Toward a Regulatory Qualification of Real-World Mobility Performance Biomarkers in Parkinson’s Patients Using Digital Mobility Outcomes
Abstract
:1. Introduction
2. Qualification Strategy
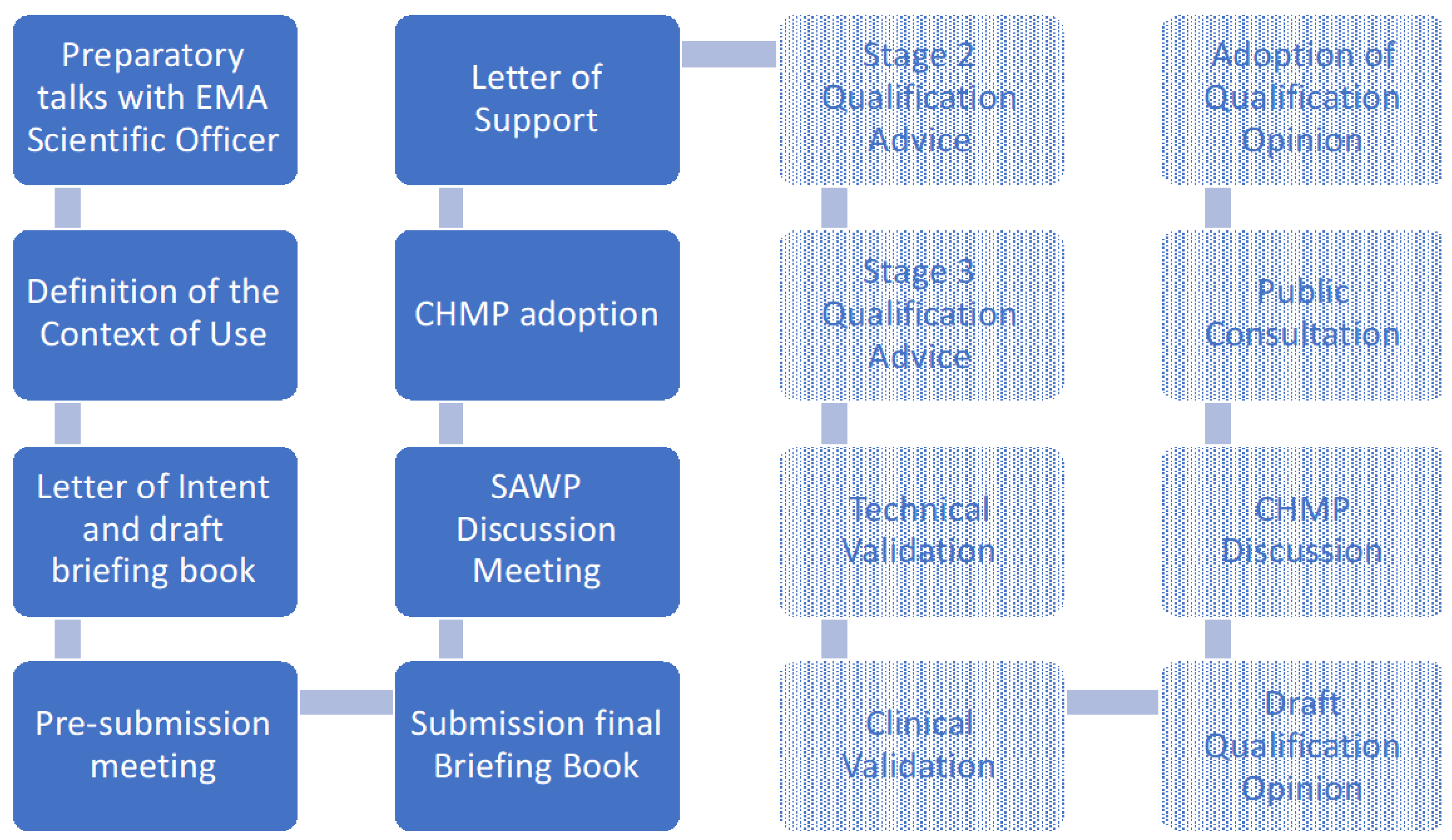
3. Qualification Process
4. Results
4.1. Context of Use
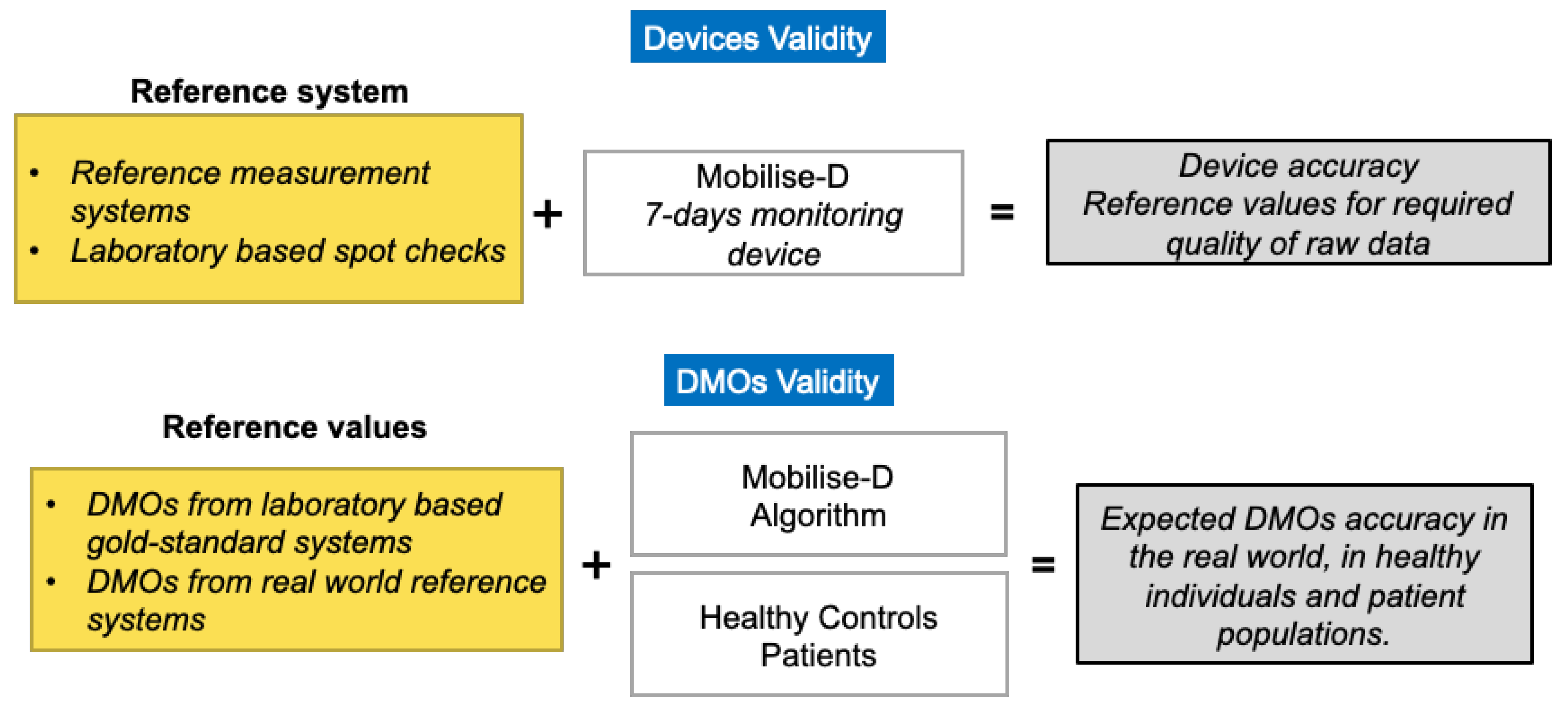
4.2. Technical Validation
4.3. Clinical Validation
4.4. Secure Data Management
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fritz, S.; Lusardi, M. White Paper: “Walking Speed: The Sixth Vital Sign”. J. Geriatr. Phys. Ther. 2009, 32, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, S.; Patel, K.V.; Rosano, C.; Rubin, S.M.; Satterfield, S.; Harris, T.; Ensrud, K.; Orwoll, E.; Lee, C.G.; Chandler, J.M.; et al. Gait Speed Predicts Incident Disability: A Pooled Analysis. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2015, 71, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lu, K.; Forsman, M.; Lindecrantz, K.; Seoane, F.; Ekblom, Ö.; Eklund, J. Evaluation of physiological workload assessment methods using heart rate and accelerometry for a smart wearable system. Ergonomics 2019, 62, 694–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekelund, U.; Tarp, J.; Steene-Johannessen, J.; Hansen, B.H.; Jefferis, B.; Fagerland, M.W.; Whincup, P.; Diaz, K.M.; Hooker, S.P.; Chernofsky, A.; et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: Systematic review and harmonised meta-analysis. BMJ 2019, 366, l4570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saint-Maurice, P.F.; Troiano, R.P.; Bassett, D.R.; Graubard, B.I.; Carlson, S.A.; Shiroma, E.J.; Fulton, J.E.; Matthews, C.E. Association of Daily Step Count and Step Intensity with Mortality Among US Adults. JAMA 2020, 323, 1151–1160. [Google Scholar] [CrossRef]
- Boehme, P.; Hansen, A.; Roubenoff, R.; Scheeren, J.; Herrmann, M.; Mondritzki, T.; Ehlers, J.; Truebel, H. How soon will digital endpoints become a cornerstone for future drug development? Drug Discov. Today 2019, 24, 16–19. [Google Scholar] [CrossRef]
- Espay, A.J.; Hausdorff, J.M.; Sánchez-Ferro, Á.; Klucken, J.; Merola, A.; Bonato, P.; Paul, S.S.; Horak, F.B.; Vizcarra, J.A.; Mestre, T.A.; et al. A roadmap for implementation of patient-centered digital outcome measures in Parkinson’s disease obtained using mobile health technologies. Mov. Disord. 2019, 34, 657–663. [Google Scholar] [CrossRef]
- Merchant, K.; Cedarbaum, J.M.; Brundin, P.; Dave, K.D.; Eberling, J.; Espay, A.J.; Hutten, S.J.; Javidnia, M.; Luthman, J.; Maetzler, W.; et al. A Proposed Roadmap for Parkinson’s Disease Proof of Concept Clinical Trials Investigating Compounds Targeting Alpha-Synuclein. J. Park. Dis. 2019, 9, 31–61. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.-Y.; Verma, V.K.; Lee, M.-Y.; Lin, H.-C.; Lai, C.-S. Prediction of 30-Day Readmission for COPD Patients Using Accelerometer-Based Activity Monitoring. Sensors 2020, 20, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, J.F.; Sandroff, B.M.; Motl, R.W. Therapies for mobility disability in persons with multiple sclerosis. Expert Rev. Neurother. 2018, 18, 493–502. [Google Scholar] [CrossRef]
- Benzinger, P.; Lindemann, U.; Becker, C.; Aminian, K.; Jamour, M.; Flick, S. Geriatric rehabilitation after hip fracture. Zeitschrift für Gerontologie und Geriatrie 2013, 47, 236–242. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Maetzler, W.; Liepelt, I.; Berg, D. Progression of Parkinson’s disease in the clinical phase: Potential markers. Lancet Neurol. 2009, 8, 1158–1171. [Google Scholar] [CrossRef]
- Horváth, K.; Aschermann, Z.; Kovács, M.; Makkos, A.; Harmat, M.; Janszky, J.; Komoly, S.; Karádi, K.; Kovács, N. Minimal clinically important differences for the experiences of daily living parts of movement disorder society-sponsored unified Parkinson’s disease rating scale. Mov. Disord. 2017, 32, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Blázquez, C.; Alvarez, M.; Arakaki, T.; Arillo, V.C.; Chaná, P.; Fernández, W.; Garretto, N.; Martínez-Castrillo, J.C.; Oroz, M.C.R.; Serrano-Dueñas, M.; et al. Self-Assessment of Disability in Parkinson’s Disease: The MDS-UPDRS Part II Versus Clinician-Based Ratings. Mov. Disord. Clin. Pr. 2017, 4, 529–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meguro, M.; Barley, E.A.; Spencer, S.; Jones, P.W. Development and Validation of an Improved, COPD-Specific Version of the St. George Respiratory Questionnaire. Chest 2007, 132, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Huijsmans, R.J.; De Haan, A.; Hacken, N.N.T.; Straver, R.V.; Hul, A.J.V. The clinical utility of the GOLD classification of COPD disease severity in pulmonary rehabilitation. Respir. Med. 2008, 102, 162–171. [Google Scholar] [CrossRef] [Green Version]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444. [Google Scholar] [CrossRef] [Green Version]
- European Medicine Agency Guideline on Clinical Investigation of Medicinal Products in the Treatment of Chronic Obstructive Pulmonary Disease (COPD). 2012. Available online: https://www.ema.europa.eu/en/clinical-investigation-medicinal-products-treatment-chronic-obstructive-pulmonary-disease-copd (accessed on 12 October 2020).
- Fischer, J.S.; Rudick, R.A.; Cutter, G.R.; Reingold, S.C.; National MS Society Clinical Outcomes Assessment Task Force. The Multiple Sclerosis Functional Composite measure (MSFC): An integrated approach to MS clinical outcome assessment. Mult. Scler. J. 1999, 5, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Freire, A.N.; Guerra, R.O.; Alvarado, B.; Guralnik, J.M.; Zunzunegui, M.V. Validity and Reliability of the Short Physical Performance Battery in Two Diverse Older Adult Populations in Quebec and Brazil. J. Aging Heal. 2012, 24, 863–878. [Google Scholar] [CrossRef] [PubMed]
- Prestmo, A.; Hagen, G.; Sletvold, O.; Helbostad, J.L.; Thingstad, P.; Taraldsen, K.; Lydersen, S.; Halsteinli, V.; Saltnes, T.; E Lamb, S.; et al. Comprehensive geriatric care for patients with hip fractures: A prospective, randomised, controlled trial. Lancet 2015, 385, 1623–1633. [Google Scholar] [CrossRef] [Green Version]
- Del Din, S.; Godfrey, A.; Mazzà, C.; Lord, S.; Rochester, L. Free-living monitoring of Parkinson’s disease: Lessons from the field. Mov. Disord. 2016, 31, 1293–1313. [Google Scholar] [CrossRef] [PubMed]
- Del Din, S.; Elshehabi, M.; Galna, B.; Hobert, M.A.; Warmerdam, E.; Sünkel, U.; Brockmann, K.; Metzger, F.; Hansen, C.; Berg, D.; et al. Gait analysis with wearables predicts conversion to Parkinson disease. Ann. Neurol. 2019, 86, 357–367. [Google Scholar] [CrossRef]
- FDA-NIH Biomarker Working Group BEST (Biomarkers, EndpointS, and Other Tools) Resource; Food and Drug Administration (US): Silver Spring (MD). 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK326791/ (accessed on 12 October 2020).
- Lowe, S.A.; Ólaighin, G. Monitoring human health behaviour in one’s living environment: A technological review. Med. Eng. Phys. 2014, 36, 147–168. [Google Scholar] [CrossRef]
- Feehan, L.M.; Geldman, J.; Sayre, E.C.; Park, C.; Ezzat, A.M.; Yoo, J.Y.; Hamilton, C.B.; Li, L.C. Accuracy of Fitbit Devices: Systematic Review and Narrative Syntheses of Quantitative Data. JMIR mHealth uHealth 2018, 6, e10527. [Google Scholar] [CrossRef] [Green Version]
- Straiton, N.; Alharbi, M.; Bauman, A.; Neubeck, L.; Gullick, J.; Bhindi, R.; Gallagher, R. The validity and reliability of consumer-grade activity trackers in older, community-dwelling adults: A systematic review. Maturitas 2018, 112, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Kristoffersson, A.; Linden, M. A Systematic Review on the Use of Wearable Body Sensors for Health Monitoring: A Qualitative Synthesis. Sensors 2020, 20, 1502. [Google Scholar] [CrossRef] [Green Version]
- Evenson, K.R.; Goto, M.M.; Furberg, R.D. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Storm, F.; Heller, B.W.; Mazzà, C. Step Detection and Activity Recognition Accuracy of Seven Physical Activity Monitors. PLoS ONE 2015, 10, e0118723. [Google Scholar] [CrossRef] [PubMed]
- Del Din, S.; Godfrey, A.; Rochester, L. Validation of an Accelerometer to Quantify a Comprehensive Battery of Gait Characteristics in Healthy Older Adults and Parkinson’s Disease: Toward Clinical and at Home Use. IEEE J. Biomed. Heal. Inform. 2015, 20, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Hickey, A.; Gunn, E.; Alcock, L.; Del Din, S.; Godfrey, A.; Rochester, L.; Galna, B. Validity of a wearable accelerometer to quantify gait in spinocerebellar ataxia type 6. Physiol. Meas. 2016, 37, N105–N117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijkstra, B.; Zijlstra, W.; Scherder, E.; Kamsma, Y. Detection of walking periods and number of steps in older adults and patients with Parkinson’s disease: Accuracy of a pedometer and an accelerometry-based method. Age Ageing 2008, 37, 436–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furlanetto, K.C.; Bisca, G.W.; Oldemberg, N.; Sant’Anna, T.J.; Morakami, F.K.; Camillo, C.A.; Cavalheri, V.; Hernandes, N.A.; Probst, V.S.; Ramos, E.M.C.; et al. Step Counting and Energy Expenditure Estimation in Patients With Chronic Obstructive Pulmonary Disease and Healthy Elderly: Accuracy of 2 Motion Sensors. Arch. Phys. Med. Rehabil. 2010, 91, 261–267. [Google Scholar] [CrossRef]
- Del Din, S.; Hickey, A.; Woodman, S.; Hiden, H.; Morris, R.; Watson, P.; Nazarpour, K.; Catt, M.; Rochester, L.; Godfrey, A. Accelerometer-based gait assessment: Pragmatic deployment on an international scale. In Proceedings of the 2016 IEEE Statistical Signal Processing Workshop (SSP), Palma de Mallorca, Spain, 26–29 June 2016; pp. 1–5. [Google Scholar]
- Storm, F.; Nair, K.; Clarke, A.J.; Van Der Meulen, J.M.; Mazzà, C. Free-living and laboratory gait characteristics in patients with multiple sclerosis. PLoS ONE 2018, 13, e0196463. [Google Scholar] [CrossRef] [Green Version]
- Weiss, A.; Sharifi, S.; Plotnik, M.; Van Vugt, J.P.P.; Giladi, N.; Hausdorff, J.M. Toward Automated, At-Home Assessment of Mobility Among Patients with Parkinson Disease, Using a Body-Worn Accelerometer. Neurorehabilit. Neural Repair 2011, 25, 810–818. [Google Scholar] [CrossRef]
- European Medicine Agency Qualification Opinion on Stride Velocity 95th Centile as a Secondary Endpoint in Duchenne Muscular Dystrophy Measured by a Valid and Suitable Wearable Device. 2019. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/qualification-opinion-stride-velocity-95th-centile-secondary-endpoint-duchenne-muscular-dystrophy_en.pdf (accessed on 12 October 2020).
- European Medicine Agency Qualification Opinion on Proactive in COPD. 2018. Available online: https://www.ema.europa.eu/en/documents/comments/overview-comments-received-qualification-opinion-proactive-chronic-obstructive-pulmonary-disease_en.pdf (accessed on 12 October 2020).
- Gimeno-Santos, E.; Raste, Y.; Demeyer, H.; Louvaris, Z.; De Jong, C.; Rabinovich, R.A.; Hopkinson, N.S.; Polkey, M.I.; Vogiatzis, I.; Tabberer, M.; et al. The PROactive instruments to measure physical activity in patients with chronic obstructive pulmonary disease. Eur. Respir. J. 2015, 46, 988–1000. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food & Drug Administration Review of the Letter of Intent (LOI) Submission for DDT COA #000106. 2018. Available online: https://www.fda.gov/drugs/clinical-outcome-assessment-coa-qualification-program/ddt-coa-000106-actibeltr-multiple-sclerosis (accessed on 12 October 2020).
- IEEE Standard IEEE 2700-2017—IEEE Standard for Sensor Performance Parameter Definitions. 2017. Available online: https://standards.ieee.org/standard/2700-2017.html (accessed on 12 October 2020).
- El-Sheimy, N.; Hou, H.; Niu, X. Analysis and Modeling of Inertial Sensors Using Allan Variance. IEEE Trans. Instrum. Meas. 2007, 57, 140–149. [Google Scholar] [CrossRef]
- Hussen, A.A.; Jleta, I.N. Low-Cost Inertial Sensors Modeling Using Allan Variance. Int. J. Electr. Comput. Eng. 2015, 9, 1237–1242. [Google Scholar]
- Chiari, L.; Della Croce, U.; Leardini, A.; Cappozzo, A. Human movement analysis using stereophotogrammetry. Gait Posture 2005, 21, 197–211. [Google Scholar] [CrossRef]
- Di Marco, R.; Rossi, S.; Castelli, E.; Patanè, F.; Mazzà, C.; Cappa, P. Effects of the calibration procedure on the metrological performances of stereophotogrammetric systems for human movement analysis. Measurement 2017, 101, 265–271. [Google Scholar] [CrossRef]
- Bertuletti, S.; Salis, F.; Cereatti, A.; Angelini, L.; Buckley, E.; Nair, K.P.S.; Mazza, C.; Della Croce, U. Inter-leg Distance Measurement as a Tool for Accurate Step Counting in Patients with Multiple Sclerosis. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; Volume 2019, pp. 6413–6417. [Google Scholar]
- Bertuletti, S.; Cereatti, A.; Comotti, D.; Caldara, M.; Della Croce, U. Static and Dynamic Accuracy of an Innovative Miniaturized Wearable Platform for Short Range Distance Measurements for Human Movement Applications. Sensors 2017, 17, 1492. [Google Scholar] [CrossRef] [Green Version]
- Salis, F.; Bertuletti, S.; Caruso, M.; Della Croce, U.; Mazzà, C.; Cereatti, A. Multi-sensor integration and data fusion for enhancing gait assessment in and out of the laboratory. Gait Posture 2019, 74, 34. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 1967, 17, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cliff, N. Dominance statistics: Ordinal analyses to answer ordinal questions. Psychol. Bull. 1993, 114, 494–509. [Google Scholar] [CrossRef]
- World Health Organisation Guidelines on Good Data and Record Management Practices. 2018. Available online: https://www.who.int/medicines/publications/pharmprep/WHO_TRS_996_annex05.pdf (accessed on 12 October 2020).
- European Parliament Directive 2006/24/EC of the European Parliament and of the Council on the Retention of Data Generated or Processed in Connection with the Provision of Publicly Available Electronic Communications Services or of Public Communications Networks and Amending Directive 2002/58/EC. 2006. Available online: https://eur-lex.europa.eu/legal-content/GA/TXT/?uri=CELEX:32006L0024 (accessed on 12 October 2020).
- European Parliament Regulation (EU) 2016/679 of the European Parliament and of the Council on the Protection of Natural Persons with Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95/46/EC (General Data Protection Regulation). 2016. Available online: https://eur-lex.europa.eu/eli/reg/2016/679/oj (accessed on 12 October 2020).
- Hiden, H.; Woodman, S.; Watson, P.; Cala, J. Developing cloud applications using the e-Science Central platform. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20120085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerreta, F.; Ritzhaupt, A.; Metcalfe, T.; Askin, S.; Duarte, J.; Berntgen, M.; Vamvakas, S. Digital technologies for medicines: Shaping a framework for success. Nat. Rev. Drug Discov. 2020, 19, 573–574. [Google Scholar] [CrossRef] [PubMed]
- European Medicine Agency Questions and Answers: Qualification of Digital Technology-based Methodologies to Support Approval of Medicinal Product. 2020. Available online: https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-advice-protocol-assistance/qualification-novel-methodologies-medicine-development-0 (accessed on 12 October 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viceconti, M.; Hernandez Penna, S.; Dartee, W.; Mazzà, C.; Caulfield, B.; Becker, C.; Maetzler, W.; Garcia-Aymerich, J.; Davico, G.; Rochester, L. Toward a Regulatory Qualification of Real-World Mobility Performance Biomarkers in Parkinson’s Patients Using Digital Mobility Outcomes. Sensors 2020, 20, 5920. https://doi.org/10.3390/s20205920
Viceconti M, Hernandez Penna S, Dartee W, Mazzà C, Caulfield B, Becker C, Maetzler W, Garcia-Aymerich J, Davico G, Rochester L. Toward a Regulatory Qualification of Real-World Mobility Performance Biomarkers in Parkinson’s Patients Using Digital Mobility Outcomes. Sensors. 2020; 20(20):5920. https://doi.org/10.3390/s20205920
Chicago/Turabian StyleViceconti, Marco, Sabina Hernandez Penna, Wilhelmus Dartee, Claudia Mazzà, Brian Caulfield, Clemens Becker, Walter Maetzler, Judith Garcia-Aymerich, Giorgio Davico, and Lynn Rochester. 2020. "Toward a Regulatory Qualification of Real-World Mobility Performance Biomarkers in Parkinson’s Patients Using Digital Mobility Outcomes" Sensors 20, no. 20: 5920. https://doi.org/10.3390/s20205920





