Benthic Diatom Composition in Coastal Zone of Black Sea, Sasyk Reservoir (Ukraine)
Abstract
:1. Introduction
2. Materials and Methods
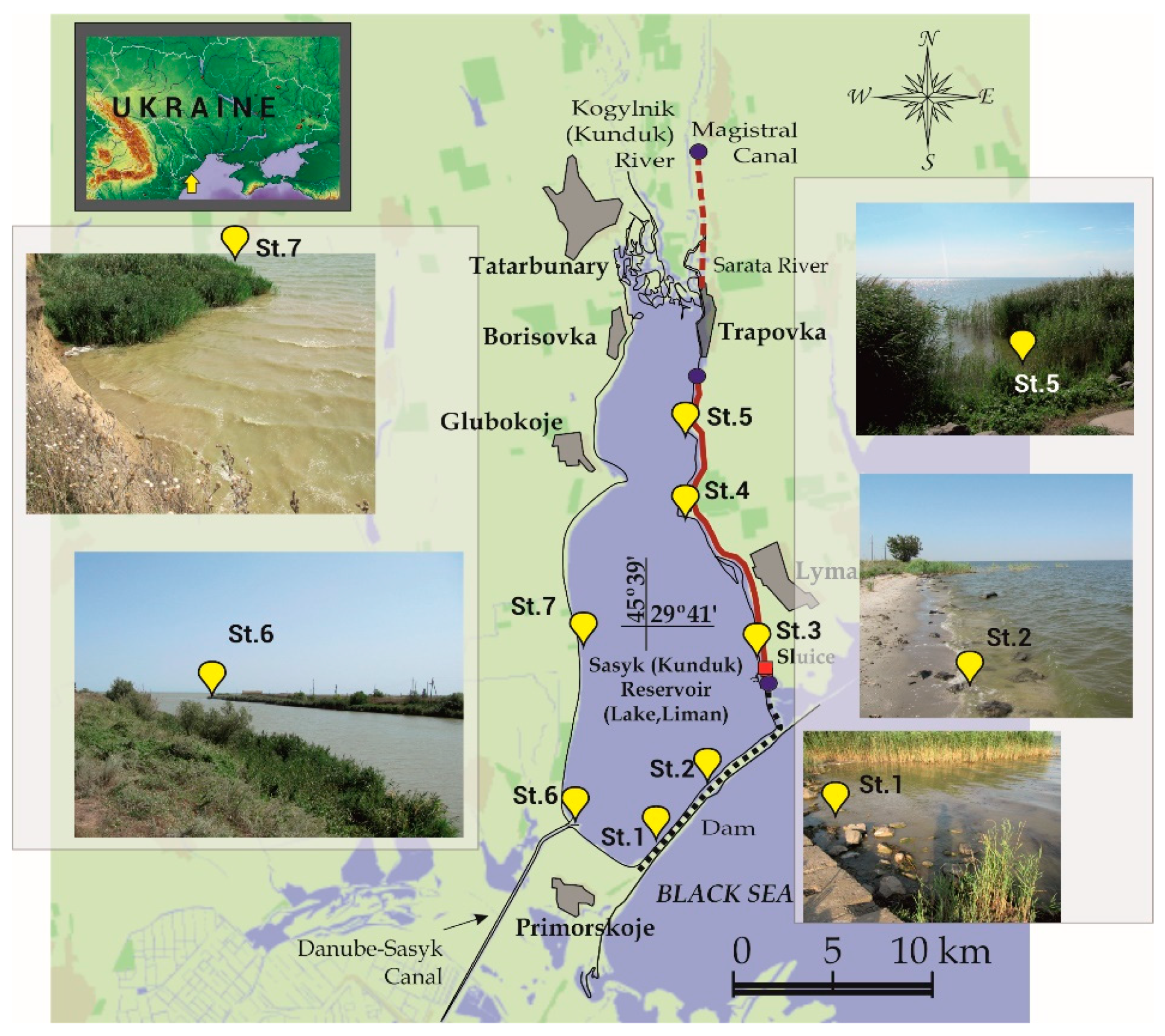
2.1. Study Area
2.2. Sampling Strategy
2.3. Statistical Data Analysis
3. Results
3.1. Benthic Diatom Diversity
3.2. Ecological Characteristics of the Sasyk Sites
3.3. Bioindication of Salinity in Sasyk Reservoir
4. Discussion
4.1. Biodiversity of the Sasyk Reservoir
4.2. Ecological Characteristic of Sasyk
4.3. Salinity Changes Over the Sasyk Reservoir
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Besse-Lototskaya, A.; Verdonschot, P.F.M.; Coste, M.; Van de Vijver, B. A new perspective for phytobenthos in the European water framework directive. Ecol. Indic. 2012, 18, 705–708. [Google Scholar] [CrossRef]
- Schaumburg, J.; Schranz, C.; Hofmann, G.; Stelzer, D.; Schneider, S.; Schmedtje, U. Macrophytes and phytobenthos as indicators of ecological status in German lakes—A contribution to the implementation of the water framework directive. Limnologica 2004, 34, 302–314. [Google Scholar] [CrossRef] [Green Version]
- Bennion, H.; Kelly, M.G.; Juggins, S.; Yallop, M.L.; Burgess, A.; Jamieson, J.; Krokowski, J. Assessment of ecological status in UK lakes using benthic diatoms. Freshw. Sci. 2014, 33, 639–654. [Google Scholar] [CrossRef] [Green Version]
- Kelly, M.; Juggins, S.; Guthrie, R.; Pritchard, S.; Jamieson, J.; Rippey, B.; Hirst, H.; Yallop, M. Assessment of ecological status in U.K. rivers using diatoms. Freshw. Biol. 2008, 53, 403–422. [Google Scholar] [CrossRef]
- Rimet, F. Recent views on river pollution and diatoms. Hydrobiologia 2012, 683, 1–24. [Google Scholar] [CrossRef]
- Stenger-Kovács, C.; Lengyel, E.; Sebestyén, V.; Szabó, B. Effects of land use on streams: Traditional and functional analyses of benthic diatoms. Hydrobiologia 2020, 847, 2933–2946. [Google Scholar] [CrossRef]
- Lengyel, E.; Szabó, B.; Stenger-Kovács, C. Realized ecological niche-based occupancy—Abundance patterns of benthic diatom traits. Hydrobiologia 2020, 847, 3115–3127. [Google Scholar] [CrossRef]
- Kelly, M. Data rich, information poor? Phytobenthos assessment and the water framework directive. Eur. J. Phycol. 2013, 48, 437–450. [Google Scholar] [CrossRef]
- Ponader, K.C.; Charles, D.F. Understanding the Relationship Between Natural Conditions and Loadings on Eutrophication: Algal Indicators of Eutrophication for New Jersey Streams. Final Report Year 3; Patrick Center for Environmental Research, Academy of Natural Sciences: Philadelphia, PA, USA, 2004; pp. 1–56. [Google Scholar]
- Van Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from The Netherlands. Neth. J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar] [CrossRef]
- Pardoa, I.; Delgadoa, C.; Abraína, R.; Gómez-Rodríguezb, C.; García-Rosellóc, E.; Garcíaa, L.; Reynoldsond, T.B. A predictive diatom-based model to assess the ecological status of streams and rivers of Northern Spain. Ecol. Indic. 2018, 90, 519–528. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Pan, Y. Assessing environmental conditions in rivers and streams with diatoms. In The Diatoms: Applications for the Environmental and Earth Sciences; Stoermer, S., Smol, J.P., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 11–40. [Google Scholar]
- Ács, É.; Bíró, T.; Berta, C.; Duleba, M.; Földi, A.; Grigorszky, I.; Hidas, A.; Knisz, J.; Korponai, J.L.; Trábert, Z.; et al. Long-term changes of species composition and functional traits of epiphytic diatoms in the Szigetköz region (Hungary) of the Danube river. Water 2020, 12, 776. [Google Scholar] [CrossRef] [Green Version]
- Bennett, M.G.; Lee, S.S.; Schofield, K.A.; Ridley, C.; Norton, S.B.; Webb, J.A.; Nichols, S.J.; Ogden, R.; Collins, A. Using systematic review and evidence banking to increase uptake and use of aquatic science in decision-making. Limnol. Oceanogr. Bull. 2018, 27, 103–109. [Google Scholar] [CrossRef]
- Witkowski, A.; Lange-Bertalot, H.; Metzelin, D. Diatom flora of marine coasts I. In Iconographia diatomologica; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag K.G.: Ruggell, Liechtenstein, 2000; Volume 7, pp. 1–925. [Google Scholar]
- Wilson, J.; Basset, A.; West, R. Estuarine and lagoon biodiversity and their natural goods and services. Estuar. Coast. Shelf Sci. 2013, 132, 1–108. [Google Scholar] [CrossRef]
- Lilitskaya, G.G.; Tsarenko, P.M.; Maslov, I.I. Bacillariophyta of lake Donuzlav (Crimea, Ukraine). Int. J. Algae 2013, 15, 135–152. [Google Scholar] [CrossRef]
- Bilous, O.P.; Ivanova, N.O. Phytoplankton characteristics of the Sasyk reservoir (Ukraine). Int. J. Algae 2018, 20, 277–288. [Google Scholar] [CrossRef]
- Newton, A.; Brito, A.C.; Icely, J.D.; Derolez, V.; Clara, I.; Angus, S.; Schernewski, G.; Inácio, M.; Lillebø, A.I.; Sousa, A.I.; et al. Assessing, quantifying and valuing the ecosystem services of coastal lagoons. J. Nat. Conserv. 2018, 44, 50–65. [Google Scholar] [CrossRef]
- Marcos, C.; Gamito, S.; Umgiesser, G.; Pérez-Ruzafa, A. Coastal lagoons: Environmental processes, biological functioning and anthropogenic impacts. Estuar. Coast. Shelf Sci. 2019, 216, 1–240. [Google Scholar] [CrossRef]
- Quintino, V.; Sangiorgio, F.; Mamedea, R.; Ricardoa, F.; Sampaioa, L.; Martinsa, R.; Freitasa, R.; Rodrigues, A.M.; Basset, A. The leaf-bag and the sediment sample: Two sides of the same ecological quality story? Estuar. Coast. Shelf Sci. 2011, 95, 326–337. [Google Scholar] [CrossRef]
- Alekseenko, E.; Roux, B. Contribution to remediation of brackish lagoon: 3D simulation of salinity, bottom currents and resuspension of bottom sediments by strong winds. Estuar. Coast. Shelf Sci. 2019, 216, 27–37. [Google Scholar] [CrossRef]
- Ivanova, N.O. Vodoshovishe Sasik—ekologo-gidrologichni problemi isnuvannya (Sasyk reservoir—ecological and hydrological problems of existence). Hydrol. Hydrochem. Hydroecol. 2009, 17, 113–117. (In Ukrainian) [Google Scholar]
- Kharchenko, T.A.; Timchenko, V.I.; Ivanov, A.I.; Kolesnik, M.P.; Novikov, B.I.; Enaki, I.G.; Ryabov, A.K.; Sirenko, L.A.; Klokov, V.M.; Bashmakova, I.C.; et al. Bioproductivnost’ i kachestvo vody Sasykskogo vodokhranilischa v usloviyakh ego opresneniya. In Bioproductivity and Water Quality Conditions in the Sasyk reservoir on its Desalination; Braginsky, L.P., Ed.; Nauk. Dumka: Kiev, Ukraine, 1990; pp. 1–276. (In Russian) [Google Scholar]
- Lyashenko, A.V.; Zorina-Sakharova, Y.Y. Hydroecological characteristics of the Sasyk liman and the Sasyk reservoir. Hydrobiol. J. 2017, 53, 26–43. [Google Scholar] [CrossRef]
- Lyashenko, A.V.; Zorina-Sakharova, Y.Y. Succession of hydrobiocenoses of the Sasyk liman and the Sasyk reservoir. Hydrobiol. J. 2018, 54, 14–29. [Google Scholar] [CrossRef]
- Timchenko, V.M.; Ivanova, N.O. Ecological and hydrological view on the problems of the Sasyk estuary. In Proceedings of the North-Western Black Sea Coast: Current Hydroecological Problems and Ways to Solve Them, Materials All-Ukrainian scientific practice conference, Odessa, Ukraine, 12–14 September 2012; pp. 147–150. [Google Scholar]
- Kharchenko, T.A. Sasyk Reservoir: Environmental problems of desalinated estuary. Bull. Acad. Sci. USSR 1988, 4, 63–67. [Google Scholar]
- Timchenko, V.M.; Timchenko, O.V. Ecological aspects of hydrology in a navigable channel “The Danube—The Black Sea” in Ukraine. In Proceedings of the 37th IAD Conference, Chisinau, Moldova, 29 October–1 November 2008; pp. 229–231. [Google Scholar]
- Vasenko, O.G.; Yeremenko, E.V. UkrNDIEP NAS of Ukraine. In Development of Socio-Economic and Environmental Justification for the Restoration of the Hydrological Regime of Lake Sasyk: Report under the Agreement (Final); Agreement № 11/1180/19/2; Research Institution “Ukrainian Research Institute of Environmental Problems” (UKRNDIEP): Kharkiv, Ukraine, 2004; pp. 1–215. [Google Scholar]
- Rubel, O.E.; Burkinsky, B.V. Polipshennya ekologichnogo stanu ozera Sasyk: Zvit po Report on GDR/IPREED NAS of Ukraine. In Improving the Ecological State of Lake Sasyk; № DR0108U008999. without inv. №. 2009; Institute of Market Problems and Economic-Ecological Research of the National Academy of Sciences of Ukraine: Odessa, Ukrainian, 2008; pp. 1–60. (In Ukrainian) [Google Scholar]
- Sasyk Lake. Available online: http://www.ramsar.org/sasyk-lake (accessed on 23 April 2020).
- Bilous, O.P.; Barinova, S.S.; Ivanova, N.O.; Huliaieva, O.A. The use of phytoplankton as an indicator of internal hydrodynamics of a large seaside reservoir—Case of the Sasyk reservoir, Ukraine. Ecohydrol. Hydrobiol. 2016, 16, 160–174. [Google Scholar] [CrossRef]
- European Committee for Standardization. Water Quality—Guidance Standard for the Routine Sampling and Pretreatment of Benthic Diatoms from Rivers; CEN EN 13946; Comité Européen de Normalisation: Geneva, Switzerland, 2003; pp. 1–22. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 1. Teil: Naviculaceae. In Süßwasserflora von Mitteleuropa; Ettl, H., Gerlo, J., Heynig, H., Mollenhauer, D., Eds.; VEB Gustav Fisher Verlag: Jena, Germany, 1986; pp. 1–876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 2. Teil: Epithemiaceae, Bacillariaceae, Surirellaceae. In Süßwasserflora von Mitteleuropa; Ettl, H., Gerlo, J., Heynig, H., Mollenhauer, D., Eds.; VEB Gustav Fisher Verlag: Jena, Germany, 1989; pp. 1–596. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 3. Teil: Eunoticeae. In Süßwasserflora von Mitteleuropa; Ettl, H., Gerlo, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher Verlag: Stuttgart, Germany, 1991; pp. 1–576. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 4. Teil: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. Teil 1–4. In Süßwasserflora von Mitteleuropa; Ettl, H., Gärtner, G., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher Verlag: Stuttgart, Germany, 1991; pp. 1–437. [Google Scholar]
- Bąk, M.; Witkowski, A.; Żelazna-Wieczorek, J.; Wojtal, A.Z.; Szczepocka, E.; Szulc, A.; Szulc, B. Klucz do oznaczania okrzemek w fitobentosie na potrzeby oceny stanu ekologicznego wód powierzchniowych w Polsce; Główny Inspektorat Ochrony Środowiska: Warszawa, Poland, 2012; pp. 1–452. (In Polish)
- Kulikovskiy, M.S.; Glushchenko, A.M.; Genkal, S.I.; Kuznetsova, I.V. Identification Book of Diatoms from Russia; Filigran: Yaroslavl, Russia, 2016; pp. 1–804. (In Russian) [Google Scholar]
- Lange-Bertalot, H.; Hofmann, G.; Werum, M.; Cantonati, M. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment; Koeltz Botanical Books: Schmitten-Oberreifenberg, Germany, 2017; pp. 1–942. [Google Scholar]
- Medlin, L.K.; Kaczmarska, I. Evolution of the diatoms: V. morphological and cytological support for the major clades and a taxonomic revision. Phycologia 2004, 43, 245–270. [Google Scholar] [CrossRef] [Green Version]
- Guiry, M.D.; Guiry, G.M. AlgaeBase, World-wide Electronic Publication; National University of Ireland Press: Galway, Ireland, 2020. [Google Scholar]
- Bielczyńska, A. Bioindication on the basis of benthic diatoms: Advantages and disadvantages of the Polish phytobenthos lake assessment method (IOJ—the Diatom Index for Lakes)/Bioindykacja na podstawie okrzemek bentosowych: Mocne i słabe strony polskiej metody oceny jezior na podstawie fitobentosu (IOJ—Indeks Okrzemkowy Jezior) Environmental Protection and Natural Resources. J. Inst. Environ. Prot. Natl. Res. Inst. 2015, 26, 48–55. (In Polish) [Google Scholar] [CrossRef] [Green Version]
- Rozporządzenie Ministra Gospodarki Morskiej i Żeglugi Śródlądowej z dnia 9 października 2019 r. w sprawie form i sposobu prowadzenia monitoringu jednolitych części wód powierzchniowych i jednolitych części wód podziemnych. Dz. Ustaw Rzeczyposp. Pol. 2019, 2147, 1–98. (In Polish)
- Hustedt, F. Systematische und ökologische Untersuchungen über die Diatomeen-Flora von Java, Bali und Sumatra nach dem Material der Deutschen Limnologischen Sunda-Expedition. I. Systematischer Teil. Archiv Hydrobiol. 1938, XV, 131–177. [Google Scholar]
- Barinova, S.S.; Bilous, O.P.; Tsarenko, P.M. Algoindicatsiya vodnykh ob’ektov Ukrainy: Metody i perspektyvy (Algal indication of water bodies in Ukraine: Methods and perspectives); University of Haifa Publisher: Haifa, Israel; Kiev, Ukraine, 2019; pp. 1–367. [Google Scholar]
- Jonsson, J.; Smedfors, K.; Nyholm, L.; Thornell, G. Towards chip-based salinity measurements for small submersibles and biologgers. Int. J. Oceanogr. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Kundu, S.S.; Tariq, H.; Kewalramani, N.; Yadav, R.K. Impact of total dissolved solids in drinking water on nutrient utilisation and growth performance of Murrah buffalo calves. Livest. Sci. 2017, 198, 17–23. [Google Scholar] [CrossRef]
- Malmberg, C.G. Electrical conductivity of dilute solutions of ‘sea water’ from 5 to 120. C. J. Res. Natl. Bur. Stand. A Phys. Chem. 1965, 69, 39–43. [Google Scholar] [CrossRef]
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, A.J.; Ly, A.; Gronau, Q.F.; Smira, M.; Epskamp, S.; et al. JASP: Graphical statistical software for common statistical designs. J. Stat. Softw. 2019, 88, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Kivrak, E.; Uygun, A. The structure and diversity of the epipelic diatom community in a heavily polluted stream (the Akarçay, Turkey) and their relationship with environmental variables. J. Freshw. Ecol. 2012, 27, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Snigireva, A.A.; Kovaleva, G.V. Diatom algae of sandy spits of the northwestern part of the Black Sea (Ukraine). Int. J. Algae 2015, 17, 107–130. [Google Scholar] [CrossRef]
- Van Dam, H.; Stenger-Kovács, C.; Ács, É.; Borics, G.; Buczkó, K.; Hajnal, É.; Soróczki-Pinter, É.; Várbiró, G.; Tóthmérész, B.; Padisák, J. Implementation of the European water framework directive: Development of a system for water quality assessment of Hungarian running waters with diatoms. Large Rivers 2007, 17, 339–364. [Google Scholar] [CrossRef]
- Timchenko, V.M. Ecological Hydrology of Waterbodies in Ukraine; Naukova Dumka: Kyiv, Ukraine, 2006; pp. 1–384. (In Ukranian) [Google Scholar]
- Timchenko, V.M.; Litvinov, A.S.; Kolesnyk, M.P.; Poddubny, S.A. Water circulation in Sasyk Reservoir. Hydrob. J. 1988, 24, 67–73. (In Russian) [Google Scholar]
- Ivanova, N.O. Water exchange as a factor of the formation of modern conditions and the function of the Sasyk Reservoir ecosystem. Series: Biology. Special issue: Assessment of the ecological state of waterbodies and adaptation of hydrobionts. Sci. Notes Ternopil Nat. Pedagogical Univ. V. Hnatyuk 2015, 3–4, 274–277. (In Ukranian) [Google Scholar]
- Ivanova, N.O. Pecularities of the water masses mixing at the Sasyk Reservoir. In Proceedings of the Meteorology, Hydrology, Monitoring of Environment in the Context of Ecological Challenges of Nowadays; Materials of All-Ukrainian conference of young people, Kyiv, Ukraine, 16–17 November 2016; pp. 27–29. (In Ukranian). [Google Scholar]
- Myronjuk, A.N.; Tkachenko, F.P. Composition of algae indicators of small rivers in the northwestern of the Black Sea. Bulletin Kharkiv Nat. Agric. Univ. Biol. Ser. 2013, 2, 93–102. [Google Scholar]
- Environmental Monitoring in the Black Sea. National Pilot Monitoring Studies and Joint Open Sea Surveys in Georgia, Russian Federation and Ukraine. Draft Final Scientific Report, 2017; Slobodnik, J., Alexandrov, B., Komorin, V., Mikaelyan, A., Guchmanidze, A., Arabidze, M., Korshenko, A., Eds.; EU/UNDP Project: Improving Environmental Monitoring in the Black Sea—Phase II (EMBLAS-II) ENPI/2013/313-169; 2018; pp. 1–573. Available online: http://emblasproject.org/wp-content/uploads/2019/07/EMBLAS-II_NPMS_JOSS_2017_ScReport_FinDraft2.pdf (accessed on 30 November 2020).
- Solak, C.N.; Peszek, Ł.; Yilmaz, E.; Ergül, H.A.; Kayal, M.; Ekmekçi, F.; Várbíró, G.; Yüce, A.M.; Canli, O.; Binici, M.S.; et al. Use of diatoms in monitoring the Sakarya river basin, Turkey. Water 2020, 12, 703. [Google Scholar] [CrossRef] [Green Version]
- Tsarenko, P.M.; Ennan, A.A.; Shikhaleyeva, G.N.; Barinova, S.S.; Gerasimiuk, V.P.; Ryzhko, V.E. Cyanoprokaryota of the Kuyalnik estuary ecosystem (Ukraine). Int. J. Algae 2016, 18, 337–352. [Google Scholar] [CrossRef]
- Gerasimiuk, V.P.; Gerasymova, O.V.; Struk (Konischuk), M.O.; Terenko, G.V.; Tsarenko (Bilous), O.P.; Tsarenko, P.M.; Wasser, S.P. Algae of Ukraine: Diversity, Nomenclature, Taxonomy, Ecology and Geography Bacillariophyta; Tsarenko, P.M., Wasser, S.P., Nevo, E., Eds.; A.R.G. Gartner Verlag Kommandit Geselschaft: Ruggel, Liechtenstein, 2009; Volume 2, pp. 1–413. [Google Scholar]
- Berezovskaya, V.Y. Species diversity of algae of the Kiev upland rivers (Ukraine). Int. J. Algae 2019, 21, 43–66. [Google Scholar] [CrossRef]
- Picińska-Fałtynowicz, J.; Błachuta, J. Methodological Guidelines for Assessing the Ecological Status of Bodies of Rivers and Lakes and the Ecological Potential of Artificial and Heavily Modified Bodies of Running Waters in Poland on the Basis of Phytobenthos Surveys/Wytyczne Metodyczne Do Przeprowadzenia Oceny Stanu Ekologicznego Jednolitych Części Wód Rzek I Jezior Oraz Potencjału Ekologicznego Sztucznych I Silnie Zmienionych Jednolitych Części Wód Płynących Polski Na Podstawie Badań Fitobentosu; Chief Inspectorate for Environmental Protection: Wrocław, Poland, 2010; pp. 1–79. (In Polish)
- Kelly, M.; Ács, É.; Bertrin, V.; Bennion, H.; Borics, G.; Burgess, A.; Denys, L.; Ecke, F.; Kahlert, M.; Karjalainen, S.M.; et al. Water Framework Directive Intercalibration Technical Report: Lake Phytobenthos Ecological Assessment Methods; Poikane, S., Ed.; Publications Office of the European Union: Luxembourg, 2014; pp. 1–140. ISBN 978-92-79-35468-7. [Google Scholar]
- Fritz, S.; Cumming, B.; Gasse, F.; Laird, K.R. Diatoms as indicators of hydrologic and climatic change in saline lakes. In The Diatoms: Applications for the Environmental and Earth Sciences, 2nd ed.; Smol, J.P., Stoermer, E.F., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 186–208. [Google Scholar] [CrossRef]
- Fritz, S.C. Salinity and Climate Reconstructions from Continental Lakes. In Encyclopedia of Quaternary Science, 2nd ed.; Elias, S.A., Mock, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 507–515. [Google Scholar]
- Kopyrina, L.; Pshennikova, E.; Barinova, S. Diversity and ecological characteristic of algae and cyanobacteria of thermokarst lakes in Yakutia (northeastern Russia). Oceanol. Hydrobiol. Stud. 2020, 49, 99–122. [Google Scholar] [CrossRef]
- Jiyenbekov, A.; Barinova, S.; Bigaliev, A.; Nurashov, S.; Sametova, E.; Fahima, T. Ecological diversity of algae in the Alakol Lake Natural Reserve, Kazakhstan. Bot. Pac. A J. Plant Sci. Conserv. 2019, 8, 63–74. [Google Scholar] [CrossRef]



| Site | Coordinates | Water Temperature, °C | Salinity, g·L−1 (ppt) | Conductivity mS cm−1 | |
|---|---|---|---|---|---|
| 1 | 45°32’25.41″N | 29°39’17.76″E | 22.35 ± 0.17 | 2.06 ± 0.54 | 2.81 ± 0.52 |
| 2 | 45°34’19.54″N | 29°41’41.17″E | 23.70 ± 0.12 | 2.27 ± 0.57 | 3.08 ± 0.57 |
| 3 | 45°37’20.65″N | 29°44’1.51″E | 25.25 ± 0.17 | 2.31 ± 0.54 | 3.09 ± 0.57 |
| 4 | 45°41’39.63″N | 29°41’30.76″E | 26.70 ± 0.12 | 2.16 ± 0.49 | 2.84 ± 0.48 |
| 5 | 45°43’36.85″N | 29°41’16.14″E | 26.80 ± 0.12 | 2.29 ± 0.55 | 3.07 ± 0.55 |
| 6 | 45°33’30.44″N | 29°36’26.56″E | 26.60 ± 0.23 | 0.49 ± 0.15 | 0.71 ± 0.16 |
| 7 | 45°36’12.09″N | 29°36’23.76″E | 26.40 ± 0.23 | 2.19 ± 0.52 | 2.93 ± 0.52 |
| Halobien Indicators | Sites | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| mh (mesohalobes) | 5 | 4 | 4 | 2 | 3 | 2 | 4 |
| hl (oligohalobes-halophiles) | 5 | 2 | 1 | 2 | 3 | 5 | 6 |
| i (oligohalobes-indifferents) | 36 | 20 | 28 | 28 | 23 | 30 | 24 |
| hb (oligohalobes-halophobes) | 1 | 1 | 2 | 1 | 1 | 2 | 1 |
| Total | 47 | 27 | 35 | 33 | 30 | 39 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilous, O.P.; Wojtal, A.Z.; Ivanova, N.O.; Tsarenko, P.M.; Burova, O.V.; Barinova, S. Benthic Diatom Composition in Coastal Zone of Black Sea, Sasyk Reservoir (Ukraine). Diversity 2020, 12, 458. https://doi.org/10.3390/d12120458
Bilous OP, Wojtal AZ, Ivanova NO, Tsarenko PM, Burova OV, Barinova S. Benthic Diatom Composition in Coastal Zone of Black Sea, Sasyk Reservoir (Ukraine). Diversity. 2020; 12(12):458. https://doi.org/10.3390/d12120458
Chicago/Turabian StyleBilous, Olena P., Agata Z. Wojtal, Natalia O. Ivanova, Petro M. Tsarenko, Olga V. Burova, and Sophia Barinova. 2020. "Benthic Diatom Composition in Coastal Zone of Black Sea, Sasyk Reservoir (Ukraine)" Diversity 12, no. 12: 458. https://doi.org/10.3390/d12120458









