Preliminary Results. In Proceedings of the International Conference on the Development of Biomedical Engineering, Ho Chi Minh, Vietnam, 27–29 December 2022; pp. 1102–1112. [Google Scholar]
- Imakiire, R.; Fujisawa, T.; Nagao, M.; Tokuda, R.; Hattori, T.; Kainuma, K.; Kawano, Y. Basophil activation test based on CD203c expression in the diagnosis of fish allergy. Allergy Asthma Immunol. Res. 2020, 12, 641. [Google Scholar] [CrossRef]
- JASP, version 0.18.3. [Computer software]. JASP: Amsterdam, The Netherlands, 2024.
- Duan, L.; Celik, A.; Hoang, J.A.; Schmidthaler, K.; So, D.; Yin, X.; Ditlof, C.M.; Ponce, M.; Upton, J.E.; Lee, J.S. Basophil activation test shows high accuracy in the diagnosis of peanut and tree nut allergy: The Markers of Nut Allergy Study. Allergy 2021, 76, 1800–1812. [Google Scholar] [CrossRef]
- Santos, A.F.; Alpan, O.; Hoffmann, H.J. Basophil activation test: Mechanisms and considerations for use in clinical trials and clinical practice. Allergy 2021, 76, 2420–2432. [Google Scholar] [CrossRef]
- Chinthrajah, R.S.; Purington, N.; Andorf, S.; Rosa, J.S.; Mukai, K.; Hamilton, R.; Smith, B.M.; Gupta, R.; Galli, S.J.; Desai, M. Development of a tool predicting severity of allergic reaction during peanut challenge. Ann. Allergy Asthma Immunol. 2018, 121, 69–76.e2. [Google Scholar] [CrossRef]
- Santos, A.F.; Du Toit, G.; Douiri, A.; Radulovic, S.; Stephens, A.; Turcanu, V.; Lack, G. Distinct parameters of the basophil activation test reflect the severity and threshold of allergic reactions to peanut. J. Allergy Clin. Immunol. 2015, 135, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Rubio, A.; Vivinus-Nébot, M.; Bourrier, T.; Saggio, B.; Albertini, M.; Bernard, A. Benefit of the basophil activation test in deciding when to reintroduce cow’s milk in allergic children. Allergy 2011, 66, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Lötzsch, B.; Dölle, S.; Vieths, S.; Worm, M. Exploratory analysis of CD63 and CD203c expression in basophils from hazelnut sensitized and allergic individuals. Clin. Transl. Allergy 2016, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Kleine-Tebbe, J.; Erdmann, S.; Knol, E.F.; MacGlashan, D.W., Jr.; Poulsen, L.K.; Gibbs, B.F. Diagnostic tests based on human basophils: Potentials, pitfalls and perspectives. Int. Arch. Allergy Immunol. 2006, 141, 79–90. [Google Scholar] [CrossRef]
- Gámez, C.; Sanchez-Garcia, S.; Ibáñez, M.; López, R.; Aguado, E.; López, E.; Sastre, B.; Sastre, J.; Del Pozo, V. Tropomyosin IgE-positive results are a good predictor of shrimp allergy. Allergy 2011, 66, 1375–1383. [Google Scholar] [CrossRef]
- Yadzir, Z.H.; Misnan, R.; Abdullah, N.; Bakhtiar, F.; Arip, M.; Murad, S. Identification of the major allergen of Macrobrachium rosenbergii (giant freshwater prawn). Asian Pac. J. Trop. Biomed. 2012, 2, 50–54. [Google Scholar] [CrossRef]
- Lv, L.; Wei, F.; Liu, L.; Song, F.; Hou, X.; Yang, Q. Study on the Allergenicity of Tropomyosin from Different Aquatic Products Based on Conformational and Linear Epitopes Analysis. J. Agric. Food Chem. 2025, 73, 4936–4946. [Google Scholar] [CrossRef]
- García-Orozco, K.D.; Aispuro-Hernández, E.; Yepiz-Plascencia, G.; Calderón-de-la-Barca, A.M.; Sotelo-Mundo, R.R. Molecular characterization of arginine kinase, an allergen from the shrimp Litopenaeus vannamei. Int. Arch. Allergy Immunol. 2007, 144, 23–28. [Google Scholar] [CrossRef]
- Wai, C.Y.Y.; Leung, N.Y.H. Comprehending the allergen repertoire of shrimp for precision molecular diagnosis of shrimp allergy. Allergy 2022, 77, 3041–3051. [Google Scholar] [CrossRef]
- Schiaffino, S.; Rossi, A.C.; Smerdu, V.; Leinwand, L.A.; Reggiani, C. Developmental myosins: Expression patterns and functional significance. Skelet. Muscle 2015, 5, 22. [Google Scholar] [CrossRef]
- Daghlas, S.A.; Mohiuddin, S.S. Biochemistry, Glycogen; StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Chiumiento, I.R.; Ituarte, S.; Sun, J.; Qiu, J.W.; Heras, H.; Dreon, M.S. Hemocyanin of the caenogastropod Pomacea canaliculata exhibits evolutionary differences among gastropod clades. PLoS ONE 2020, 15, e0228325. [Google Scholar] [CrossRef] [PubMed]
- Morales-Amparano, M.B.; Teran, M.G.; Huerta-Ocampo, J.Á.; Teran, L.M. Impact of enolase in allergic disease. Curr. Allergy Asthma Rep. 2024, 24, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Windle, C.L.; Berry, A.; Nelson, A. Aldolase-catalysed stereoselective synthesis of fluorinated small molecules. Curr. Opin. Chem. Biol. 2017, 37, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, B.M.; Ribeiro, V.G.; do Nascimento Campos, N.; Mota, L.G.d.S.R.; Moreira, M.F. A comprehensive review of arginine kinase proteins: What we need to know? Biochem. Biophys. Rep. 2024, 40, 101837. [Google Scholar] [CrossRef]
- Manstein, D.J.; Meiring, J.; Hardeman, E.; Gunning, P.W. Actin–tropomyosin distribution in non-muscle cells. J. Muscle Res. Cell Motil. 2020, 41, 11–22. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, T.; Li, M.; Yun, X.; Huan, F.; Liu, Q.; Hu, M.; Wei, X.; Zheng, P.; Liu, G. Crystal structure analysis of sarcoplasmic-calcium-binding protein: An allergen in Scylla paramamosain. J. Agric. Food Chem. 2023, 71, 1214–1223. [Google Scholar] [CrossRef]
- Nziengui, H.; Bouhidel, K.; Pillon, D.; Der, C.; Marty, F.; Schoefs, B. Reticulon-like proteins in Arabidopsis thaliana: Structural organization and ER localization. FEBS Lett. 2007, 581, 3356–3362. [Google Scholar] [CrossRef]
- Warren, W.G.; Osborn, M.; Duffy, P.; Yates, A.; O’Sullivan, S.E. Potential safety implications of fatty acid-binding protein inhibition. Toxicol. Appl. Pharmacol. 2024, 491, 117079. [Google Scholar] [CrossRef]
- Santos, A.F.; Douiri, A.; Bécares, N.; Wu, S.Y.; Stephens, A.; Radulovic, S.; Chan, S.M.; Fox, A.T.; Du Toit, G.; Turcanu, V.; et al. Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J. Allergy Clin. Immunol. 2014, 134, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Jaumdally, H.; Kwok, M.; Jama, Z.; Hesse-Lamptey, R.; McKendry, R.; Galvez, O.; Daniel, Y.; Santos, A.F. Basophil activation test has high reproducibility and is feasible in the clinical setting. Pediatr. Allergy Immunol. 2022, 33, e13870. [Google Scholar] [CrossRef]
- Wai, C.Y.Y.; Leung, N.Y.H.; Leung, A.S.Y.; Wong, G.W.K.; Leung, T.F. Seafood Allergy in Asia: Geographical Specificity and Beyond. Front. Allergy 2021, 2, 676903. [Google Scholar] [CrossRef] [PubMed]
- Le, K.-M.; Trinh, H.K.T.; Van, T.N.V.; Le Pham, D.; Nguyen, H.T.; Tran, T.-T.; Thi, M.N.T.; Pham, B.Y.; Pham, D.M. Hemocyanin is a major allergen in local mud crab Scylla paramamosain in Viet Nam. Biomed. Res. Ther. 2024, 11, 6891–6899. [Google Scholar] [CrossRef]
- Wood, R.A.; Chinthrajah, R.S.; Rudman Spergel, A.K.; Babineau, D.C.; Sicherer, S.H.; Kim, E.H.; Shreffler, W.G.; Jones, S.M.; Leung, D.Y.M.; Vickery, B.P.; et al. Protocol design and synopsis: Omalizumab as Monotherapy and as Adjunct Therapy to Multiallergen OIT in Children and Adults with Food Allergy (OUtMATCH). J. Allergy Clin. Immunology. Glob. 2022, 1, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Groetch, M.; Mudd, K.; Woch, M.; Schaible, A.; Gray, B.E.; Babineau, D.C.; Bird, J.A.; Jones, S.; Kim, E.H.; Lanser, B.J.; et al. Retail Food Equivalents for Post-Oral Immunotherapy Dosing in the Omalizumab as Monotherapy and as Adjunct Therapy to Multi-Allergen Oral Immunotherapy in Food-Allergic Children and Adults (OUtMATCH) Clinical Trial. J. Allergy Clin. Immunology. Pract. 2023, 11, 572–580.e2. [Google Scholar] [CrossRef]
- Nguyen, N.N.Q.; Nguyen, T.H.; Le, M.K.; Duong, B.T.; Tran, T.T.; Pham, D.L.; Trinh, T.H.K. In CD63 expression-based basophil activation test is potential in discovering shellfish allergy. In Proceedings of the Annual Conference of Ho Chi Minh Society of Asthma, Allergy and Clinical Immunology, Nha Trang, Vietnam, 30–31 May 2025. [Google Scholar]
| Characteristics | Total (N = 43) |
|---|---|
| Age | 29 ± 8.66 (*) |
| Sex (female, %) | 29 (67.44%) |
| Comorbid fish allergy | 3 (6.98%) |
| Total IgE | 1222 ± 3216.82 (*) |
| Wheal diameter of SPT to Der P (mm) | 5 ± 5.10 (*) |
| Wheal diameter of SPT to Der F (mm) | 5 ± 6.27 (*) |
| Shrimp allergy | 32 (74.41%) |
| Specific allergy to shrimp | 9 (26.5%) |
| Prawn allergy | 25 (58.14%) |
| Specific allergy to prawn | 2 (5.9%) |
| Both shrimp and prawn allergy | 23 (67.6%) |
| Sensitivity | Specificity | Cutoff | p-Value | PPV | NPV | LR+ | LR− | |
|---|---|---|---|---|---|---|---|---|
| Shrimp | 0.77 | 0.90 | 13.95% | <0.001 * | 0.96 ± 0.04 (0.84–0.99) | 0.56 ± 0.12 (0.33–0.77) | 7.5 | 0.28 |
| Prawn | 0.88 | 0.77 | 23.9% | <0.001 * | 0.83 ± 0.07 (0.66–0.95) | 0.74 ± 0.1 (0.52–0.89) | 3.64 | 0.26 |
| Allergens | Molecular Weight | Family | Characteristics |
|---|---|---|---|
| Myosin heavy chain | 98 kDa | Class II myosins |
|
| Glycogen phosphorylase | 95 kDa | Glycosyl hydrolase family 65 (GH65) in the CAZy (Carbohydrate-active enzymes) classification system |
|
| Hemocyanin | 75 kDa | Type-3 copper proteins |
|
| Enolase | 50 kDa | 2-phosphopyruvate hydratase |
|
| Aldolase | 40 kDa | Aldolase |
|
| Arginine kinase | 40 kDa | Phosphagen kinase |
|
| Tropomyosin | 40 kDa | Tropomyosin |
|
| Sarcoplasmic calcium-binding protein | 22 kDa | EF-hand calcium-binding protein |
|
| Reticulon-like protein | 20 kDa | Reticulon |
|
| Fatty acid-binding protein | 18 kDa | Fatty acid-binding protein |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.N.Q.; Nguyen, T.H.; Le, M.K.; Duong, T.B.; Pham, D.L.; Tran, T.T.; Trinh, T.H.K. Integrated Diagnostic Approach Using Basophil Activation Test and IgE Assays for Shrimp and Prawn Allergy. Medicina 2025, 61, 1040. https://doi.org/10.3390/medicina61061040
Nguyen NNQ, Nguyen TH, Le MK, Duong TB, Pham DL, Tran TT, Trinh THK. Integrated Diagnostic Approach Using Basophil Activation Test and IgE Assays for Shrimp and Prawn Allergy. Medicina. 2025; 61(6):1040. https://doi.org/10.3390/medicina61061040
Chicago/Turabian StyleNguyen, Nhu N. Q., Thao H. Nguyen, Minh K. Le, Tram B. Duong, Duy L. Pham, Tai T. Tran, and Tu H. K. Trinh. 2025. "Integrated Diagnostic Approach Using Basophil Activation Test and IgE Assays for Shrimp and Prawn Allergy" Medicina 61, no. 6: 1040. https://doi.org/10.3390/medicina61061040
APA StyleNguyen, N. N. Q., Nguyen, T. H., Le, M. K., Duong, T. B., Pham, D. L., Tran, T. T., & Trinh, T. H. K. (2025). Integrated Diagnostic Approach Using Basophil Activation Test and IgE Assays for Shrimp and Prawn Allergy. Medicina, 61(6), 1040. https://doi.org/10.3390/medicina61061040







