Wearable Systems for Monitoring Mobility-Related Activities in Chronic Disease: A Systematic Review
Abstract
:1. Introduction
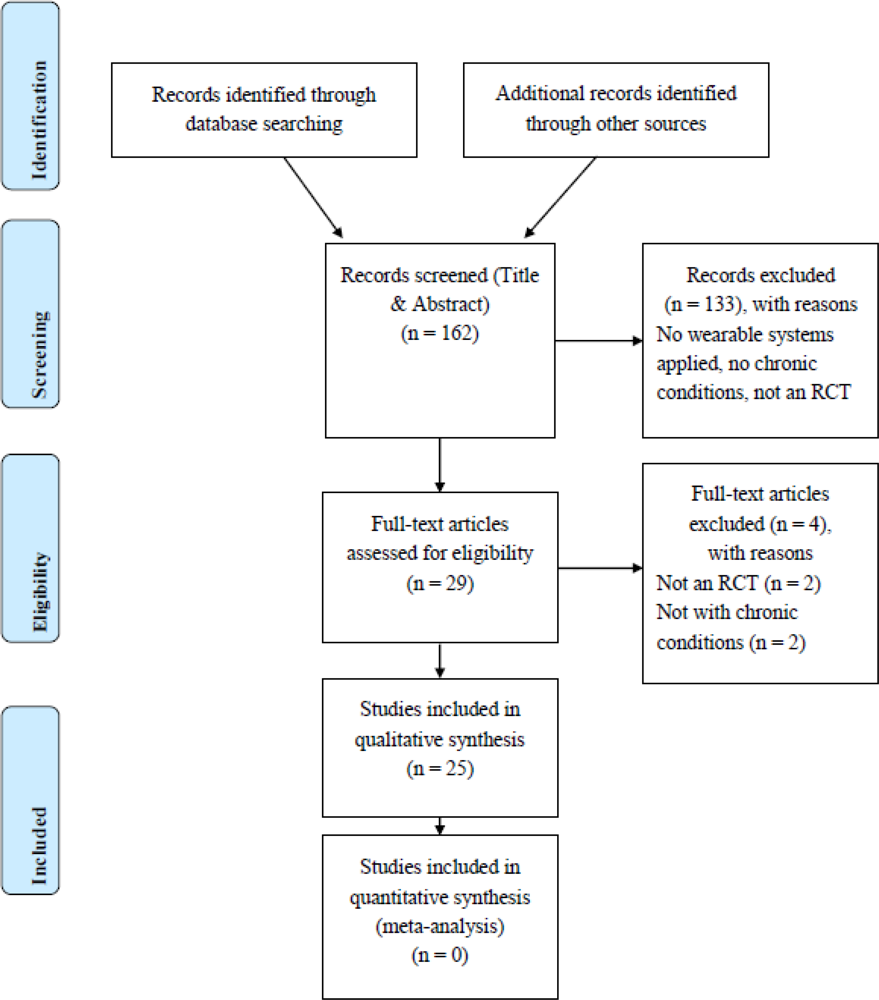
2. Methods
2.1. First Selection Based on Abstracts
2.2. Method for Quality Assessment in Selected Full Text Articles
3. Results and Discussion
3.1. Technologies and Applications
3.2. Mode of Application
3.3. Feasibility and Adherence
3.4. Clinical Relevance
3.4.1. Characteristics of Osteoarthritis Studies
3.4.2. Characteristics of CVD Studies
3.4.3. Characteristics of type 2 Diabetes Mellitus Studies
3.4.4. Characteristics of COPD Studies
3.5. Discussion
3.6. Other Reviews
4. Conclusions
References
- Beaglehole, R; Ebrahim, S; Reddy, S; Voute, J; Leeder, S. Prevention of Chronic Diseases: A Call to Action. Lancet 2007, 370, 2152–2157. [Google Scholar]
- Yach, D; Hawkes, C; Gould, CL; Hofman, KJ. The Global Burden of Chronic Diseases: Overcoming Impediments to Prevention and Control. JAMA 2004, 291, 2616–2622. [Google Scholar]
- Johnson, RJ; Wolinsky, FD. The Structure of Health Status Among Older Adults: Disease, Disability, Functional Limitation, and Perceived Health. J. Health Soc. Behav 1993, 34, 105–121. [Google Scholar]
- Malmberg, J; Miilunpalo, S; Pasanen, M; Vuori, I; Oja, P. Characteristics of Leisure Time Physical Activity Associated with Risk of Decline in Perceived Health—a 10-year Follow-up of Middle-Aged and Elderly Men and Women. Prev. Med 2005, 41, 141–150. [Google Scholar]
- Malmberg, JJ; Miilunpalo, SI; Vuori, IM; Pasanen, ME; Oja, P; Haapanen-Niemi, NA. A Health-Related Fitness and Functional Performance Test Battery for Middle-Aged and Older Adults: Feasibility and Health-Related Content Validity. Arch. Phys. Med. Rehabil 2002, 83, 666–677. [Google Scholar]
- Katzmarzyk, PT; Janssen, I. The Economic Costs Associated with Physical Inactivity and Obesity in Canada: an Update. Can. J. Appl. Physiol 2004, 29, 90–115. [Google Scholar]
- Chodzko-Zajko, WJ; Proctor, DN; Fiatarone Singh, MA; Minson, CT; Nigg, CR; Salem, GJ; Skinner, JS. American College of Sports Medicine Position Stand. Exercise and Physical Activity for Older Adults. Med. Sci. Sport. Exerc 2009, 41, 1510–1530. [Google Scholar]
- Stuart, M; Chard, S; Benvenuti, F; Steinwachs, S. Community Exercise: A Vital Component to Healthy Aging. Healthc. Pap 2009, 10, 23–28. [Google Scholar]
- Physical Activity Fundamental to Preventing Disease. In Services; Department of Health & Human Services: Washington, DC, USA, 2002.
- Ferrucci, L; Baldasseroni, S; Bandinelli, S; de Alfieri, W; Cartei, A; Calvani, D; Baldini, A; Masotti, G; Marchionni, N. Disease Severity and Health-Related Quality of Life Across Different Chronic Conditions. J. Am. Geriatr. Soc 2000, 48, 1490–1495. [Google Scholar]
- de Bruin, ED; Hartmann, A; Uebelhart, D; Murer, K; Zijlstra, W. Wearable Systems for Monitoring Mobility-Related Activities in Older People: A Systematic Review. Clin. Rehabil 2008, 22, 878–895. [Google Scholar]
- Myers, AM; Holliday, PJ; Harvey, KA; Hutchinson, KS. Functional Performance Measures: Are They Superior to Self-Assessments? J. Gerontol 1993, 48, M196–206. [Google Scholar]
- Magaziner, J; Zimmerman, SI; Gruber-Baldini, AL; Hebel, JR; Fox, KM. Proxy Reporting in Five Areas of Functional Status. Comparison with Self-Reports and Observations of Performance. Am. J. Epidemiol 1997, 146, 418–428. [Google Scholar]
- Ferrucci, L; Guralnik, JM; Studenski, S; Fried, LP; Cutler, GB, Jr; Walston, JD. Designing Randomized, Controlled Trials Aimed at Preventing or Delaying Functional Decline and Disability in Frail, Older Persons: a Consensus Report. J. Am. Geriatr. Soc 2004, 52, 625–634. [Google Scholar]
- Zijlstra, W; Aminian, K. Mobility Assessment in Older People: New Possibilities and Challenges. Eur. J. Ageing 2007, 4, 3–12. [Google Scholar]
- Dahl, TH. International Classification of Functioning, Disability and Health: An Introduction and Discussion of its Potential Impact on Rehabilitation Services and Research. J. Rehabil. Med 2002, 34, 201–204. [Google Scholar]
- International Classification of Functioning, Disability and Health (ICF); WHO; World Health Organization: Geneva, Switzerland, 2001.
- Preventing Chronic Diseases: A Vital Investment; WHO Global Report; NLM Classification: WT 500; WHO; World Health Organization: Geneva, Switzerland, 2005.
- Giglia, E. PEDro: This Well-Known, Unknown. Physiotherapy Evidence Database. Eur. J. Phys. Rehabil. Med 2008, 44, 477–480. [Google Scholar]
- Maher, CG; Sherrington, C; Herbert, RD; Moseley, AM; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther 2003, 83, 713–721. [Google Scholar]
- Verhagen, AP; de Vet, HC; de Bie, RA; Kessels, AG; Boers, M; Bouter, LM; Knipschild, PG. The Delphi List: A Criteria List for Quality Assessment of Randomized Clinical Trials for Conducting Systematic Reviews Developed by Delphi Consensus. J. Clin. Epidemiol 1998, 51, 1235–1241. [Google Scholar]
- PEDro Physiotherapy Evidence Database. Available online: http://www.pedro.org.au.
- Landis, JR; Koch, GG. An Application of Hierarchical Kappa-Type Statistics in the Assessment of Majority Agreement among Multiple Observers. Biometrics 1977, 33, 363–374. [Google Scholar]
- van Tulder, MW; Assendelft, WJ; Koes, BW; Bouter, LM. Method Guidelines for Systematic Reviews in the Cochrane Collaboration Back Review Group for Spinal Disorders. Spine (Phila Pa 1976) 1997, 22, 2323–2330. [Google Scholar]
- Moher, D; Liberati, A; Tetzlaff, J; Altman, DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009, 6, 1000097. [Google Scholar]
- Talbot, LA; Gaines, JM; Huynh, TN; Metter, EJ. A Home-Based Pedometer-Driven Walking Program to Increase Physical Activity in Older Adults with Osteoarthritis of the Knee: A Preliminary Study. J. Am. Geriatr. Soc 2003, 51, 387–392. [Google Scholar]
- Toda, Y; Toda, T; Takemura, S; Wada, T; Morimoto, T; Ogawa, R. Change in Body Fat, but not Body Weight or Metabolic Correlates of Obesity, is Related to Symptomatic Relief of Obese Patients with Knee Osteoarthritis after a Weight Control Program. J. Rheumatol 1998, 25, 2181–2186. [Google Scholar]
- Bauldoff, GS; Hoffman, LA; Zullo, TG; Sciurba, FC. Exercise Maintenance Following Pulmonary Rehabilitation: Effect of Distractive Stimuli. Chest 2002, 122, 948–954. [Google Scholar]
- de Blok, BM; de Greef, MH; ten Hacken, NH; Sprenger, SR; Postema, K; Wempe, JB. The Effects of a Lifestyle Physical Activity Counseling Program with Feedback of a Pedometer during Pulmonary Rehabilitation in Patients with COPD: A Pilot Study. Patient Educ. Couns 2006, 61, 48–55. [Google Scholar]
- Sewell, L; Singh, SJ; Williams, JE; Collier, R; Morgan, MD. Can Individualized Rehabilitation Improve Functional Independence in Dlderly Patients with COPD? Chest 2005, 128, 1194–1200. [Google Scholar]
- Steele, BG; Belza, B; Cain, KC; Coppersmith, J; Lakshminarayan, S; Howard, J; Haselkorn, JK. A Randomized Clinical Trial of an Activity and Exercise Adherence Intervention in Chronic Pulmonary Disease. Arch. Phys. Med. Rehabil 2008, 89, 404–412. [Google Scholar]
- Coghill, N; Cooper, AR. The Effect of a Home-Based Walking Program on Risk Factors for Coronary Heart Disease in Hypercholesterolaemic Men. A Randomized Controlled Trial. Prev. Med 2008, 46, 545–551. [Google Scholar]
- Hughes, AR; Mutrie, N; Macintyre, PD. Effect of an Exercise Consultation on Maintenance of Physical Activity after Completion of Phase III Exercise-Based Cardiac Rehabilitation. Eur. J. Cardiovasc. Prev. Rehabil 2007, 14, 114–121. [Google Scholar]
- Moreau, KL; Degarmo, R; Langley, J; McMahon, C; Howley, ET; Bassett, DR, Jr; Thompson, DL. Increasing Daily Walking Lowers Blood Pressure in Postmenopausal Women. Med. Sci. Sport. Exerc 2001, 33, 1825–1831. [Google Scholar]
- Price, HC; Tucker, L; Griffin, SJ; Holman, RR. The Impact of Individualised Cardiovascular Disease (CVD) Risk Estimates and Lifestyle Advice on Physical Activity in Individuals at High Risk of CVD: A Pilot 2 × 2 Factorial Understanding Risk Trial. Cardiovasc Diabetol 2008, 7, 21:1–21:7. [Google Scholar]
- Sohn, AJ; Hasnain, M; Sinacore, JM. Impact of Exercise (walking) on Blood Pressure Levels in African American Adults with Newly Diagnosed Hypertension. Ethn. Dis 2007, 17, 503–507. [Google Scholar]
- Witham, MD; Gray, JM; Argo, IS; Johnston, DW; Struthers, AD; McMurdo, ME. Effect of a Seated Exercise Program to Improve Physical Function and Health Status in Frail Patients ≥70 Years of Age with Heart Failure. Am. J. Cardiol 2005, 95, 1120–1124. [Google Scholar]
- Araiza, P; Hewes, H; Gashetewa, C; Vella, CA; Burge, MR. Efficacy of a Pedometer-Based Physical Activity Program on Parameters of Diabetes Control in Type 2 Diabetes Mellitus. Metabolism 2006, 55, 1382–1387. [Google Scholar]
- Bjorgaas, M; Vik, JT; Saeterhaug, A; Langlo, L; Sakshaug, T; Mohus, RM; Grill, V. Relationship between Pedometer-Registered Activity, Aerobic Capacity and Self-Reported Activity and Fitness in Patients with Type 2 Diabetes. Diabetes Obes. Metab 2005, 7, 737–744. [Google Scholar]
- Bjorgaas, MR; Vik, JT; Stolen, T; Lydersen, S; Grill, V. Regular Use of Pedometer does not Enhance Beneficial Outcomes in a Physical Activity Intervention Study in Type 2 Diabetes Mellitus. Metabolism 2008, 57, 605–611. [Google Scholar]
- Engel, L; Lindner, H. Impact of Using a Pedometer on Time Spent Walking in Older Adults with Type 2 Diabetes. Diabetes Educ 2006, 32, 98–107. [Google Scholar]
- Keyserling, TC; Samuel-Hodge, CD; Ammerman, AS; Ainsworth, BE; Elasy, TA; Henriquez-Roldan, CF; Skelly, AH; Johnston, LF; Bangdiwala, SI. A Randomized Trial of an Intervention to Improve Self-Care Behaviors of African-American Women with Type 2 Diabetes: Impact on Physical Activity. Diabetes Care 2002, 25, 1576–1583. [Google Scholar]
- Kirk, A; Barnett, J; Leese, G; Mutrie, N. A Randomized Trial Investigating the 12-month Changes in Physical Activity and Health Outcomes Following a Physical Activity Consultation Delivered by a Person or in Written Form in Type 2 Diabetes: Time2Act. Diabetic Med 2009, 26, 293–301. [Google Scholar]
- Kirk, A; Mutrie, N; MacIntyre, P; Fisher, M. Increasing Physical Activity in People with Type 2 Diabetes. Diabetes Care 2003, 26, 1186–1192. [Google Scholar]
- Kirk, A; Mutrie, N; MacIntyre, P; Fisher, M. Effects of a 12-month Physical Activity Counselling Intervention on Glycaemic Control and on the Status of Cardiovascular Risk Factors in People with Type 2 Diabetes. Diabetologia 2004, 47, 821–832. [Google Scholar]
- Kirk, AF; Higgins, LA; Hughes, AR; Fisher, BM; Mutrie, N; Hillis, S; MacIntyre, PD. A Randomized, Controlled Trial to Study the Effect of Exercise Consultation on the Promotion of Physical Activity in People with Type 2 Diabetes: A Pilot Study. Diabetic Med 2001, 18, 877–882. [Google Scholar]
- Kirk, AF; Mutrie, N; Macintyre, PD; Fisher, MB. Promoting and Maintaining Physical Activity in People with Type 2 Diabetes. Am. J. Prev. Med 2004, 27, 289–296. [Google Scholar]
- Lemaster, JW; Mueller, MJ; Reiber, GE; Mehr, DR; Madsen, RW; Conn, VS. Effect of Weight-Bearing Activity on Foot Ulcer Incidence in People with Diabetic Peripheral Neuropathy: Feet First Randomized Controlled Trial. Phys. Ther 2008, 88, 1385–1398. [Google Scholar]
- Tudor-Locke, C; Bell, RC; Myers, AM; Harris, SB; Ecclestone, NA; Rodger, NW; Lauzon, N. Controlled Outcome Evaluation of the First Step Program: A Daily Physical Activity Intervention for Individuals with Type II Diabetes. Int. J. Obes. Relat. Metab. Disord 2004, 28, 113–119. [Google Scholar]
- Yates, T; Davies, M; Gorely, T; Bull, F; Khunti, K. Rationale, Design and Baseline Data from the Pre-Diabetes Risk Education and Physical Activity Recommendation and Encouragement (PREPARE) Programme Study: A Randomized Controlled Trial. Patient Educ. Couns 2008, 73, 264–271. [Google Scholar]
- Bravata, DM; Smith-Spangler, C; Sundaram, V; Gienger, AL; Lin, N; Lewis, R; Stave, CD; Olkin, I; Sirard, JR. Using Pedometers to Increase Physical Activity and Improve Health: A Systematic Review. JAMA 2007, 298, 2296–2304. [Google Scholar]
- Knols, RH; de Bruin, ED; Shirato, K; Uebelhart, D; Aaronson, NK. Physical Activity Interventions to Improve Daily Walking Activity in Cancer Survivors. BMC Cancer 2010, 10, 406:1–406:10. [Google Scholar]
- Crouter, SE; Schneider, PL; Karabulut, M; Bassett, DR, Jr. Validity of 10 Electronic Pedometers for Measuring Steps, Distance, and Energy Cost. Med. Sci. Sport. Exerc 2003, 35, 1455–1460. [Google Scholar]
- Schneider, PL; Crouter, SE; Lukajic, O; Bassett, DR, Jr. Accuracy and Reliability of 10 Pedometers for Measuring Steps over a 400-m Walk. Med. Sci. Sport. Exerc 2003, 35, 1779–1784. [Google Scholar]
- Crouter, SE; Schneider, PL; Bassett, DR, Jr. Spring-Levered versus Piezo-Electric Pedometer Accuracy in Overweight and Obese Adults. Med. Sci. Sport. Exerc 2005, 37, 1673–1679. [Google Scholar]
- Tudor-Locke, CE; Bell, RC; Myers, AM; Harris, SB; Lauzon, N; Rodger, NW. Pedometer-Determined Ambulatory Activity in Individuals with Type 2 Diabetes. Diabetes Res. Clin. Pract 2002, 55, 191–199. [Google Scholar]
- Corder, K; Brage, S; Ekelund, U. Accelerometers and Pedometers: Methodology and Clinical Application. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 597–603. [Google Scholar]
- Cyarto, EV; Myers, AM; Tudor-Locke, C. Pedometer Accuracy in Nursing Home and Community-Dwelling Older Adults. Med. Sci. Sport. Exerc 2004, 36, 205–209. [Google Scholar]
- Pearson, OR; Busse, ME; van Deursen, RW; Wiles, CM. Quantification of Walking Mobility in Neurological Disorders. QJM 2004, 97, 463–475. [Google Scholar]
- Chen, KY; Bassett, DR, Jr. The Technology of Accelerometry-Based Aactivity Monitors: Current and Future. Med. Sci. Sport. Exerc 2005, 37, S490–500. [Google Scholar]
- Busse, ME; Pearson, OR; van Deursen, R; Wiles, CM. Quantified Measurement of Activity Provides Insight into Motor Function and Recovery in Neurological Disease. J. Neurol. Neurosurg. Psychiat 2004, 75, 884–888. [Google Scholar]
- Coleman, KL; Smith, DG; Boone, DA; Joseph, AW; del Aguila, MA. Step Activity Monitor: Long-Term, Continuous Recording of Ambulatory Function. J. Rehabil. Res. Dev 1999, 36, 8–18. [Google Scholar]
- Duncan, PW; Sullivan, KJ; Behrman, AL; Azen, SP; Wu, SS; Nadeau, SE; Dobkin, BH; Rose, DK; Tilson, JK. Protocol for the Locomotor Experience Applied Post-stroke (LEAPS) Trial: A Randomized Controlled Trial. BMC Neurol 2007, 7, 39:1–39:23. [Google Scholar]
- Shepherd, EF; Toloza, E; McClung, CD; Schmalzried, TP. Step Activity Monitor: Increased Accuracy in Quantifying Ambulatory Activity. J. Orthop. Res 1999, 17, 703–708. [Google Scholar]
- de Bruin, ED; Najafi, B; Murer, K; Uebelhart, D; Aminian, K. Quantification of Everyday Motor Function in a Geriatric Population. J. Rehabil. Res. Dev 2007, 44, 417–428. [Google Scholar]
- Grant, PM; Ryan, CG; Tigbe, WW; Granat, MH. The Validation of a Novel Activity Monitor in the Measurement of Posture and Motion during Everyday Activities. Br. J. Sport. Med 2006, 40, 992–997. [Google Scholar]
- Ryan, CG; Grant, PM; Tigbe, WW; Granat, MH. The Validity and Reliability of a Novel Activity Monitor as a Measure of Walking. Br. J Sport. Med 2006, 40, 779–784. [Google Scholar]
- Brage, S; Brage, N; Ekelund, U; Luan, J; Franks, PW; Froberg, K; Wareham, NJ. Effect of Combined Movement and Heart Rate Monitor Placement on Physical Activity Estimates during Treadmill Locomotion and Free-Living. Eur. J. Appl. Physiol 2006, 96, 517–524. [Google Scholar]
- Brage, S; Brage, N; Franks, PW; Ekelund, U; Wareham, NJ. Reliability and Validity of the Combined Heart Rate and Movement Sensor Actiheart. Eur. J. Clin. Nutr 2005, 59, 561–570. [Google Scholar]
- Crouter, SE; Churilla, JR; Bassett, DR, Jr. Accuracy of the Actiheart for the Assessment of Energy Expenditure in Adults. Eur. J. Clin. Nutr 2008, 62, 704–711. [Google Scholar]
- Zhang, W; Moskowitz, RW; Nuki, G; Abramson, S; Altman, RD; Bierma-Zeinstra, S; Arden, N; Brandt, KD; Croft, P; Doherty, M; Dougados, M; Hochberg, M; Hunter, DJ; Kwoh, K; Lohmander, LS; Tugwell, P. OARSI Recommendations for the Management of Hip and Knee Osteoarthritis, Part I: Critical Appraisal of Existing Treatment Guidelines and Systematic Review of Current Research Evidence. Osteoarthritis Cartilage 2007, 15, 981–1000. [Google Scholar]
- Bosomworth, NJ. Exercise and Knee Osteoarthritis: Benefit or Hazard? Can. Fam. Physician 2009, 55, 871–878. [Google Scholar]
- Culhane, KM; O'Connor, M; Lyons, D; Lyons, GM. Accelerometers in Rehabilitation Medicine for Older Adults. Age Ageing 2005, 34, 556–560. [Google Scholar]
- Mathie, MJ; Coster, AC; Lovell, NH; Celler, BG. Accelerometry: Providing an Integrated, Practical Method for Long-Term, Ambulatory Monitoring of Human Movement. Physiol. Meas 2004, 25, R1–20. [Google Scholar]
- Steele, BG; Belza, B; Cain, K; Warms, C; Coppersmith, J; Howard, J. Bodies in Motion: Monitoring Daily Activity and Exercise with Motion Sensors in People with Chronic Pulmonary Disease. J. Rehabil. Res. Dev 2003, 40, 45–58. [Google Scholar]
- Berlin, JE; Storti, KL; Brach, JS. Using Activity Monitors to Measure Physical Activity in Free-Living Conditions. Phys. Ther 2006, 86, 1137–1145. [Google Scholar]
- Tudor-Locke, CE; Myers, AM. Challenges and Opportunities for Measuring Physical Activity in Sedentary Adults. Sports Med 2001, 31, 91–100. [Google Scholar]
- Warms, C. Physical Activity Measurement in Persons with Chronic and Disabling Conditions: Methods, Strategies, and Issues. Fam. Community Health 2006, 29, 78S–88S. [Google Scholar]
- Tudor-Locke, C; Williams, JE; Reis, JP; Pluto, D. Utility of Pedometers for Assessing Physical Activity: Convergent Validity. Sports Med 2002, 32, 795–808. [Google Scholar]
- Pitta, F; Troosters, T; Probst, VS; Spruit, MA; Decramer, M; Gosselink, R. Quantifying Physical Activity in Daily Life with Questionnaires and Motion Sensors in COPD. Eur. Respir. J 2006, 27, 1040–1055. [Google Scholar]
| Author | PEDRo Quality score |
|---|---|
| Osteoarthritis | |
| Talbot et al., 2003 [26] | 6 |
| Toda et al., 1998 [27] | 6 |
| COPD | |
| Bauldoff et al., 2002 [28] | 6 |
| de Blok et al., 2005 [29] | 6 |
| Sewell et al., 2005 [30] | 6 |
| Steele et al., 2008 [31] | 7 |
| CVD | |
| Coghill et al., 2008 [32] | 8 |
| Hughes et al., 2007 [33] | 7 |
| Moreau et al., 2001 [34] | 7 |
| Sohn et al., 2007 [36] | 5 |
| Witham et al., 2005 [37] | 8 |
| Diabetes mellitus | |
| Araiza et al., 2006 [38] | 6 |
| Bjorgaas et al., 2005 [39] | 6 |
| Bjorgaas et al., 2008 [40] | 5 |
| Engel et al., 2006 [41] | 6 |
| Keyserling et al., 2002 [42] | 8 |
| LeMaster et al., 2008 [48] | 9 |
| Tudor-Locke et al., 2004 [49] | 5 |
| Kirk et al., 2001 [46] | 7 |
| Kirk et al. 2003, 2004 [44,45,47] | 7/6/8 |
| Kirk et al., 2009 [43] | 9 |
| Yates et al., 2008 [50] | 4 |
| Device type | Outcome Measure(s)/Placement |
|---|---|
| Pedometers | |
| Yamax Digi-walker Modell SW-200 [26,29,34,36,49,50], SW 700 [41], ML AW-320 [40], (Yamax, Tokyo, Japan), SW 701 [38] (New Lifestyles, Kansas City, MI) | Step counts, distance, Energy Expenditure/Waist |
| Sportline Distance Pedometer Model 342 (Sportline, Campbell CA) [28] | Distance/Waist |
| Pedometer (Seiko, Tokyo, Japan) (no further specifications) [27] | Step Counts/Waist |
| Accusplit Eagle 170 (Pleasanton, CA) [48] | Step counts, distance, Energy Expenditure/Waist |
| NL-800 (New Lifestyles, USA) | Step Counts/Waist |
| Uniaxial Accelerometers | |
| Z80/32KV1 activity monitor (Gaehwiler Electronics; Hombrechtikon, Switzerland) [30] | Activity counts/Waist |
| Caltrac Accelerometer (Muscle Dynamics, Torrance, CA, USA) | Energy Expenditure/Waist |
| MIT Accelerometer Modell 7164 (MIT, Shalimar, Florida, USA) [33] | Activity counts/Ankle |
| Computer Science and Applications (CSA) uniaxial Accelerometer, (Computer Science and Applications, Shalimar, Florida, USA) [44,45,47] | Activity counts/Waist |
| Multiaxial Accelerometers | |
| Step Activity Monitor (SAM) (Prosthetic Research Study, Seattle, WA, USA)/StepWatch Activity Monitor (OrthoCare Innovations, Washington DC) [48,63] | Step counts, step rate/Ankle |
| RT3 Accelerometer (Stayhealthy Inc, Monrovia, CA, USA) [31,37] | Activity counts, vector magnitude, energy expenditure/Waist |
| ActiGraph model GT1M (ActiGraph LLC, Pensacola, FL, USA)[43]; Manufacturing Technology, Fort Walton Beach, FL) [35] | Step counts, activity counts, energy expenditure/Wrist, waist, ankle |
| Tritrac-R3D (Hemokinetics, Madison, WI, USA)[26] | Activity counts, vector magnitude, energy expenditure/Waist |
| Author | Type/Brand | Problems reported |
|---|---|---|
| Toda et al., 1998 [27] | Pedometer (Seiko, Tokyo, Japan) (no further specifications) | Three of 18 participants in the control group (17%; see Table 1) had forgotten to attach pedometers for several days and were excluded from the study. The remainder of this group (n = 15) were evaluated [27]. |
| Steele et al., 2008 [31] | RT3 Accelerometer (Stayhealthy Inc., Monrovia, CA, USA) | The RT3 data had a high signal-to-noise ratio that swamped any differences in daily activity between the groups. This finding was evidenced by large day-to-day variations in VMU (Table 2), further contributing to low power of the RT3 measurement of daily activity. The authors suspect that an arduous side-by-side comparison of the performance characteristics of the TriTrac-R3D and the RT3 might have shown that the TriTrac-R3D had a better signal-to-noise ratio, which would help explain why this device was able to detect subtle differences in daily activity in people who were exercising for greater durations. This was not done, because preventive maintenance and recalibration of the TriTrac-R3D units in possession of the authors were no longer available, and the TriTrac-R3D could no longer be purchased [31]. |
| Keyserling et al., 2002 [42] | Caltrac Accelerometer (Muscle Dynamics, Torrance, CA, USA) | One limitation mentioned is possible bias in PA measurement, which may have resulted from differences in actual Caltrac wearing time by treatment group. This might indicate possible problems with compliance wearing the device. There were, however, no differences in reported wearing time between treatment groups. It is possible that the actual PA energy expenditure was underestimated by the Caltrac, since it does not detect non-ambulatory PA (e.g., arm swinging). However, this bias is consistent for all subjects and is a limitation in the use of vertically oriented accelerometers as a direct measure of PA [42]. |
| Hughes et al., 2007 [33] | Uniaxial MIT accelerometer, Modell GT1M (Manufacturing Technology, Fort Walton Beach, FL) | The decline in total activity counts/week measured by accelerometers did not parallel the marked decrease in self-reported physical activity in the controls. The authors speculate that this discrepancy may be due to limitations of accelerometers since these devices cannot record water activities, activities that increase energy expenditure without a proportional increase in bodily acceleration (e.g., walking uphill) and those requiring a large amount of upper body movement (e.g., washing windows) [33]. The decrease in self-reported activity in the controls could reflect a decline in these activities, which would not be detected by accelerometers. |
| Moreau et al., 2001 [34] | Yamax Digi-Walker, SW-200 (Yamax, Tokyo, Japan) | The authors were unable to determine the intensity of the amount of daily walking that the women included in the study performed [34]. |
| LeMaster et al., 2008 [48] | StepWatch (OrthoCare Innovations, Washington) | With respect to protocol adherence there were some participants that attached the StepWatch in the reverse direction causing loss of physical activity data [48]. |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Allet, L.; Knols, R.H.; Shirato, K.; Bruin, E.D.d. Wearable Systems for Monitoring Mobility-Related Activities in Chronic Disease: A Systematic Review. Sensors 2010, 10, 9026-9052. https://doi.org/10.3390/s101009026
Allet L, Knols RH, Shirato K, Bruin EDd. Wearable Systems for Monitoring Mobility-Related Activities in Chronic Disease: A Systematic Review. Sensors. 2010; 10(10):9026-9052. https://doi.org/10.3390/s101009026
Chicago/Turabian StyleAllet, Lara, Ruud H. Knols, Kei Shirato, and Eling D. de Bruin. 2010. "Wearable Systems for Monitoring Mobility-Related Activities in Chronic Disease: A Systematic Review" Sensors 10, no. 10: 9026-9052. https://doi.org/10.3390/s101009026