Abstract
A new zirconium trichloride complex, supported by a monoanionic, pyrrole-based bisphosphinimine NNN-pincer ligand, [LZrCl3] (L = 2,5-[iPr2P=N(4-iPrC6H4)]2NH(C6H2) (1), is reported. Comparison with a related iminopincer complex reveals significant differences in bond lengths and angles between the atoms around the metal centre, largely due to the more electron donating phosphinimine (R3P=NR (R = alkyl, aryl)) functionality. The P=N bonds in complex (1•benzene) are longer than in the proteo ligand HL (L = 2,5-[Ph2P=N(4-iPrC6H4)]2NH(C6H2)), which is consistent with phosphinimine coordination to a metal. This is the only reported zirconium complex with this specific ligand scaffold; no analogous complexes have been reported for other group 4 metals. This structure expands the library of Zr pincer complexes that bear tridentate ligand frameworks and sets the stage for the preparation of related complexes.
1. Introduction
The use of multidentate ligands to stabilize zirconium is a common practice; its coordination chemistry has been extensively studied [,,]. Pincer ligands have been increasingly used in recent decades for numerous reasons, including their capacity to stabilize a wide array of thermally robust complexes, and the ease with which most can be fine-tuned [,,,]. There have been a plethora of zirconium pincer complexes reported [,,,,,,,]; typical examples include the PNP and CNN complexes, [Zr(1,9–CH2P(iPr2)–3,6–tBu)C12H4N]Cl–κ3–P,N,P [UQUPED] [] and [Zr{2–C6H5–6–(4–CH3–2–C6H5–(C4HN))C5H3N}2]Cl–κ3–C,N,N [SINCIE] [], reported by Gade and Milsmann.
The Hayes group reported the monoanionic pyrrole-based bisphosphinimine ligand, L (L = 2,5-[iPr2P=N(4-iPrC6H4)]2NH(C6H2); Ar = para-isopropylphenyl (Pipp), 2,6-diisopropylphenyl (Dipp), 2,4,6-trimethylphenyl (Mes)), that can stabilize a broad array of metal chlorides (e.g., Ga, In, Th) (Figure 1) [,]. The subtle structural differences imposed by varied N-aryl groups are exemplified in [OSAHIC, OSAHOI, OSAQAD, OSAQEH] []. For example, the P1–N2 bond lengths for LPippInCl2 (1.619(2) Å), LMesInCl2. (1.623(2) Å) and LDippInCl2 (1.6287(18) Å), are highly similar, though the increasing size of the aryl group (Pipp > Mes > Dipp) appears to lead to slight P=N elongation. The lack of ortho substituents on the Pipp group leads to slighter larger Nphosphinimine–In–Npyrrole angles (LPippInCl2: 77.52(8)°, 77.35(8)°, LMesInCl2: 76.21(8)° and 76.12(8)°, LDippInCl2: 76.83(6)°, 76.52(7)°). This difference is substantially more pronounced when comparing the Nphosphinimine–In–Nphosphinimine angles, (LPippInCl2: 154.61(7)°, LMesInCl2: 148.34(8)°, LDippInCl2: 148.26(6)°). Correspondingly, the In–Nphosphinimine distances exhibit the expected contraction in LPippInCl2 (2.273(2) Å, 2.270(2) Å) relative to LMesInCl2 (2.283(2) Å, 2.298(2) Å) and LDippInCl2 (2.287(2) Å, 2.289(2) Å).
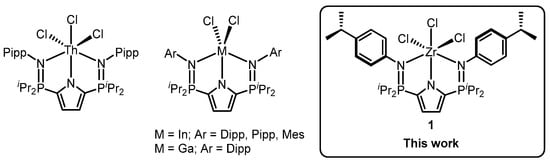
Figure 1.
Previously reported chloride complexes supported by L, and complex (1•benzene) (Pipp = para-isopropylphenyl; Dipp = 2,6-diisopropylphenyl; Mes = 2,4,6-trimethylphenyl).
In this work, we report the synthesis of the NNN-pincer complex [Zr{2,5–(4–iPrC6H4N=PiPr2)C4H2N}Cl3]–κ3–N,N,N (1) (Scheme 1). This structure expands the variety of Zr pincer complexes featuring tridentate ligand frameworks. The identity and structure of this compound were established by multinuclear NMR spectroscopic experiments and X-ray diffraction studies.
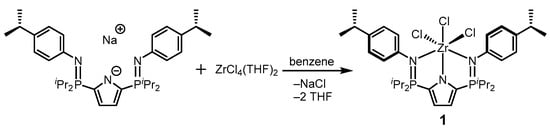
Scheme 1.
Synthesis of the title complex (1).
2. Results
2.1. X-Ray Crystal Structure of (1•Benzene)
Crystals of (1•benzene) suitable for an X-ray diffraction study were grown from a saturated solution of benzene at ambient temperature. The title compound was crystallized in the space group P21/n, with Z = 4 and Z’ = 1. The lattice symmetry elements include ī-centers, n-glides, and a 21-screw axis. The distances between the metal centre and Cl2 and Cl3 are similar (2.4578(9) Å and 2.4513(10) Å, respectively), while at 2.4927(9) Å the Zr–Cl1 length is significantly longer (Table 1). As anticipated, Zr–N1 and Zr–N3 are not statistically different from one another (2.181(3) Å and 2.189(3) Å, respectively). Likewise, the two phosphinimine P–N bonds are virtually identical (P1–N2 = 1.631(3) Å and P2–N2 = 1.636(3) Å). Complex (1) has six coordinates and exhibits distorted octahedral geometry, as expected because of the rigidity of the pincer ligand. The bond angles N1–Zr1–N2 and N2–Zr1–N3 are 73.39(11)° and 73.62(11)°, respectively, which deviate markedly from the ideal value of 90°. Correspondingly, the Cl–Zr–Cl angles range from 87.87(3)° to 174.67(3)° (Figure 2) (please refer to Supplementary Materials for additional information).

Table 1.
Selected intramolecular distances (Å) and angles (degrees) in [LZrCl3] and comparison to (2) (Å).
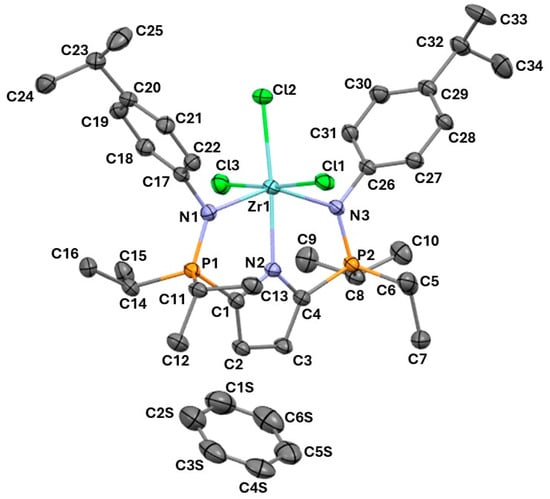
Figure 2.
The molecular structure of (1•benzene). The anisotropic displacement ellipsoids are shown at the 50% probability level. Hydrogen atoms are omitted for clarity.
2.2. Molecular Structure of (1•Benzene) and Comparison to (2)
An extensive literature search revealed that there are no previously reported zirconium NNN-pincer complexes with a phosphinimine functionality as part of the ligand scaffold. However, the CCDC contains one zirconium trichloride complex supported by an NNN-pincer ligand that features a pyrrole core (2) KORWOG [] (Figure 3). In that example, the substituents at the 2 and 5 positions of the pyrrole are anthracene-substituted pyridines. The Zr1–Cl bonds vary slightly from one another (Zr1–Cl1 = 2.4189(4) Å, Zr1–Cl2 = 2.4006(4) Å, Zr1–Cl3 = 2.4273(4) Å).
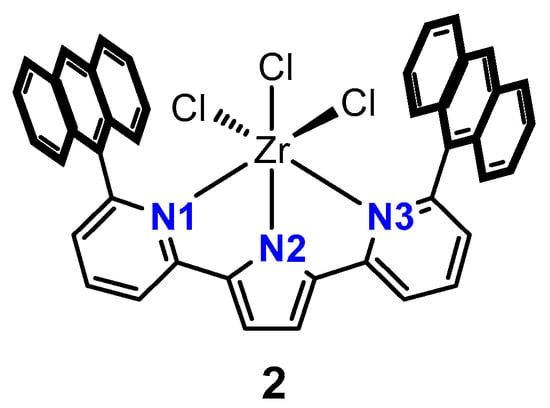
Figure 3.
Zirconium trichloride NNN-pincer complex reported by Agapie and colleagues [].
The distances between the nitrogen atoms and the metal centre in (2) (Zr1–N1 = 2.3685(11) Å, Zr1–N2 = 2.1478(11) Å, Zr1–N3 = 2.3921(11) Å) differ markedly from those in (1•benzene), which exhibits Zr1–N1, Zr1–N2 and Zr1–N3 lengths of 2.181(3) Å, 2.233(3) Å, and 2.189(3) Å, respectively (Table 1). Specifically, the metal–Npyrrole (Zr–N2) distance is substantially shorter in (2), while the bonds between the flanking donors and zirconium are much longer, presumably due to the steric bulk imparted by the anthracene substituents and the greater electron donating capacity of the phosphinimine groups in (1•benzene). For those same reasons, the Zr1–N1/N3 bond lengths in (1•benzene) are markedly shorter than those in complex (2).
Regarding bond angles, (2) exhibits N2–Zr1–N1 = 69.15(4)° and N2–Zr1–N3 = 68.47(4)°. These values are smaller than those in (1•benzene), where the corresponding angles are 73.39(11)° and 73.62(11)°, respectively. These differences may be caused by the steric constraint imposed by the two iPr groups bound to the phosphorus atoms in our pincer ligand (compared to sp2-hybridized, trigonal planar imine carbon atoms in (2)). As expected, the geometry about the 4-coordinate phosphinimine phosphorus atoms is approximately tetrahedral (N1–P1–C14 = 116.78(17)°; N1–P1–C1 = 100.57(16)°; N1–P1–C11 = 111.73(17)°; C1–P1–C14 = 108.21(18)°; C1–P1–C11 = 110.90(18)°).
Due to the absence of similar phosphinimine-bound zirconium complexes, we have elected to compare the P=N lengths in [LZrCl3] to those within the neutral proteo ligand (HL) (NAYMIL) [], which are substantially shorter (1.613(3) Å and 1.636(3) Å vs. 1.562(2) Å to 1.572(2) Å in HL). Additionally, we chose to compare (1•benzene) with LInCl2 [OSAQAD []], given that the complex is supported by the same pincer ligand and at 0.80 Å In3+ [] is close in ionic radius to Zr4+ (0.72 Å) [,]. At 1.619(2) Å and 1.615(2) Å the P=N lengths in OSAQAD are marginally shorter than those observed in (1•benzene), (1.631(3) Å and 1.636(3) Å), possibly due to the increased Lewis acidity of the latter []. The slightly contracted Zr–Nphosphinimine bonds (2.181(3) Å, 2.189(3) Å, c.f. 2.270(2) Å and 2.273(2) Å in OSAQAD) and longer Zr–Npyrrole length (2.233(3) Å vs. 2.129(2) Å) can likely be attributed to the fact that [LZrCl3] exhibits distorted octahedral geometry at zirconium while indium is distorted trigonal bipyramidal (t5 = 0.459). To the best of our knowledge, there are no reported monoanionic phosphinimine-containing NNN-pincer complexes of other group 4 metals (e.g., titanium and hafnium) that could be used for comparison.
2.3. Lattice Structure of (1•Benzene)
A depiction of the lattice structure can be seen in Figure 4. The unit cell contains four molecules of (1) and four benzene solvent molecules (Z = 4, Z′ = 1). There are four short contacts in the lattice (Table 2, Figure 4). The Cl3···C9′ contact is between the secondary carbon of a P–isopropyl group and a chlorine atom from a second molecule. Cl1···H14′ and Cl2···H12A′ are contacts between P-isopropyl methine hydrogens and chlorine atoms bonded to the metal centre, though the latter interaction is between a chlorine on one molecule and a P–isopropyl methyl from a second molecule in the unit cell. It is worth noting that the chlorine atoms do not exhibit short contacts to any other groups. There are no short contacts between the benzene solvent molecule and complex (1).
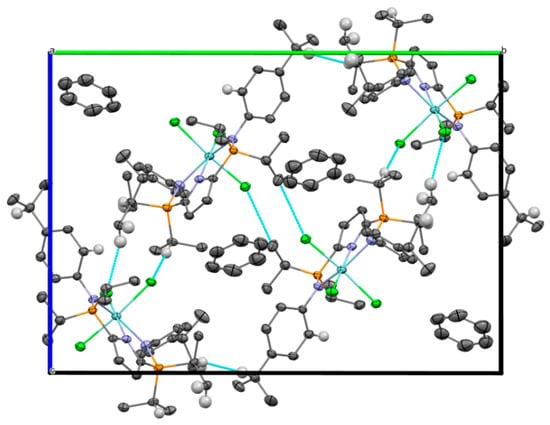
Figure 4.
Packing diagram of the unit cell of (1), viewed down the a-axis, showing intermolecular close contacts shorter than the sum of the VdW radii (displacement ellipsoids are shown at the 50% probability level). Light blue lines depict short contacts. Selected hydrogen atoms have been omitted for clarity.

Table 2.
Summary of contacts (Å) that are shorter than the sum of the VdW radii.
3. Materials and Methods
3.1. General Methods
The handling of reagents that are sensitive to air and moisture was carried out under a nitrogen atmosphere using vacuum line techniques or in an MBraun glove box. Benzene-d6, toluene and tetrahydrofuran (THF) were dried over sodium benzophenone ketyl, de-gassed via three freeze–pump–thaw cycles, distilled in vacuo and stored over 4 Å molecular sieves in glass bombs under nitrogen. All NMR spectra were recorded at ambient temperature with a Bruker Avance III NMR spectrometer (700.44 MHz for 1H, 176.13 MHz for 13C, and 283.54 MHz for 31P). Chemical shifts are reported in parts per million, relative to the external standards SiMe4 (1H, 13C) and 85% H3PO4 (31P). NaL was prepared as previously reported by the Hayes group []. [ZrCl4(THF)2] was synthesized according to a literature procedure []. [ZrCl4] was purchased from Sigma Aldrich and used without further purification.
3.2. Synthesis and Characterization
NaL (11 mg, 18 μmol) was dissolved in toluene (5 mL) at ambient temperature. The solution was added to [ZrCl4(THF)2] (6.4 mg, 17 μmol) and allowed to react for 10 min. The solution was then filtered to eliminate the NaCl by-product, followed by removal of solvent under reduced pressure. Yield 8.7 mg, 1.2 μmol (67%). 1H NMR (benzene-d6, 23 °C, 700.44 MHz): δ 7.86 (d, 3JHH = 6.8 Hz, 4H, 4-iPrC6H4), 7.01 (d, 3JHH = 6.8 Hz, 4H, 4-iPrC6H4), 6.56 (d, 3JPH = 1.5 Hz, 2H, pyrrole-H), 2.64 (sp, 3JHH = 7.0 Hz, 2H, CH(CH3)2), 2.03 (dsp, 2JPH = 14.4 Hz, 3JHH = 7.0 Hz, 4H, PCH(CH3)2), 1.06 (d, 3JHH = 7.0 Hz, 12H, CH(CH3)2), 1.04 (d, 3JHH = 7.0 Hz, 6H, PCH(CH3)2), 1.02 (septet, 3JHH = 7.0 Hz, 12H, PCH(CH3)2), 0.99 (d, 3JHH = 7.0 Hz, 6H, PCH(CH3)2). 13C{1H} NMR (benzene-d6, 23 °C, 176.13 MHz): δ 146.9 (s, aromatic ipso-C), 140.2 (s, aromatic ipso-C), 130.5 (s, aromatic CH), 127.7 (s, aromatic CH), 127.2 (d, 3JCP = 12.8 Hz, 2,5-pyrrole CH), 116.40 (dd, 2JCP = 23.9 Hz, 3JCP = 9.9 Hz, 3,4-pyrrole CH), 33.1 (s, CH(CH3)2), 27.1 (d, 1JCP = 54.6 Hz, PCH(CH3)2), 25.8 (s, CH(CH3)2), 16.6 (s, PCH(CH3)2), 16.1 (s, PCH(CH3)2). 31P{1H} NMR (benzene-d6, 23 °C, 283.54 MHz): δ 57.9. Anal Calcd. for C34H52Cl3N3P2Zr•C6H6: C, 57.16; H, 6.96; N, 5.00. Found. C, 56.78; H, 7.13; N, 4.96.
3.3. Crystallization and Refinement
Single crystals of C40H58Cl3N3P2Zr were obtained from a saturated solution of complex (1) in benzene. A suitable crystal was selected and mounted on a SuperNova, Dual, Cu at home/near, Pilatus 200K diffractometer, using Olex2 (version 1.5) []. The structure was solved using the SHELXT (version 2018/2) [] structure solution program using Intrinsic Phasing and refined with the SHELXL (version 2018/3) [] refinement package using Least Squares minimisation. One molecule of benzene co-crystallized with each molecule of [LZrCl3]. There is no notable disorder in the structure. CCDC (deposit number 2486571) contains the supplementary crystallographic data for this paper. This data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/ (accessed on 10 November 2025) (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
Crystal Data for C40H58Cl3N3P2Zr (1) (M = 840.40 g mol−1): colourless, irregular crystal, dimensions 0.065 × 0.127 × 0.236 mm, monoclinic, space group P21/c (no. 14), a = 11.6867(2) Å, b = 22.8374(4) Å, c = 17.0369(3) Å, β = 107.824(2)°, V = 4328.79(14) Å3, Z = 4, T = 100.0(4) K, μ(Cu Kα) = 4.706 mm−1, Dcalc = 1.290 g/cm3, 47598 reflections measured (6.684° ≤ 2θ ≤ 159.496°), 9338 unique (Rint = 0.0585, Rsigma = 0.0389) which were used in all calculations. The final R1 was 0.0539 (I > 2σ(I)) and wR2 was 0.1437 (all data). Goodness of fit (GooF) on F2 of 1.082.
Supplementary Materials
The following supporting information is available. Table S1. Crystal data and structure refinement for [LZrCl3], Table S2. Fractional Atomic Coordinates (×104) and Equivalent Isotropic Displacement Parameters (Å2 × 103) for [LZrCl3]. Ueq is defined as 1/3 of the trace of the orthogonalised UIJ tensor, Table S3. Anisotropic Displacement Parameters (Å2 × 103) for [LZrCl3]. The Anisotropic displacement factor exponent takes the form: −2π2[h2a*2U11 + 2hka*b*U12 + …], Table S4. Bond Lengths for [LZrCl3], Table S5. Bond Angles for [LZrCl3], Table S6. Torsion Angles for [LZrCl3], Table S7. Hydrogen Atom Coordinates (Å × 104) and Isotropic Displacement Parameters (Å2 × 103) for [LZrCl3], Figure S1. 1H NMR spectrum (700 MHz) of [LZrCl3] in benzene-d6 at 22 °C; Figure S2. 13C{1H} NMR spectrum (176 MHz) of LZrCl3 in benzene-d6 at 22 °C; Figure S3. 31P{1H} NMR spectrum (284 MHz) of [LZrCl3] in benzene-d6 at 22 °C.
Author Contributions
T.V.S.-B. contributed to investigation, methodology, formal analysis, data curation, writing—original draft, reviewing and editing. C.P.F. contributed to methodology and data acquisition. P.G.H. contributed to investigation, methodology, formal analysis, data curation, conceptualization, project administration, supervision, writing—original draft, reviewing and editing, resources and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Discovery Grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada (341687). The diffractometer at the University of Lethbridge X-ray Diffraction Facility was purchased by the University and the Faculty of Arts & Science.
Data Availability Statement
The data that supports the findings of this study are available in the Supplementary Materials.
Acknowledgments
P.G.H. thanks the University of Lethbridge for a Tier I Board of Governors Research Chair in Organometallic Chemistry. Emily L. Trew is gratefully acknowledged for her constant support as part of the Hayes group. René T. Boeré is acknowledged for his invaluable knowledge and support regarding the data analysis. T.V.S.-B. thanks SECIHTI for a Postgraduate Scholarship in Science and Humanities Abroad with CVU: 2103527.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Pipp | para-isopropylphenyl |
| Dipp | 2,6-diisopropylphenyl |
| Mes | 2,4,6-trimethylphenyl |
| SC-XRD | single crystal X-ray diffraction |
| NMR | nuclear magnetic resonance |
| THF | tetrahydrofuran |
References
- Miller, D.A.; Bereman, R.D. The chemistry of d1 complexes of niobium, tantalum, zirconium and hafnium. Coord. Chem. Rev. 1972, 9, 107–143. [Google Scholar] [CrossRef]
- Burford, R.J.; Yeo, A.; Fryzuk, M.D. Dinitrogen activation by group 4 and group 5 metal complexes supported by phosphine-amido containing ligand manifolds. Coord. Chem. Rev. 2017, 334, 84–99. [Google Scholar] [CrossRef]
- Hea, F.; Zoisc, K.P.; Tzelid, D.; Danopoulos, A.A.; Braunstein, P. N-heterocyclic carbenes as bridgehead donors in metal pincer complexes. Coord. Chem. Rev. 2023, 514, 215757. [Google Scholar] [CrossRef]
- MacNeil, C.S.; Dickie, T.K.K.; Hayes, P.G. Chapter 7: Actinide Pincer Chemistry: A New Frontier. In The Chemistry of Pincer Compounds; Morales-Morales, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 133–172. [Google Scholar] [CrossRef]
- Melen, R.L.; Gade, L.H. New Chemistry with Anionic NNN Pincer Ligands. In Topics in Organometallic Chemistry: Organometallic Pincer Chemistry II; Koten, G.V., Gossage, R.A., Eds.; Springer International Publishing: Switzerland, Cham, 2016; Volume 54, pp. 179–208. [Google Scholar]
- Morales-Morales, D.; Jensen, C.M. The Chemistry of Pincer Compounds; Elsevier Science: Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- van Koten, G.; Milstein, D. Organometallic Pincer Chemistry; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Pugh, D.; Danopoulos, A.A. Metal complexes with ‘pincer’-type ligands incorporating N-heterocyclic carbene functionalities. Coord. Chem. Rev. 2007, 251, 610–641. [Google Scholar] [CrossRef]
- Kleinhans, G.; Karhu, A.J.; Boddaert, H.; Tanweer, S.; Wunderlin, D.; Bezuidenhout, D.I. LNL-Carbazole Pincer Ligand: More than the Sum of Its Parts. Chem. Rev. 2023, 123, 8781–8858. [Google Scholar] [CrossRef] [PubMed]
- Cavell, R.G.; Kamalesh Babu, R.P.; Aparna, K. Early transition metal and lanthanide bis(iminophosphorano)methandiide complexes; ‘pincer’ and bridging bis(phosphorus) metal carbenes. J. Organomet. Chem. 2001, 617, 158–159. [Google Scholar] [CrossRef]
- Liang, L.-C. Metal complexes of chelating diarylamido phosphine ligands. Coord. Chem. Rev. 2006, 250, 1152–1177. [Google Scholar] [CrossRef]
- Zhang, D.; Zi, G. N-heterocyclic carbene (NHC) complexes of group 4 transition metals. Chem. Soc. Rev. 2015, 44, 1898–1921. [Google Scholar] [CrossRef] [PubMed]
- Plundrich, G.T.; Wadepohl, H.; Clot, E.; Gade, L.H. η6-Arene-Zirconium-PNP-Pincer Complexes: Mechanism of TheirHydrogenolytic Formation and Their Reactivity as Zirconium(II)Synthons. Chem. Eur. J. 2016, 22, 9283–9292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Petersen, J.L.; Milsmann, C. Photochemical C−C Bond Formation in Luminescent Zirconium Complexes with CNN Pincer Ligands. Organometallics 2018, 37, 4488–4499. [Google Scholar] [CrossRef]
- Chisholm, D.T.; Hayes, P.G. Synthesis and characterization of group 13 dichloride (M = Ga, In), dimethyl (M = Al) and cationic methyl aluminum complexes supported by monoanionic NNN-pincer ligands. New J. Chem. 2021, 45, 15043–15052. [Google Scholar] [CrossRef]
- Dickie, T.K.K.; Aborawi, A.A.; Hayes, P.G. Diphosphazide-Supported Trialkyl Thorium(IV) Complex. Organometallics 2020, 9, 2047–2052. [Google Scholar] [CrossRef]
- Sampson, J.; Choi, G.; Akhtar, M.N.; Jaseer, E.A.; Theravalappil, R.; Garcia, N.; Agapie, T. Early Metal Di(pyridyl) Pyrrolide Complexes with Second Coordination Sphere Arene−π Interactions: Ligand Binding and Ethylene Polymerization. ACS Omega 2019, 4, 15879–15892. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.R.D.; Hannon, M.A.; Ritch, J.S.; Hayes, P.G. Thermally stable rare earth dialkyl complexes supported by a novel bis(phosphinimine)pyrrole ligand. Dalton Trans. 2012, 41, 7873–7875. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Kelly, A.; Knowles, K.M. Appendix 7: Crystal Structure Data. In Crystallography and Crystal Defects; Wiley: Hoboken, NJ, USA, 2012; pp. 491–498. [Google Scholar] [CrossRef]
- Manzer, L.E.; Deaton, J.; Sharp, P.; Schrock, R.R. 31. Tetragtdrfuran Complexes of Selected Early Transition Metals. In Inorganic Syntheses; Fackler, J.P., Jr., Ed.; Wiley: Hoboken, NJ, USA, 1982. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystalstructure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).