One-Step Microwave-Assisted Fabrication of Carbon Dots as Efficient Fluorescent Chemosensors for Hg2+ and Fe3+ Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
2.3. Synthesis of CDs
2.4. Detection of Hg2+ and Fe3+
2.5. Measurement of Fluorescence Quantum Yield (QY)
2.6. Limit of Detection (LOD) and Limit of Quantification (LOC) Estimation
2.7. Relative Fluorescence Change (F − F0)/F0
- Fluorescence Enhancement: In some cases, the metal ion can cause an “on–off” fluorescence response, where the fluorescence increases. In this case, F > F0, and (F − F0)/F0 becomes a positive value.
- Fluorescence Quenching: When a metal ion interacts with a sensor, it can quench the sensor’s fluorescence intensity. This leads to a decrease in F0 value, making (F − F0)/F0 a negative value. (Common in heavy metal ion detection).
2.8. F/F0
2.9. Relative Fluorescence Intensity (F0 − F)/F0
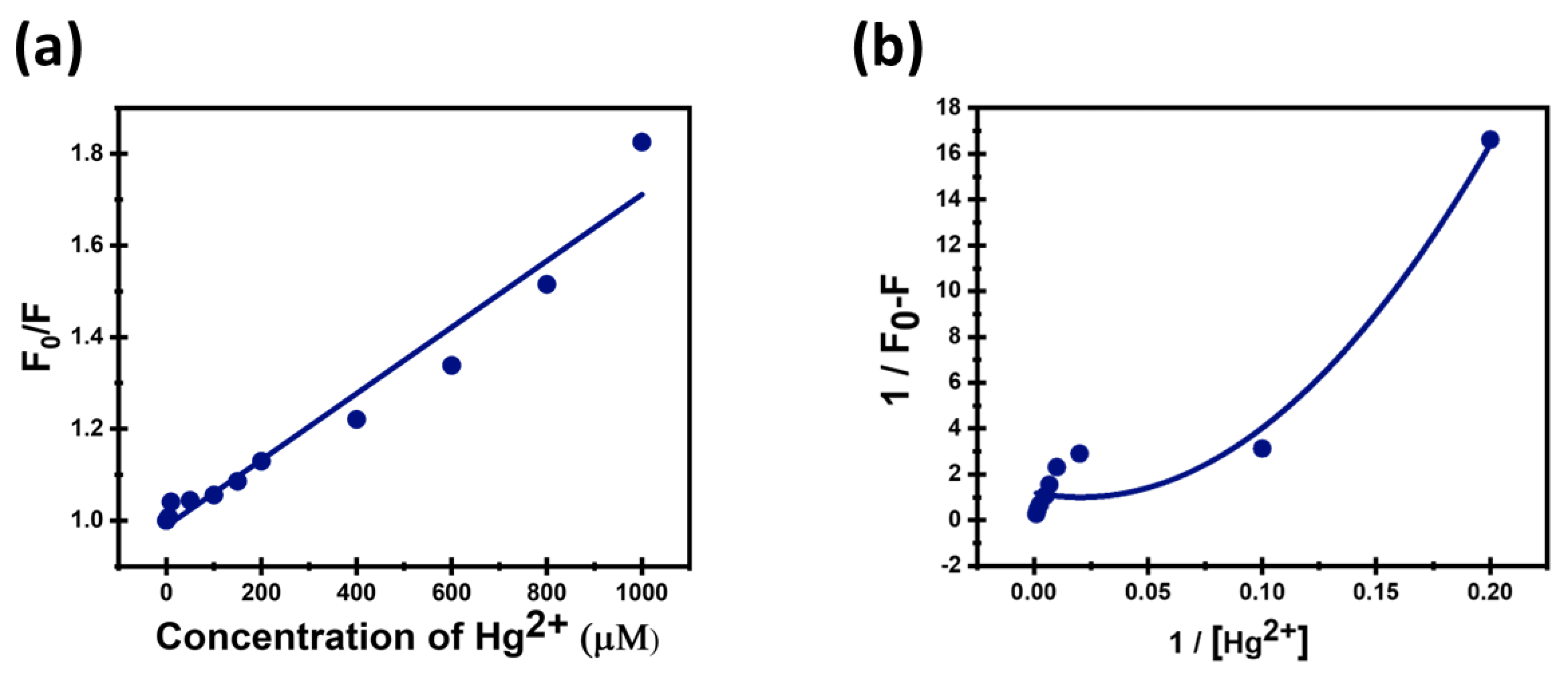
2.10. Stern–Volmer Plot
2.11. Benesi–Hildbrand Plot
2.12. Study of CD Stability
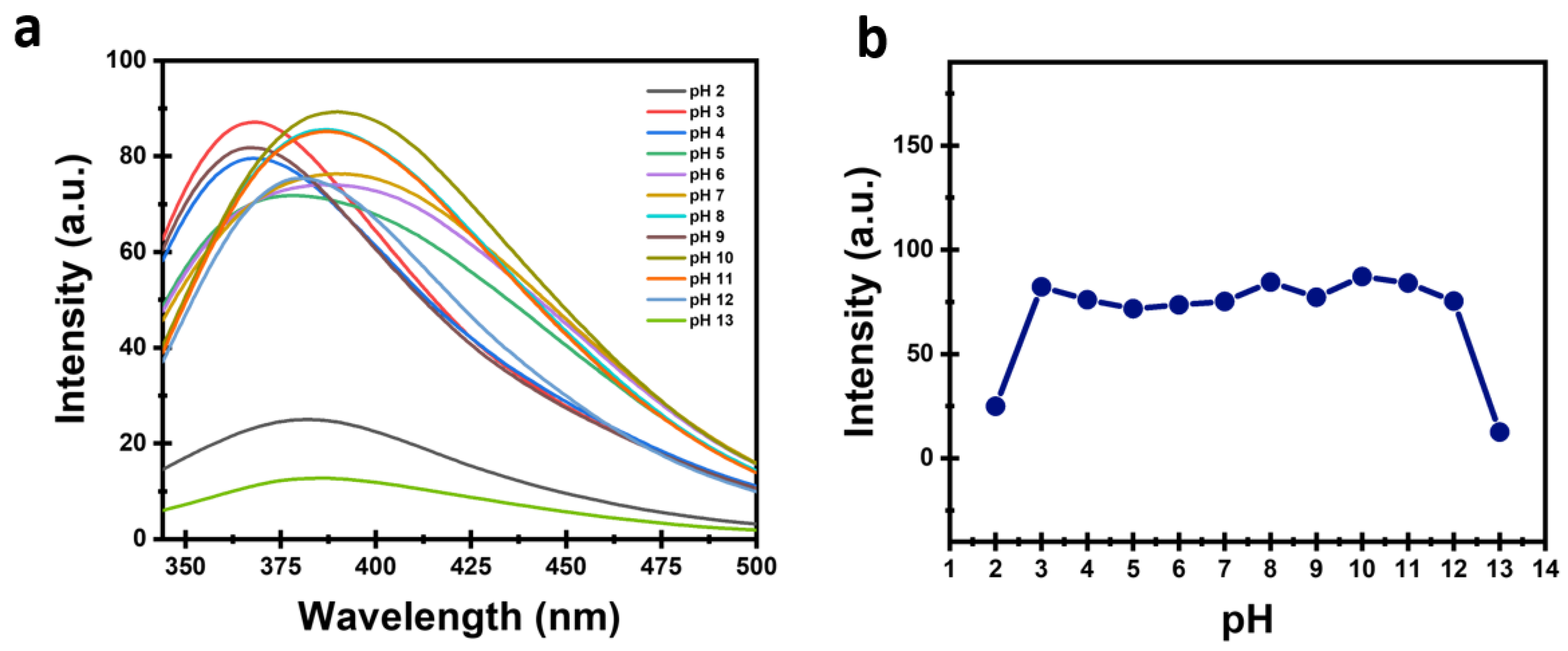
2.12.1. pH Stability
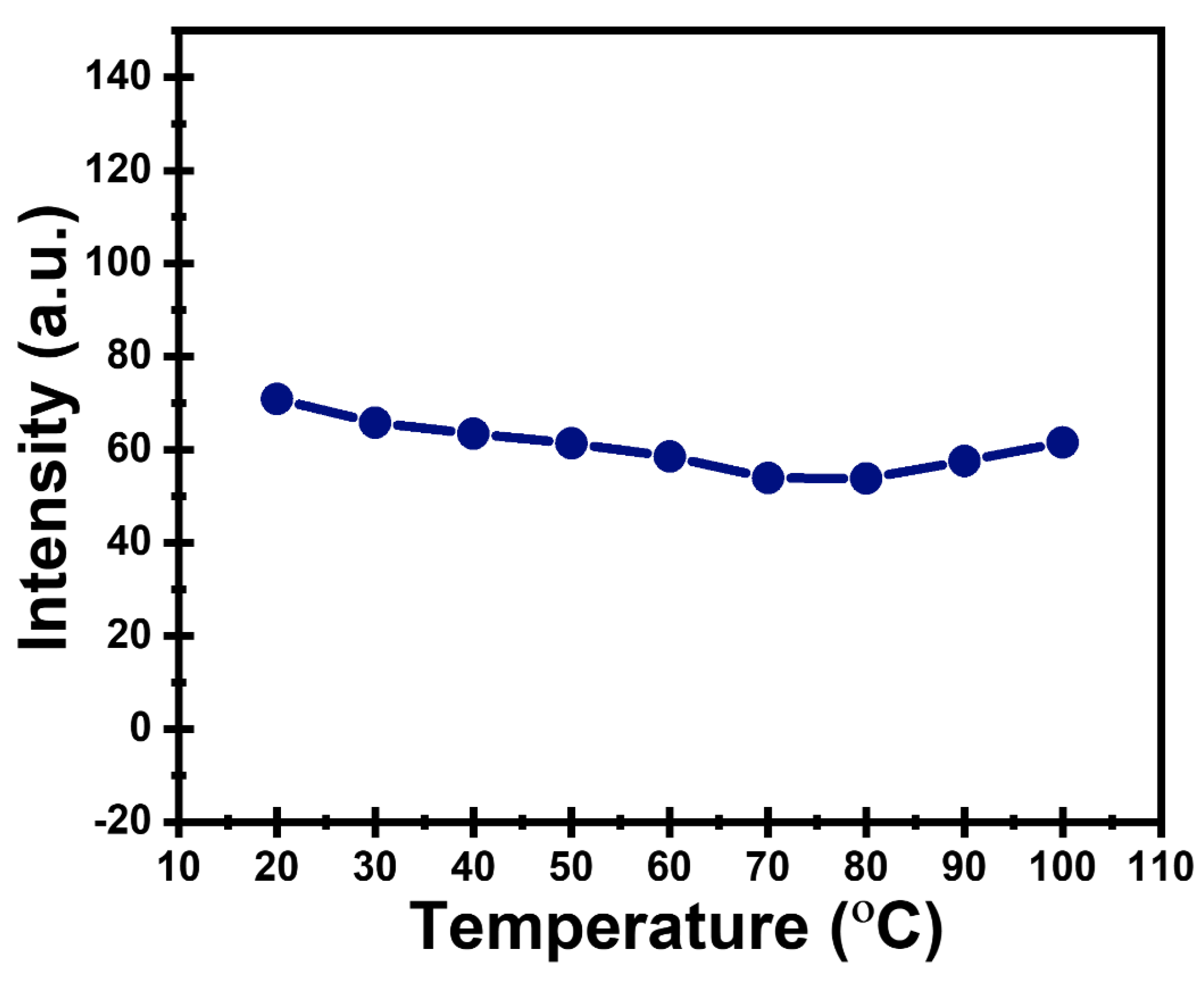
2.12.2. Thermal Stability
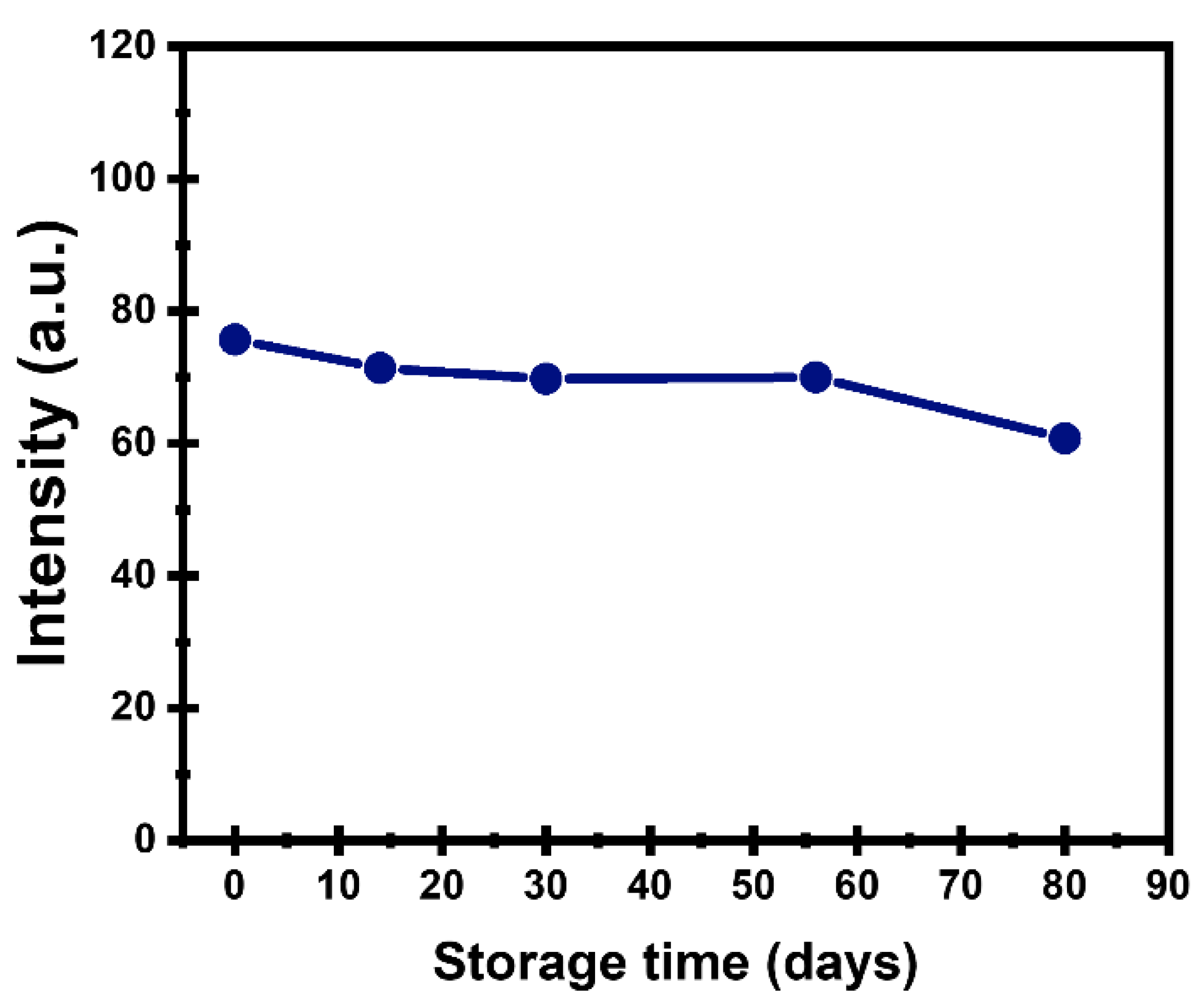
2.12.3. Storage Time Stability
3. Results and Discussion
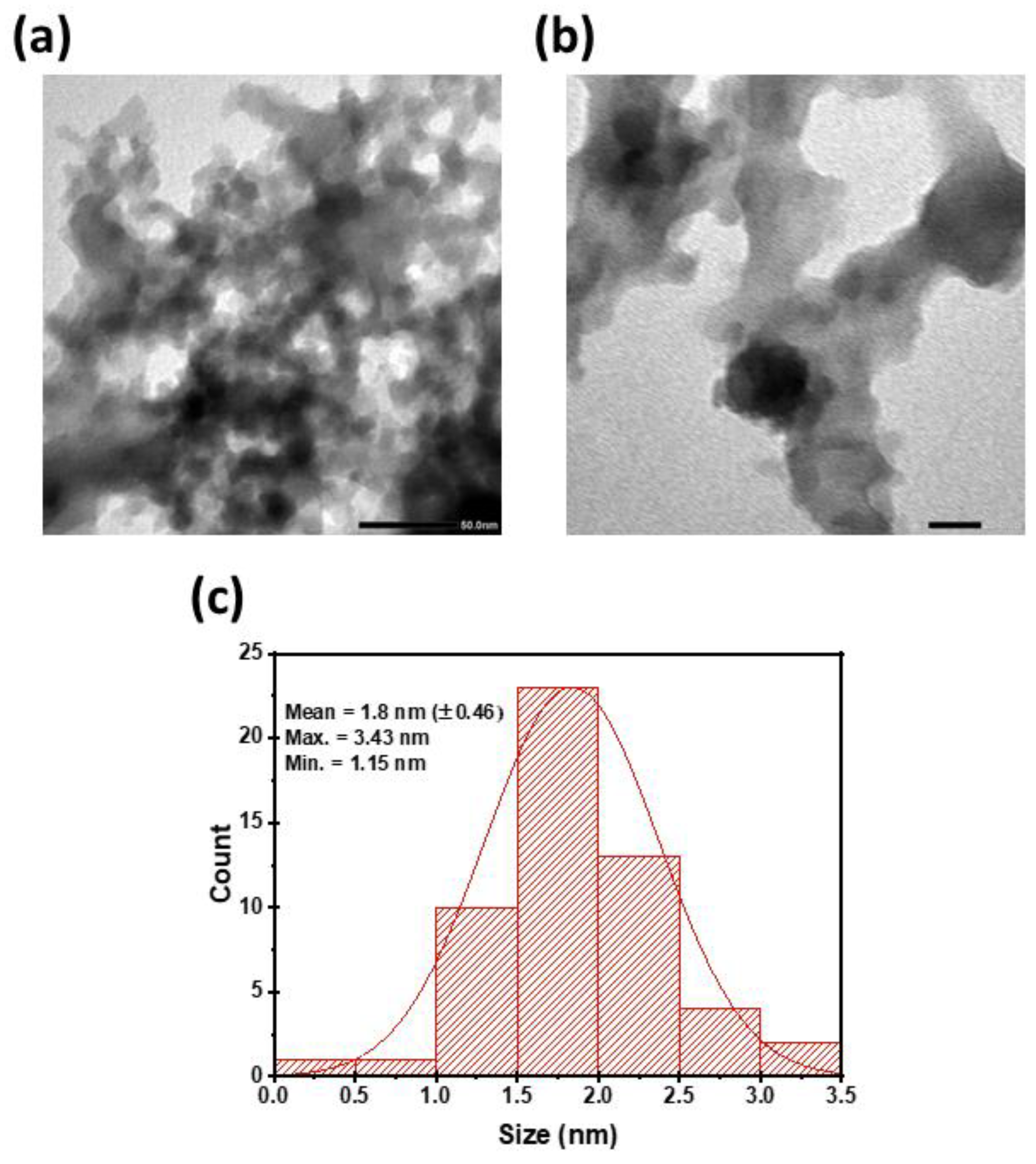
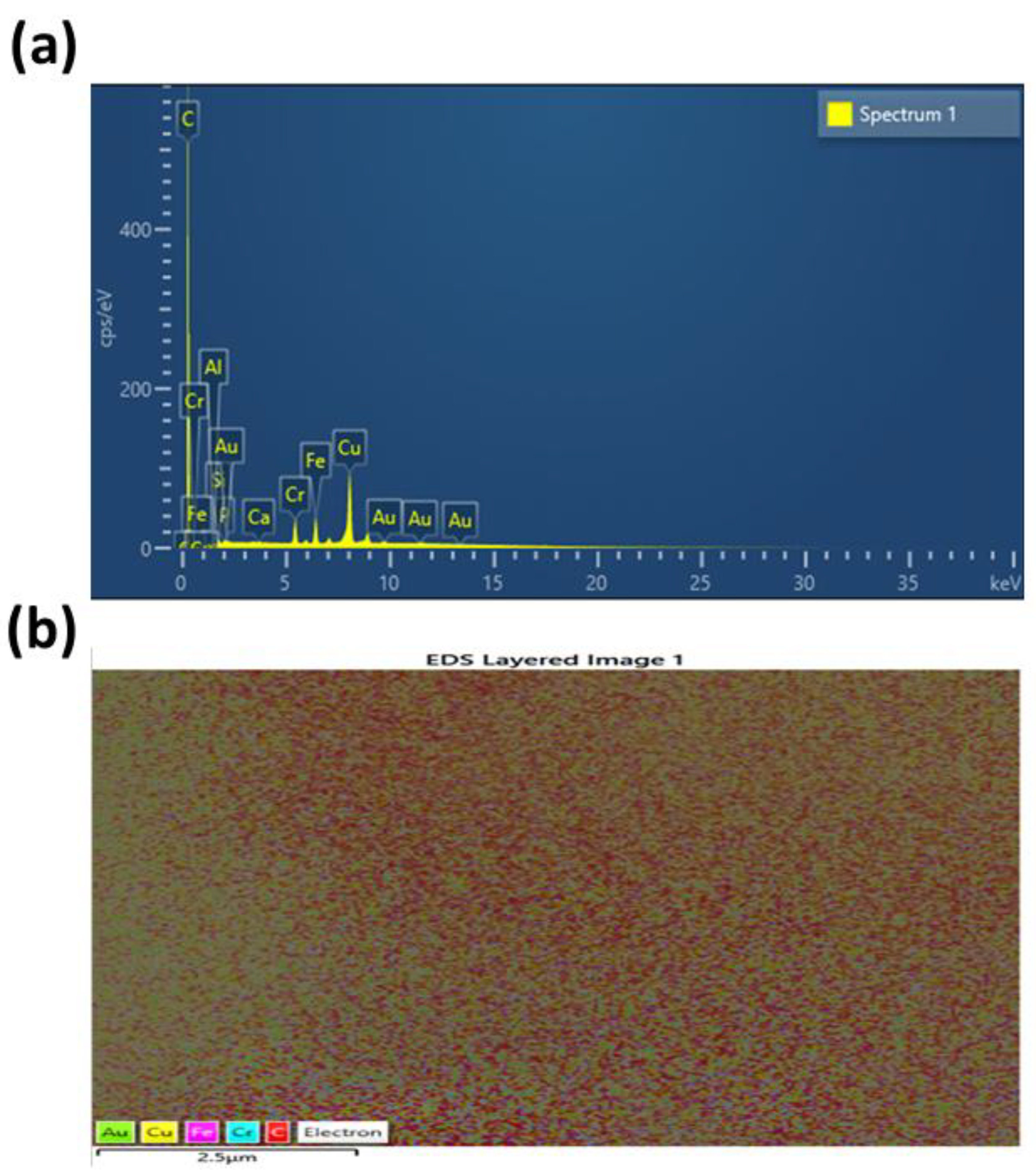
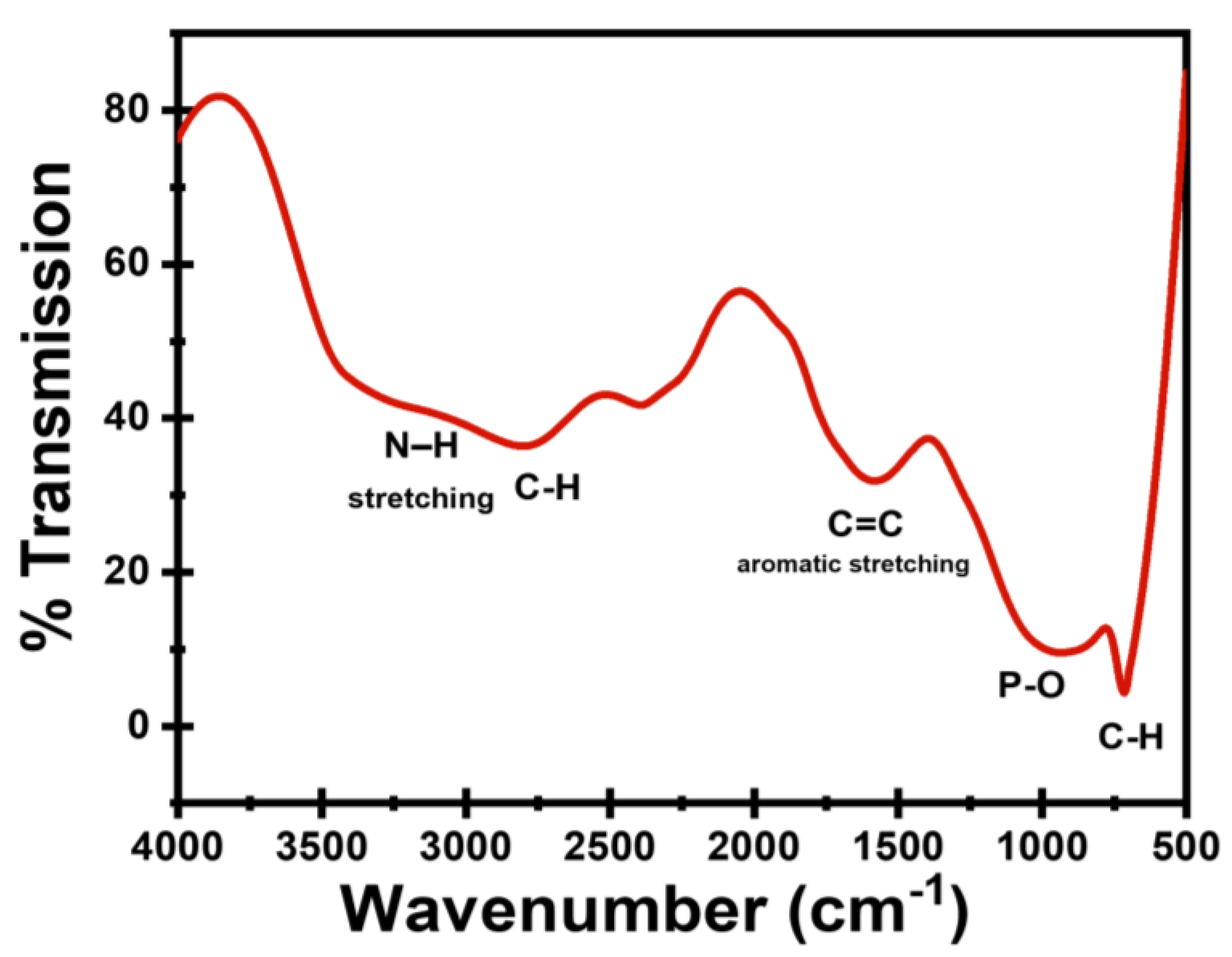
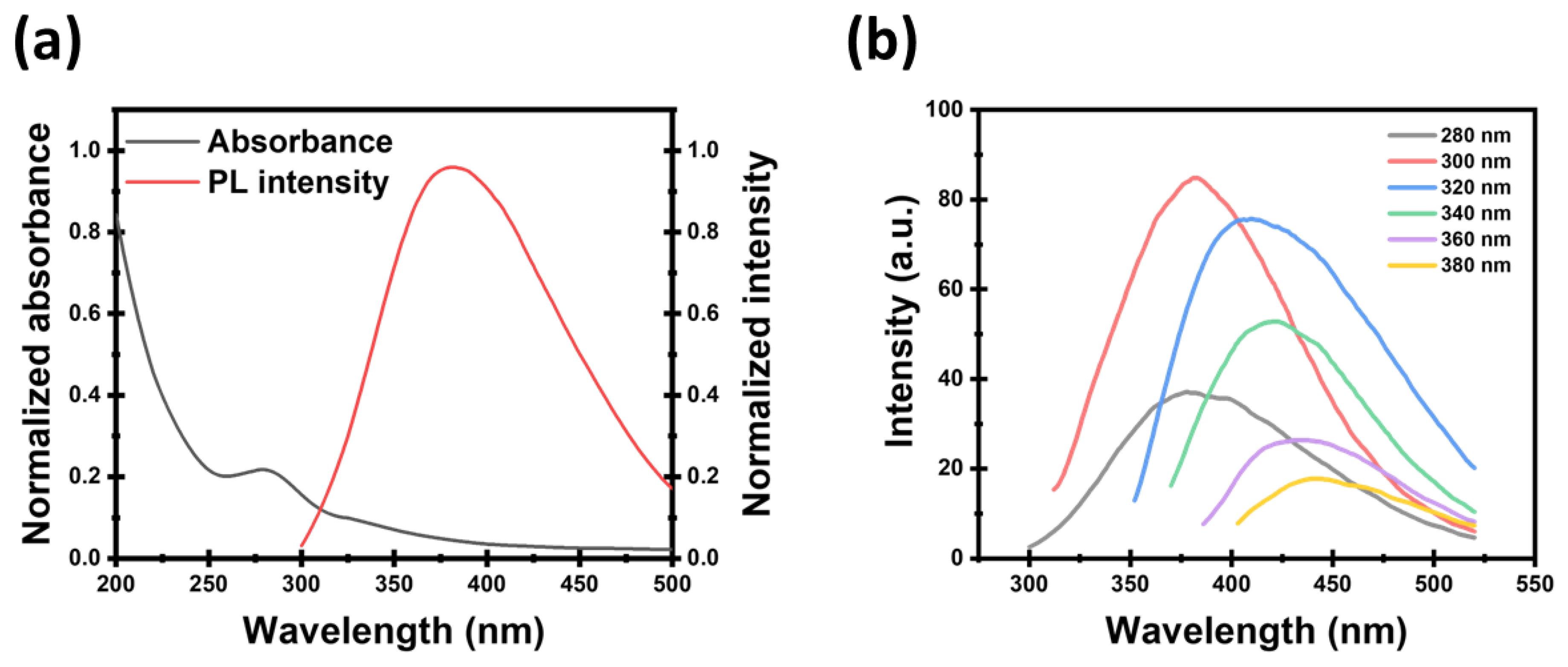
3.1. Characterization of CDs
3.2. Stability of CDs
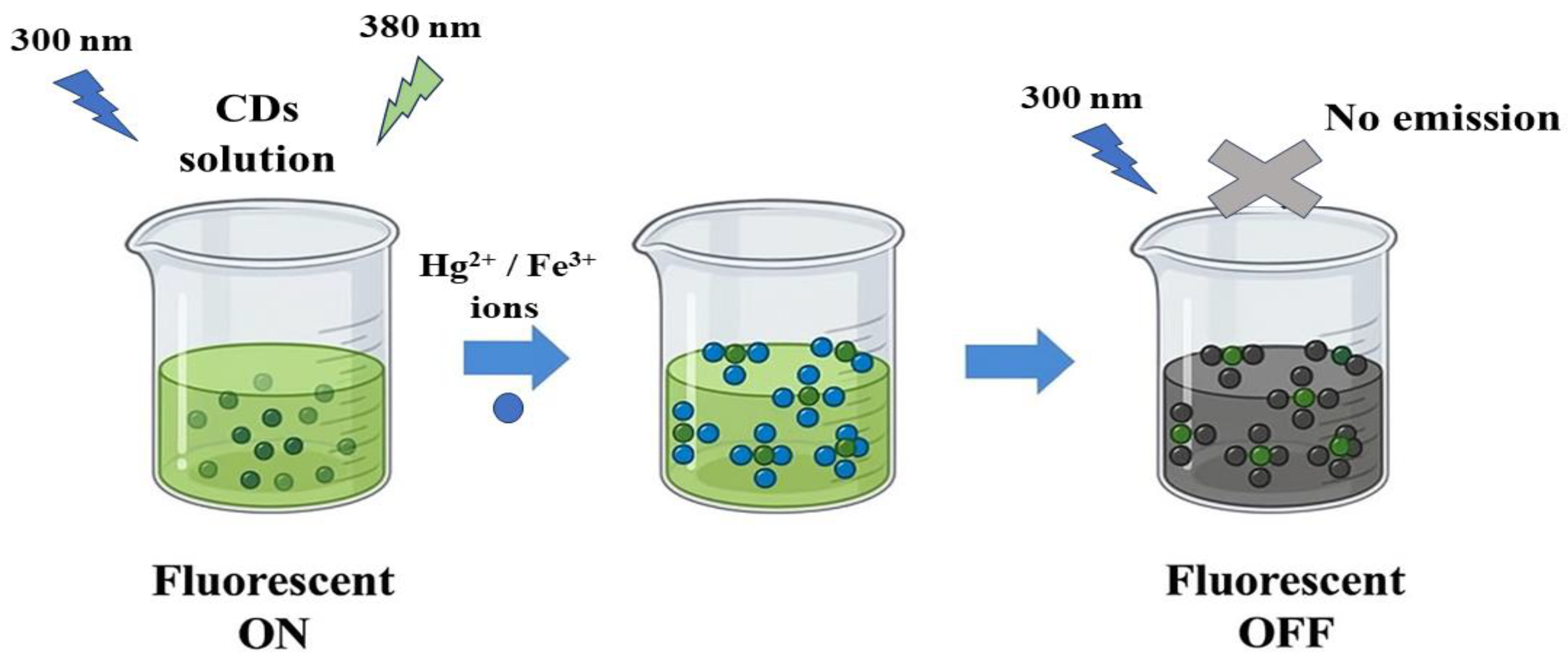
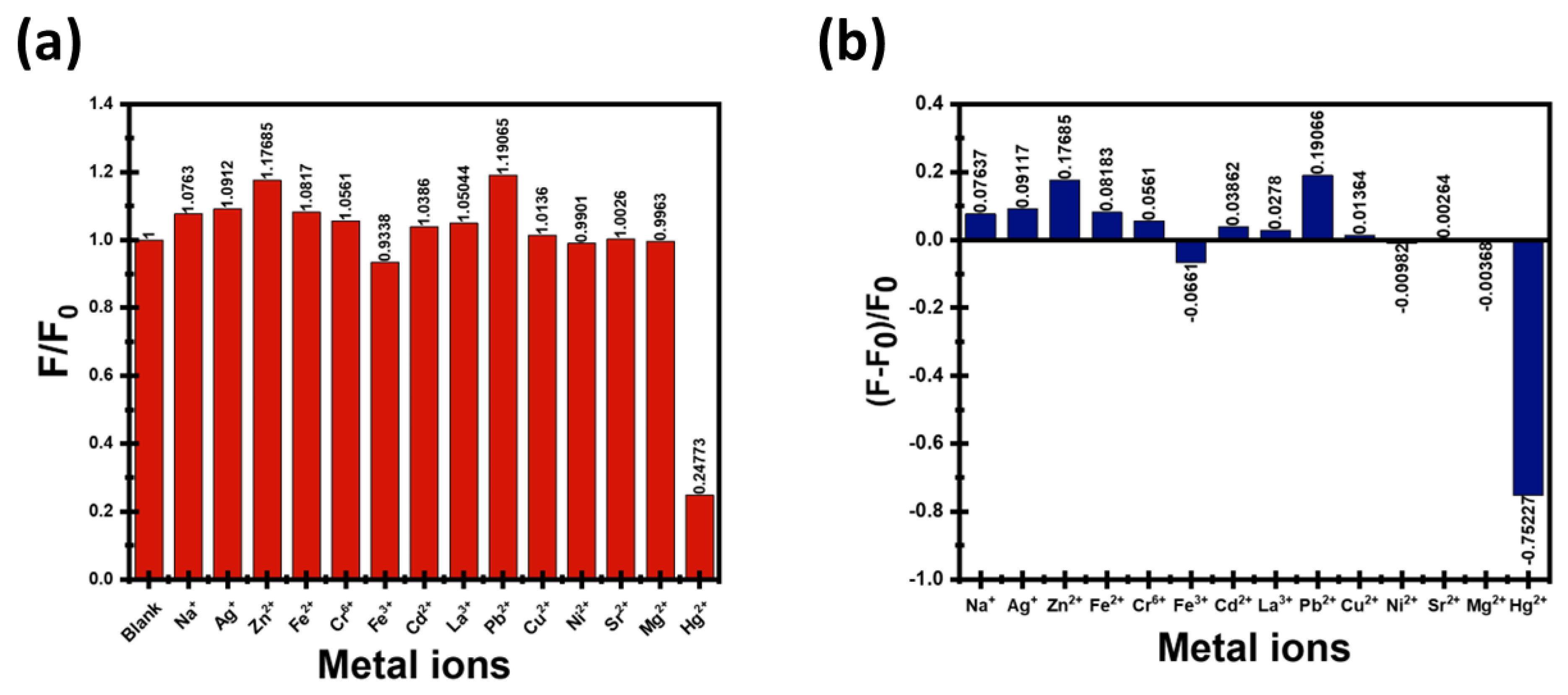
3.3. Selectivity of CDs Toward Hg2+ and Fe3+
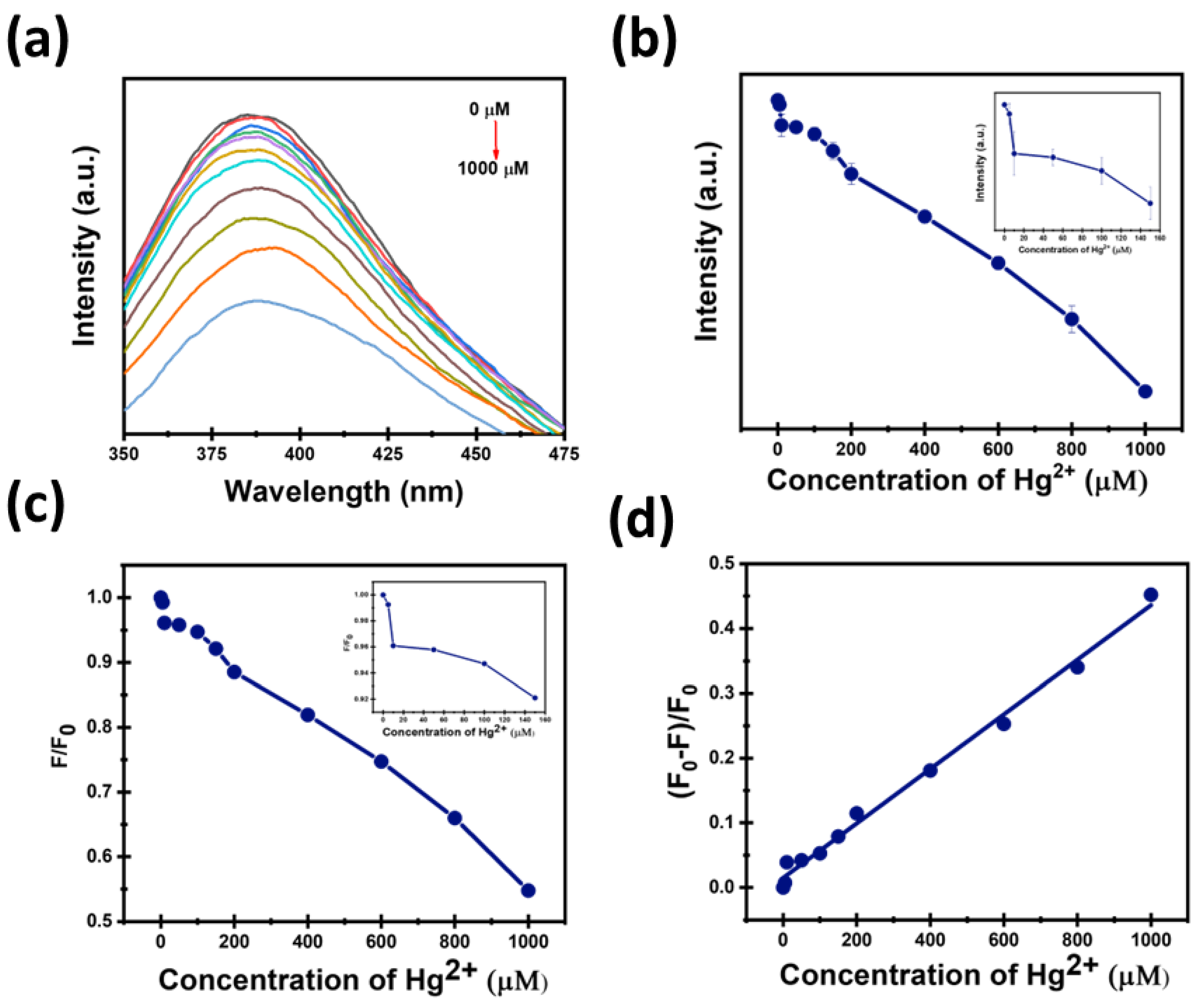
3.4. Sensitivity of CDs Toward Hg2+
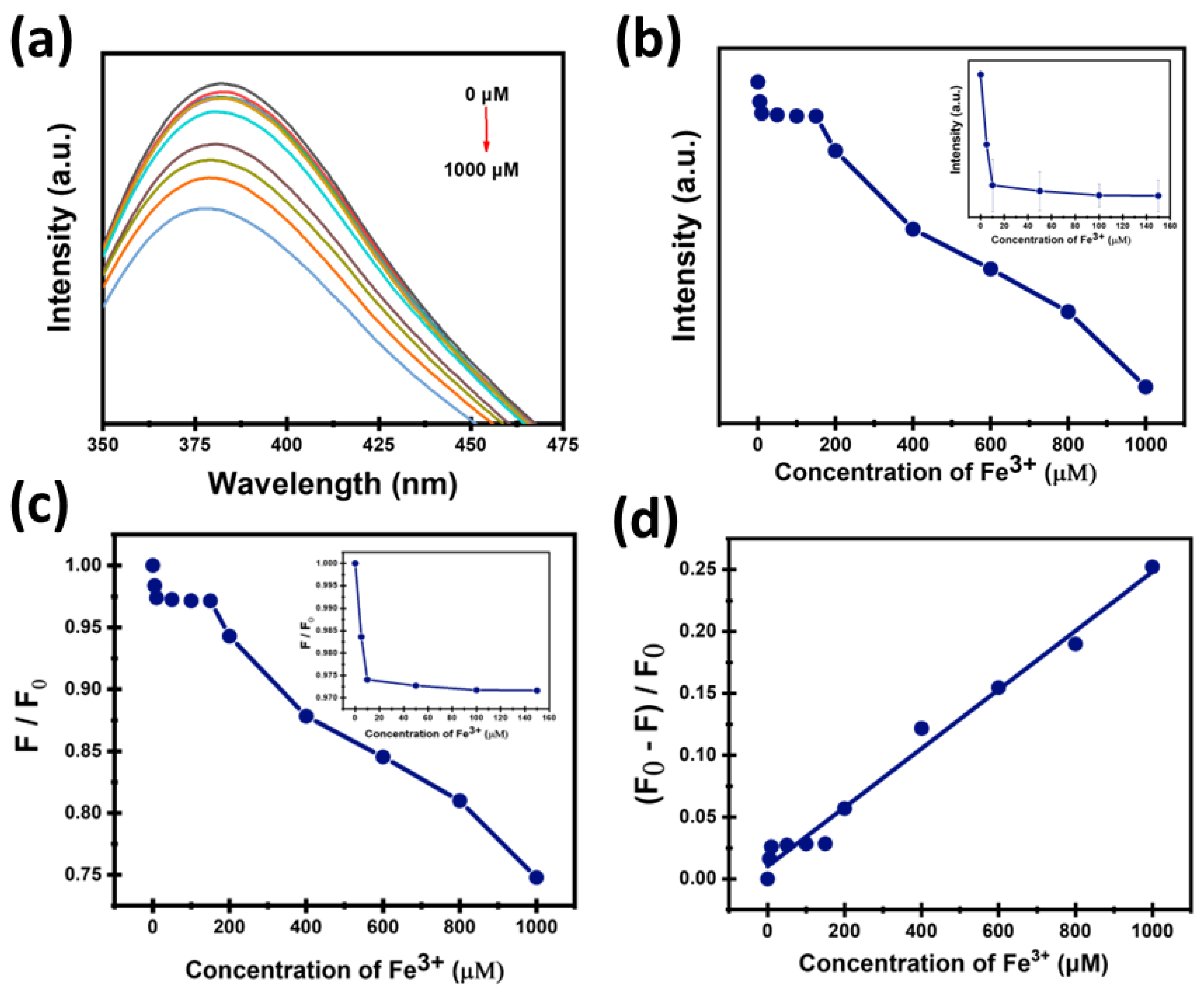
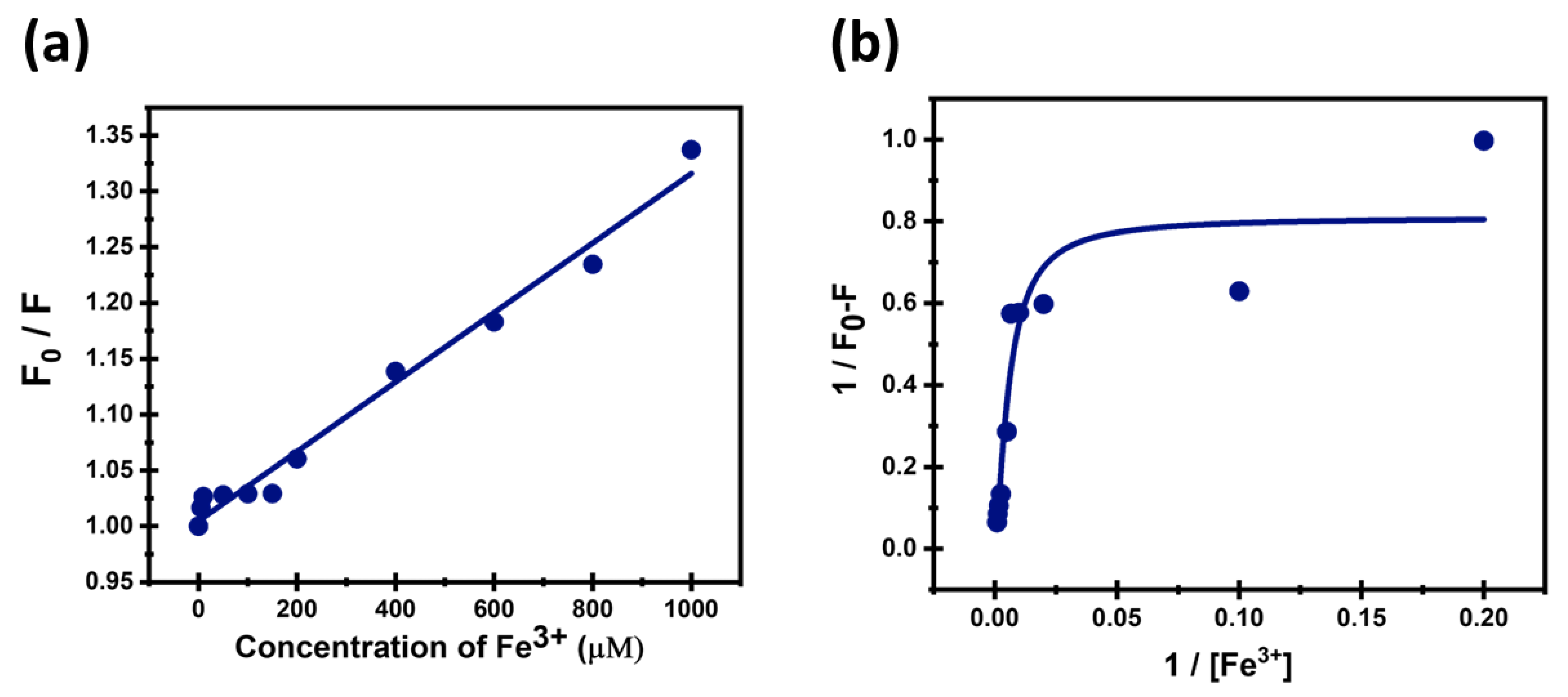
3.5. Sensitivity of CDs Toward Fe3+
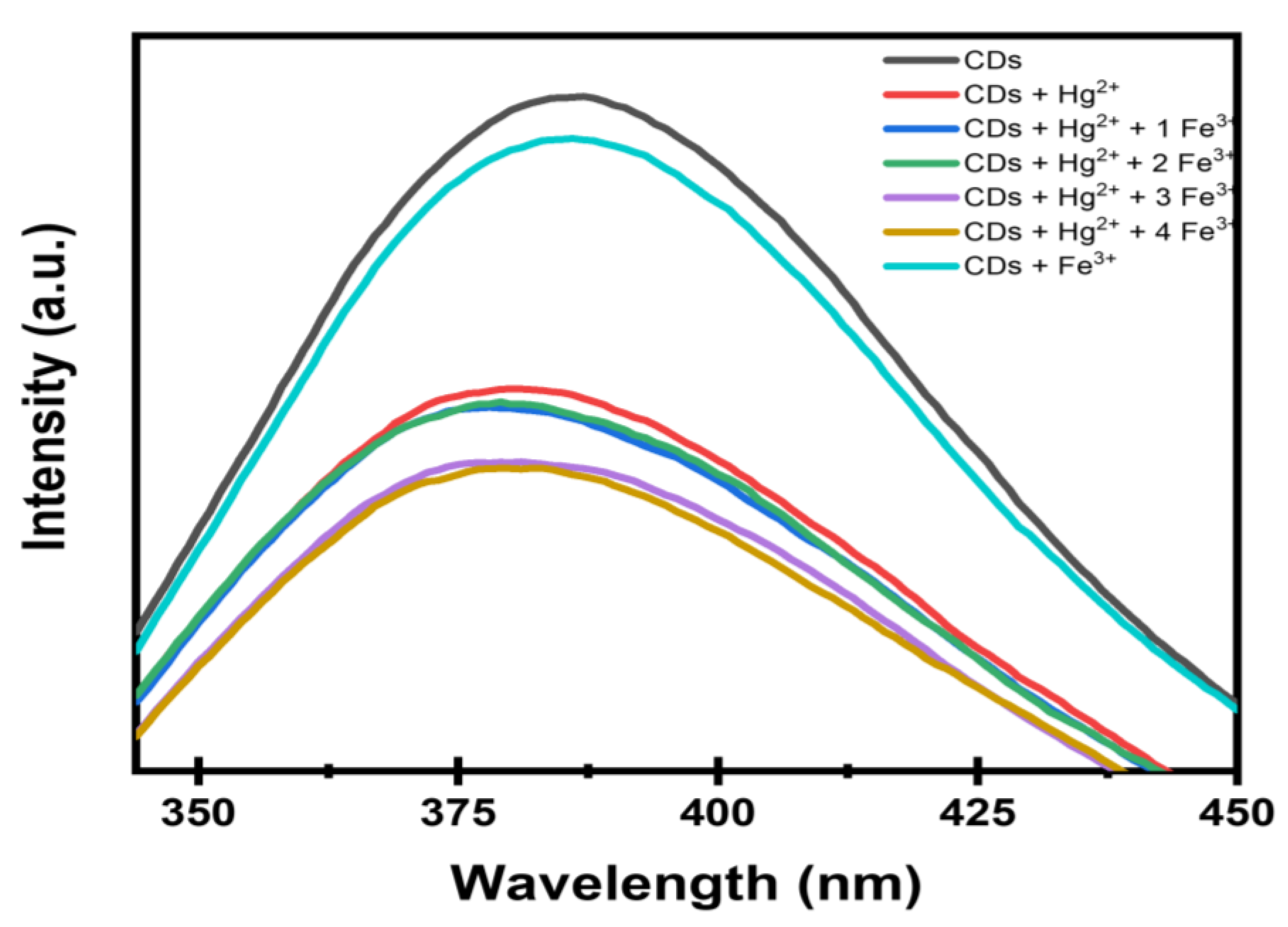
3.6. Simultaneous Detection of Hg2+ and Fe3+
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Z.; Zhou, Z. Green synthesis of nitrogen-doped carbon dots from Pueraria residues for use as a sensitive fluorescent probe for sensing Cr(VI) in water. Sensors 2025, 25, 5554. [Google Scholar] [CrossRef]
- Bajpai, S.K.; D’Souza, A.; Suhail, B. Blue light-emitting carbon dots (CDs) from a milk protein and their interaction with Spinacia oleracea leaf cells. Int. Nano Lett. 2019, 9, 203–212. [Google Scholar] [CrossRef]
- Cui, L.; Ren, X.; Sun, M.; Liu, H.; Xia, L. Carbon dots: Synthesis, properties and applications. Nanomaterials 2021, 11, 3419. [Google Scholar] [CrossRef]
- Döring, A.; Ushakova, E.; Rogach, A.L. Chiral carbon dots: Synthesis, optical properties, and emerging applications. Light Sci. Appl. 2022, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ge, J.; Liu, W.; Niu, G.; Jia, Q.; Wang, H.; Wang, P. Tunable multicolor carbon dots prepared from well-defined polythiophene derivatives and their emission mechanism. Nanoscale 2016, 8, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Travlou, N.A.; Giannakoudakis, D.A.; Algarra, M.; Labella, A.M.; Rodríguez-Castellón, E.; Bandosz, T.J. S- and N-doped carbon quantum dots: Surface chemistry dependent antibacterial activity. Carbon 2018, 135, 104–111. [Google Scholar] [CrossRef]
- Markovic, Z.M.; Labudova, M.; Danko, M.; Matijasevic, D.; Micusik, M.; Nadazdy, V.; Kovacova, M.; Kleinova, A.; Spitalsky, Z.; Pavlovic, V. Highly efficient antioxidant F-and Cl-doped carbon quantum dots for bioimaging. ACS Sustain. Chem. Eng. 2020, 8, 16327–16338. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, W.; Song, W.; Liu, J.; Ren, C.; Wu, J.; Liu, D.; Chen, H. Red emission B, N, S-co-doped carbon dots for colorimetric and fluorescent dual mode detection of Fe3+ ions in complex biological fluids and living cells. ACS Appl. Mater. Interfaces 2017, 9, 12663–12672. [Google Scholar] [CrossRef]
- Magesh, V.; Sundramoorthy, A.K.; Ganapathy, D. Recent advances on synthesis and potential applications of carbon quantum dots. Front. Mater. 2022, 9, 906838. [Google Scholar] [CrossRef]
- Mirlou-Miavagh, F.; Rezvani-Moghaddam, A.; Roghani-Mamaqani, H.; Sundararaj, U. Comparative study of synthesis of carbon quantum dots via different routes: Evaluating doping agents for enhanced photoluminescence emission. Prog. Org. Coatings 2024, 191, 108445. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, L.; Ettoumi, F.; Javed, M.; Li, L.; Lin, X.; Xu, Y.; Lu, Y.; Shao, X.; Luo, Z. Ultrasonic-assisted green extraction of peach gum polysaccharide for blue-emitting carbon dots synthesis. Sustain. Chem. Pharm. 2021, 24, 100555. [Google Scholar] [CrossRef]
- Lin, X.; Xiong, M.; Zhang, J.; He, C.; Ma, X.; Zhang, H.; Kuang, Y.; Yang, M.; Huang, Q. Carbon dots based on natural resources: Synthesis and applications in sensors. Microchem. J. 2021, 160, 105604. [Google Scholar] [CrossRef]
- Xu, J.; Cui, K.; Gong, T.; Zhang, J.; Zhai, Z.; Hou, L.; Yuan, C. Ultrasonic-assisted synthesis of N-doped, multicolor carbon dots toward fluorescent inks, fluorescence sensors, and logic gate operations. Nanomaterials 2022, 12, 312. [Google Scholar] [CrossRef]
- Chao-Mujica, F.J.; Garcia-Hernández, L.; Camacho-López, S.; Camacho-López, M.; Camacho-López, M.A.; Reyes Contreras, D.; Pérez-Rodríguez, A.; Peña-Caravaca, J.P.; Páez-Rodríguez, A.; Darias-Gonzalez, J.G. Carbon quantum dots by submerged arc discharge in water: Synthesis, characterization, and mechanism of formation. J. Appl. Phys. 2021, 129, 163301. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Hoffman, J.; Morgiel, J.; Mościcki, T.; Stobiński, L.; Szymański, Z.; Małolepszy, A. Luminescent carbon dots synthesized by the laser ablation of graphite in polyethylenimine and ethylenediamine. Materials 2021, 14, 729. [Google Scholar] [CrossRef]
- Magdy, G.; El-Deen, A.K.; Radwan, A.S.; Belal, F.; Elmansi, H. Ultrafast microwave-assisted green synthesis of nitrogen-doped carbon dots as turn-off fluorescent nanosensors for determination of the anticancer nintedanib: Monitoring of environmental water samples. Talanta Open 2025, 11, 100423. [Google Scholar] [CrossRef]
- Ng, H.K.M.; Lim, G.K.; Leo, C.P. Comparison between hydrothermal and microwave-assisted synthesis of carbon dots from biowaste and chemical for heavy metal detection: A review. Microchem. J. 2021, 165, 106116. [Google Scholar] [CrossRef]
- Otten, M.; Hildebrandt, M.; Kuhnemuth, R.; Karg, M. Pyrolysis and solvothermal synthesis for carbon dots: Role of purification and molecular fluorophores. Langmuir 2022, 38, 6148–6157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, L.; Huang, L.; Zheng, G.; Zhang, P.; Jin, Y.; Jiao, Z.; Sun, X. Study on the fluorescence properties of carbon dots prepared via combustion process. J. Lumin. 2019, 206, 608–612. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Edison, T.N.J.I.; Sundramoorthy, A.K.; Vinodh, R.; Sangaraju, S.; Kishore, S.C.; Lee, Y.R. Natural nitrogen-doped carbon dots obtained from hydrothermal carbonization of chebulic myrobalan and their sensing ability toward heavy metal ions. Sensors 2023, 23, 787. [Google Scholar] [CrossRef] [PubMed]
- Kayani, K.F.; Ghafoor, D.; Mohammed, S.J.; Shatery, O.B.A. Carbon dots: Synthesis, sensing mechanisms, and potential applications as promising materials for glucose sensors. Nanoscale Adv. 2025, 7, 42–59. [Google Scholar] [CrossRef]
- Latief, U.; Ul Islam, S.; Khan, Z.M.S.H.; Khan, M.S. A facile green synthesis of functionalized carbon quantum dots as fluorescent probes for a highly selective and sensitive detection of Fe3+ ions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 262, 120132. [Google Scholar] [CrossRef]
- Venugopalan, P.; Vidya, N. Microwave-assisted green synthesis of carbon dots derived from wild lemon (Citrus pennivesiculata) leaves as a fluorescent probe for tetracycline sensing in water. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 286, 122024. [Google Scholar] [CrossRef]
- González-González, R.B.; González, L.T.; Madou, M.; Leyva-Porras, C.; Martinez-Chapa, S.O.; Mendoza, A. Synthesis, purification, and characterization of carbon dots from non-activated and activated pyrolytic carbon black. Nanomaterials 2022, 12, 298. [Google Scholar] [CrossRef]
- Nazar, M.; Hasan, M.; Wirjosentono, B.; Gani, B.A.; Nada, C.E. Microwave synthesis of carbon quantum dots from arabica coffee ground for fluorescence detection of Fe3+, Pb2+, and Cr3+. ACS Omega 2024, 9, 20571–20581. [Google Scholar] [CrossRef] [PubMed]
- Rawat, J.; Sharma, H.; Dwivedi, C. Microwave-assisted synthesis of carbon quantum dots and their integration with TiO2 nanotubes for enhanced photocatalytic degradation. Diam. Relat. Mater. 2024, 144, 111050. [Google Scholar] [CrossRef]
- Qiu, H.; Yuan, F.; Wang, Y.; Zhang, Z.; Li, J.; Li, Y. Green-light-emitting carbon dots via eco-friendly route and their potential in ferric-ion detection and WLEDs. Mater. Adv. 2022, 3, 7339–7347. [Google Scholar] [CrossRef]
- Chung, S.; Zhang, M. Microwave-assisted synthesis of carbon dot—iron oxide nanoparticles for fluorescence imaging and therapy. Front. Bioeng. Biotechnol. 2021, 9, 711534. [Google Scholar] [CrossRef]
- Shibata, H.; Abe, M.; Sato, K.; Uwai, K.; Tokuraku, K.; Iimori, T. Microwave-assisted synthesis and formation mechanism of fluorescent carbon dots from starch. Carbohydr. Polym. Technol. Appl. 2022, 3, 100218. [Google Scholar] [CrossRef]
- Caetano, M.M. Thiamethoxam sensing using gelatin carbon dots: Influence of synthesis and purification methods. Chemosensors 2025, 13, 326. [Google Scholar] [CrossRef]
- Kamal, A.; Hong, S.; Ju, H. Carbon quantum dots: Synthesis, characteristics, and quenching as biocompatible fluorescent probes. Biosensors 2025, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cai, Z.; Liu, Y.; Fan, Y.; She, Y. Fluorescent enhanced endogenous carbon dots derived from green tea residue for multiplex detection of heavy metal ions in food. Front. Sustain. Food Syst. 2024, 8, 1431792. [Google Scholar] [CrossRef]
- Zhao, Y.; Ji, X.; Liang, J.; Gao, Y.; Xing, H.; Song, Y.; Hou, J.; Yang, G. The fabrication of fluorescent sensor for Fe3+ detection and configurable logic gate operation based on N-doped carbon dots. J. Photochem. Photobiol. A Chem. 2024, 449, 115418. [Google Scholar] [CrossRef]
- Tan, Q.; Li, X.; Wang, L.; Zhao, J.; Yang, Q.; Sun, P.; Deng, Y.; Shen, G. One-step synthesis of highly fluorescent carbon dots as fluorescence sensors for the parallel detection of cadmium and mercury ions. Front. Chem. 2022, 10, 1005231. [Google Scholar] [CrossRef]
- Xu, X.-J.; Ge, S.; Li, D.-Q.; Xu, Z.-Q.; Wang, E.-J.; Wang, S.-M. Fluorescent carbon dots for sensing metal ions and small molecules. Chin. J. Anal. Chem. 2022, 50, 103–111. [Google Scholar] [CrossRef]
- Torres Landa, S.D.; Reddy Bogireddy, N.K.; Kaur, I.; Batra, V.; Agarwal, V. Heavy metal ion detection using green precursor derived carbon dots. iScience 2022, 25, 103816. [Google Scholar] [CrossRef]
- Deng, Z.; Jin, W.; Yin, Q.; Huang, J.; Huang, Z.; Fu, H.; Yuan, Y.; Zou, J.; Nie, J.; Zhang, Y. Ultrasensitive visual detection of Hg2+ ions via the Tyndall effect of gold nanoparticles. Chem. Commun. 2021, 57, 2613–2616. [Google Scholar] [CrossRef] [PubMed]
- Arif, Z.; Sugiarti, S.; Rohaeti, E.; Batubara, I. Validation method of the cellulose triacetate-based optode membrane for Fe(III) detection in water samples. J. Kim. Sains dan Apl. 2025, 28, 146–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Swaren, L.; Wang, W. Water decontamination by reactive high-valent iron species. Eco-Environ. Health 2024, 3, 55–58. [Google Scholar] [CrossRef]
- Valarmathy, C.; Sudhaparimala, S. Carbon dots as selective fluorescent probes for metal ions-influence of Moringa oleifera leaf as a precursor. Int. J. Nanosci. Nanotechnol. 2023, 19, 263–275. [Google Scholar]
- Castillo, V.C.G.; Limpoco, T.; Enriquez, E.P. Detection of nitrogen in layer-by-layer polymeric films by energy dispersive X-ray spectroscopy. Key Eng. Mater. 2022, 913, 87–97. [Google Scholar] [CrossRef]
- Park, S.Y.; Tan, J.K.S.; Mo, X.; Song, Y.; Lim, J.; Liew, X.R.; Chung, H.; Kim, S. Carbon quantum dots with tunable size and fluorescence intensity for development of a nano-biosensor. Small 2025, 21, e2404524. [Google Scholar] [CrossRef] [PubMed]
- Šafranko, S.; Janđel, K.; Kovačević, M.; Stanković, A.; Dutour Sikirić, M.; Mandić, Š.; Széchenyi, A.; Glavaš Obrovac, L.; Leventić, M.; Strelec, I.; et al. A facile synthetic approach toward obtaining N-doped carbon quantum dots from citric acid and amino acids, and their application in selective detection of Fe(III) ions. Chemosensors 2023, 11, 21–24. [Google Scholar] [CrossRef]
- Fiori, F.; Moukham, H.; Olia, F.; Piras, D.; Ledda, S.; Salis, A.; Stagi, L.; Malfatti, L.; Innocenzi, P. Highly photostable carbon dots from citric acid for bioimaging. Materials 2022, 15, 2395. [Google Scholar] [CrossRef]
- Bai, J.; Cui, J.; Ma, Y.; Zhao, W.; Wang, Y.; Li, Z. Orange emissive N-doped carbon dots and their application in detection of water in organic solvents and the polyurethane composites. Opt. Mater. 2022, 123, 111927. [Google Scholar] [CrossRef]
- Duarte De Assuncao, T.; Broussier, A.; Plain, J.; Proust, J. Unveiling the photoluminescence of carbon quantum dots with controlled cyan or green emission: Synthesis and photophysics investigation. ACS Appl. Opt. Mater. 2024, 2, 1947–1954. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, L.; Liu, J.; Wang, Q.; Jiao, L. A label-free yellow-emissive carbon dot-based nanosensor for sensitive and selective ratiometric detection of chromium (VI) in environmental water samples. Mater. Chem. Phys. 2020, 248, 122912. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Ren, G.; Wang, W.; Wu, S.; Shen, J. Bioinspired carbon quantum dots for sensitive fluorescent detection of vitamin B12 in cell system. Anal. Chim. Acta 2018, 1032, 154–162. [Google Scholar] [CrossRef]
- Mohandoss, S.; Ahmad, N.; Khan, M.R.; Velu, K.S.; Palanisamy, S.; You, S.; Kumar, A.J.; Lee, Y.R. Nitrogen and sulfur co-doped photoluminescent carbon dots for highly selective and sensitive detection of Ag+ and Hg2+ ions in aqueous media: Applications in bioimaging and real sample analysis. Environ. Res. 2023, 228, 115898. [Google Scholar] [CrossRef]
- Liu, H.; Xu, H.; Li, H. Detection of Fe3+ and Hg2+ ions by using high fluorescent carbon dots doped with S and N as fluorescence probes. J. Fluoresc. 2022, 32, 1089–1098. [Google Scholar] [CrossRef]
- Dua, S.; Kumar, P.; Pani, B.; Kaur, A.; Khanna, M.; Bhatt, G. Stability of carbon quantum dots: A critical review. RSC Adv. 2023, 13, 13845–13861. [Google Scholar] [CrossRef]
- Aljuhani, E.; Al Zbedy, A.S.; Alqarni, S.A.; Alzahrani, S.O.; Alharbi, A.; Alfi, A.A.; Al-Bonayan, A.M.; Shahat, A. Eco-friendly nanocellulose-based sensor for sensitive and selective detection of mercury and iron ions in water samples. Int. J. Environ. Anal. Chem. 2025, 1–17. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Suriyaprakash, R.; Shunmugakani, S.; Saravanan, P.; Kumar, J.V.; Mythili, R.; Al-Sadoon, M.K.; Santhamoorthy, M. Sequential detection of Hg2+ and TNT using a nitrogen-doped polymeric carbon dots on–off–on fluorescence sensor. Polym. Adv. Technol. 2024, 35, e6608. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, H.; Li, H.; Xu, T.; Li, H.; Wang, C.; Yang, Z.; Jia, X.; Liu, X. Carbon dots as specific fluorescent sensors for Hg2+ and glutathione imaging. Microchim. Acta 2023, 190, 224. [Google Scholar] [CrossRef]
- Li, Y.; Tang, L.; Zhu, C.; Liu, X.; Wang, X.; Liu, Y. Fluorescent and colorimetric assay for determination of Cu(II) and Hg(II) using AuNPs reduced and wrapped by carbon dots. Microchim. Acta 2022, 189, 10. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, L.; Zhao, Y.; Zhang, X.; Zhang, L.; Chen, L.; Yoon, J.; Liu, S. Insight into mercury ion detection in environmental samples and imaging in living systems by a near-infrared fluorescent probe. Sensors Actuators B Chem. 2024, 411, 135768. [Google Scholar] [CrossRef]
- Yang, Y.; Zou, T.; Wang, Z.; Xing, X.; Peng, S.; Zhao, R.; Zhang, X.; Wang, Y. The fluorescent quenching mechanism of N and S co-doped graphene quantum dots with Fe3+ and Hg2+ ions and their application as a novel fluorescent sensor. Nanomaterials 2019, 9, 738. [Google Scholar] [CrossRef]
- Van Tam, T.; Hong, S.H.; Choi, W.M. Facile synthesis of cysteine–functionalized graphene quantum dots for a fluorescence probe for mercury ions. RSC Adv. 2015, 5, 97598–97603. [Google Scholar] [CrossRef]
- Wang, R.; Yu, Z. Validity and reliability of Benesi-Hildebrand method. Acta Phys.—Chim. Sin. 2007, 23, 1353–1359. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.; Pan, W.; Yu, G.; Wang, J. Fe3+-sensitive carbon dots for detection of Fe3+ in aqueous solution and intracellular imaging of Fe3+ inside fungal cells. Front. Chem. 2020, 7, 911. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, N.; Mousazadeh, M.H. Preparation of fluorescent nitrogen-doped carbon dots for highly selective on-off detection of Fe3+ ions in real samples. Opt. Mater. 2021, 121, 111515. [Google Scholar] [CrossRef]
- Wu, J.; Luo, Y.; Cui, C.; Han, Q.; Peng, Z. Carbon dots as multifunctional fluorescent probe for Fe3+ sensing in ubiquitous water environments and living cells as well as lysine detection via “on-off-on” mechanism. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 309, 123840. [Google Scholar] [CrossRef]
- Dalal, C.; Garg, A.K.; Mathur, M.; Sonkar, S.K. Fluorescent polymer carbon dots for the sensitive–selective sensing of Fe3+ metal ions and cellular imaging. ACS Appl. Nano Mater. 2022, 5, 12699–12710. [Google Scholar] [CrossRef]
- Ma, Y.; Mao, L.; Cui, C.; Hu, Y.; Chen, Z.; Zhan, Y.; Zhang, Y. Nitrogen-doped carbon dots as fluorescent probes for sensitive and selective determination of Fe3+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 316, 124347. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Yang, H.; Gong, A.-J.; Huang, X.-M.; Li, L. Sensitive and selective chemosensor for Fe3+ detection using carbon dots synthesized by microwave method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 334, 125907. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xu, G.; Hou, C.; Zhang, H. Ratiometric fluorescence nanoprobe based on nitrogen-doped carbon dots for Cu2+ and Fe3+ detection. Sci. Rep. 2025, 15, 6261. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, W.; Chen, Y.; Zheng, J.; Cao, L.; Duan, L.; Tang, T.; Wang, Y. Green synthesis of boron-doped carbon dots from Chinese herbal residues for Fe3+ sensing, anti-counterfeiting, and photodegradation applications. J. Clean. Prod. 2023, 422, 138577. [Google Scholar] [CrossRef]

| Precursor | Method | QY (%) | Reference |
|---|---|---|---|
| citric acid and amino acids | Hydrothermal synthesis | 36.45 | [43] |
| citric acid and tris (hydroxymethyl)-aminomethane | Hydrothermal synthesis | 37.00 | [44] |
| 1,4-phenylene diisocyanate | Solvothermal | 38.00 | [45] |
| di-2-pyridiylketone | Hydrothermal synthesis | 5.55 | [46] |
| o-phenylenediamine | Solvothermal treatment | 26.70 | [47] |
| cytidine diphosphate choline (CDPC) and ethylenediamine | Pyrolysis | 25.00 | [48] |
| nitazoxanide and 3-mercaptopropionic acid | Hydrothermal synthesis | 32.00 | [49] |
| citric acid (CA) and glutathione | Microwave-assisted | 10.90 | [50] |
| o-diphenylamine | Microwave-assisted | 44.69 | This work |
| Materials | Readout Mechanism | Linear Range | LOD | Reference |
|---|---|---|---|---|
| N-CDs | Turn-Off | 0–40 μM | 3.10 nM | [53] |
| N-CDs | Turn-Off | 0.01–100 μM | 6.27 nM | [54] |
| CD-wrapped AuNP | Turn-Off | 9.0 × 10−7–9.0 × 10−5 M | 281.00 nM | [55] |
| NS-CDs | Turn-On | 0–50 × 10−6 M | 6.77 × 10−7 M | [49] |
| HCYS | Turn-Off | - | 1.41 μM. | [56] |
| CDs | Turn-Off | - | 0.16 μM | [50] |
| N, S-GQDs | Turn-Off | 1–30 nM 100–1000 nM | 0.27 nM 36.85 nM | [57] |
| Cysteine-functionalized GQDs | Turn-Off | 0–10 μM | 20.00 nM | [58] |
| CDs | Turn-Off | 0–1000 μM | 0.00958 μM (9.58 nM) | This work |
| Materials | Readout Mechanism | Linear Range | LOD | Reference |
|---|---|---|---|---|
| CDs | Turn-Off | 8–80 μM | 3.80 μM | [60] |
| N-CDs | Turn-Off | 0.002–8 μM | 13.80 nM | [61] |
| CDs | Turn-Off | 0–1000 μM | 1.90 μM | [62] |
| FPCDs | Turn-Off | - | 162.00 nM | [63] |
| N-CDs | Turn-Off | 20–80 μM | 3.18 μM | [64] |
| CDs | Turn-Off | 0.2–200 μM | 62.00 nM | [65] |
| N-CDs/OPD | Turn-Off | 20–80 μM | 7.12 μM | [66] |
| B@HRCDs | Turn-Off | 0–80 μM | 1.08 μM | [67] |
| CDs (Diphenyleneamine) | Turn-Off | 0–1000 μM | 0.02 μM (22.27 nM) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alansari, R.H.; Bakhsh, E.M.; Altamimi, L.R.; Akhtar, K.; Khan, S.B. One-Step Microwave-Assisted Fabrication of Carbon Dots as Efficient Fluorescent Chemosensors for Hg2+ and Fe3+ Detection. Sensors 2025, 25, 7452. https://doi.org/10.3390/s25247452
Alansari RH, Bakhsh EM, Altamimi LR, Akhtar K, Khan SB. One-Step Microwave-Assisted Fabrication of Carbon Dots as Efficient Fluorescent Chemosensors for Hg2+ and Fe3+ Detection. Sensors. 2025; 25(24):7452. https://doi.org/10.3390/s25247452
Chicago/Turabian StyleAlansari, Rawan H., Esraa M. Bakhsh, Lenah R. Altamimi, Kalsoom Akhtar, and Sher Bahadar Khan. 2025. "One-Step Microwave-Assisted Fabrication of Carbon Dots as Efficient Fluorescent Chemosensors for Hg2+ and Fe3+ Detection" Sensors 25, no. 24: 7452. https://doi.org/10.3390/s25247452
APA StyleAlansari, R. H., Bakhsh, E. M., Altamimi, L. R., Akhtar, K., & Khan, S. B. (2025). One-Step Microwave-Assisted Fabrication of Carbon Dots as Efficient Fluorescent Chemosensors for Hg2+ and Fe3+ Detection. Sensors, 25(24), 7452. https://doi.org/10.3390/s25247452






