N-(9-Ethyl-9H-carbazol-2-yl)-N′-(1-phenylethyl)thiourea
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
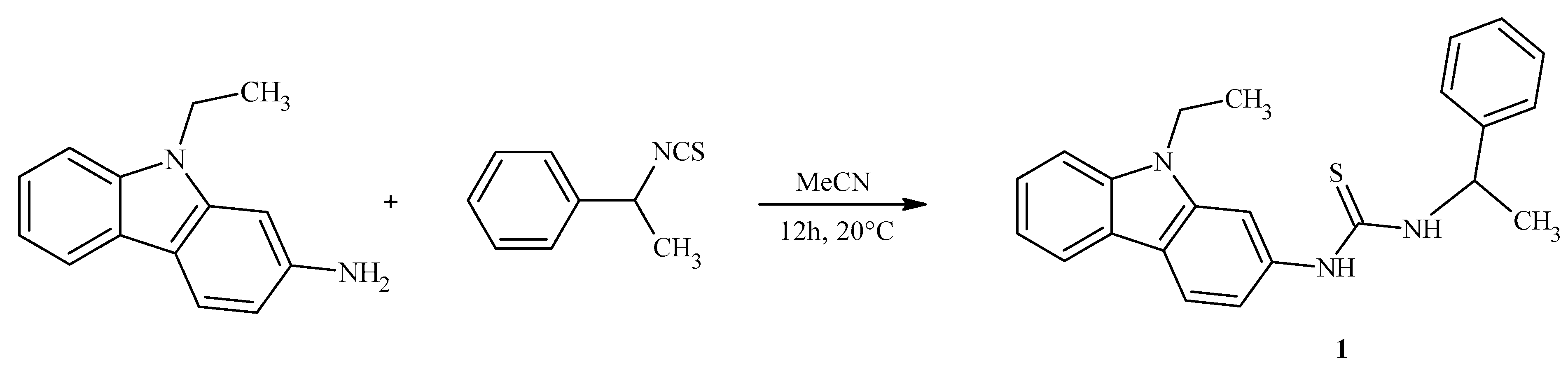
3.2. Preparation of N-(9-ethyl-9H-carbazol-2-yl)-N′-(1-phenylethyl)thiourea (1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Strzyga-Łach, P.; Chrzanowska, A.; Kiernozek-Kalińska, E.; Żyżyńska-Granica, B.; Podsadni, K.; Podsadni, P.; Bielenica, A. Proapoptotic effects of halogenated bis-phenylthiourea derivatives in cancer cells. Arch. Pharm. 2023, 356, e2300105. [Google Scholar] [CrossRef] [PubMed]
- Rana, P.; Parupalli, R.; Akhir, A.; Saxena, D.; Maitra, R.; Imran, M.; Malik, P.; Mahammad Ghouse, S.; Joshi, S.V.; Srikanth, D.; et al. Synthesis and biological evaluation of new naphthalimide-thiourea derivatives as potent antimicrobial agents active against multidrug-resistant Staphylococcus aureus and Mycobacterium tuberculosis. RSC Med. Chem. 2024, 15, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Koshizuka, T.; Majima, R.; Takahashi, K.; Ishioka, K.; Suzutani, T.; Inoue, N. Characterization of a thiourea derivative that targets viral transactivators of cytomegalovirus and herpes simplex virus type 1. Antivir. Res. 2021, 196, 105207. [Google Scholar] [CrossRef] [PubMed]
- Wagdy, R.A.; Chen, P.J.; Hamed, M.M.; Darwish, S.S.; Chen, S.H.; Abadi, A.H.; Abdel-Halim, M.; Hwang, T.L.; Engel, M. From EGFR kinase inhibitors to anti-inflammatory drugs: Optimization and biological evaluation of (4-(phenylamino)quinazolinyl)-phenylthiourea derivatives as novel NF-κB inhibitors. Bioorg. Chem. 2022, 127, 105977. [Google Scholar] [CrossRef] [PubMed]
- Kollu, U.; Avula, V.K.R.; Vallela, S.; Pasupuleti, V.R.; Zyryanov, G.V.; Neelam, Y.S.; Chamarthi, N.R. Synthesis, antioxidant activity and bioinformatics studies of L-3-hydroxytyrosine templated N-alkyl/aryl substituted urea/thioureas. Bioorg. Chem. 2021, 111, 104837. [Google Scholar] [CrossRef] [PubMed]
- Ali, U.; Ali Shah, S.W.; Khan, A.U.; Badshah, H.; Darwish, H.W.; Aschner, M.; Alam, W.; Khan, H. Preclinical and in silico studies of 3-benzothioyl-1-(3-hydroxy-3-phenyl-3-propyl)-1-methylthiourea: A promising agent for depression and anxiety. Eur. J. Pharmacol. 2025, 989, 177226. [Google Scholar] [CrossRef] [PubMed]
- Głuszynska, A. Biological potential of carbazole derivatives. Eur. J. Med. Chem. 2015, 94, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Kuo, I.-C.; Lin, J.-J.; Lu, Y.-C.; Chen, C.-T.; Back, H.-T.; Lou, P.-J.; Chang, T.-C. A novel carbazole derivative, BMVC: A potential antitumor agent and fluorescence marker of cancer cells. Chem. Biodivers. 2004, 1, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-J.; Chao, Y.; Chang, Y.-H.; Ho, F.-M.; Huang, L.-J.; Huang, Y.-L.; Luh, T.-Y.; Chen, C.-P.; Lin, W.-W. Cell apoptosis induced by a synthetic carbazole compound LCY-2-CHO is mediated through activation of caspase and mitochondrial pathways. Biochem. Pharmacol. 2005, 70, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wong, Y.; Ng, O.T.W.; Bai, L.-P.; Kwong, D.W.J.; Ke, Y.; Jiang, Z.-H.; Li, H.-W.; Yung, K.K.L.; Wong, M.S. Inhibition of beta-amyloid peptide aggregation by multifunctional carbazole-based fluorophores. Angew. Chem. Int. Ed. 2012, 51, 1804–1810. [Google Scholar]
- Tasset, I.; Espínola, C.; Medina, F.J.; Feijoo, M.; Ruiz, C.; Moreno, E.; Gomez, M.M.; Collado, J.A.; Munoz, C.; Muntane, J.; et al. Neuroprotective effect of carvedilol and melatonin on 3-nitropropionic acid-induced neurotoxicity in neuroblastoma. J. Physiol. Biochem. 2009, 65, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Chen, M.; Li, M.; Luo, B.; Zhao, Y.; Huang, P.; Xue, F.; Rapposelli, S.; Pi, R.; Wen, S. Discovery of novel N-substituted carbazoles as neuroprotective agents with potent anti-oxidative activity. Eur. J. Med. Chem. 2013, 68, 81–88. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksymiuk-Kłos, A.; Słapa, R.; Bartkowiak, M.; Michalik, R.; Pazura-Turowska, M.; Szubińska-Lelonkiewicz, D.; Bielenica, A. N-(9-Ethyl-9H-carbazol-2-yl)-N′-(1-phenylethyl)thiourea. Molbank 2025, 2025, M2103. https://doi.org/10.3390/M2103
Maksymiuk-Kłos A, Słapa R, Bartkowiak M, Michalik R, Pazura-Turowska M, Szubińska-Lelonkiewicz D, Bielenica A. N-(9-Ethyl-9H-carbazol-2-yl)-N′-(1-phenylethyl)thiourea. Molbank. 2025; 2025(4):M2103. https://doi.org/10.3390/M2103
Chicago/Turabian StyleMaksymiuk-Kłos, Agnieszka, Rafał Słapa, Marek Bartkowiak, Radosław Michalik, Monika Pazura-Turowska, Dorota Szubińska-Lelonkiewicz, and Anna Bielenica. 2025. "N-(9-Ethyl-9H-carbazol-2-yl)-N′-(1-phenylethyl)thiourea" Molbank 2025, no. 4: M2103. https://doi.org/10.3390/M2103
APA StyleMaksymiuk-Kłos, A., Słapa, R., Bartkowiak, M., Michalik, R., Pazura-Turowska, M., Szubińska-Lelonkiewicz, D., & Bielenica, A. (2025). N-(9-Ethyl-9H-carbazol-2-yl)-N′-(1-phenylethyl)thiourea. Molbank, 2025(4), M2103. https://doi.org/10.3390/M2103





