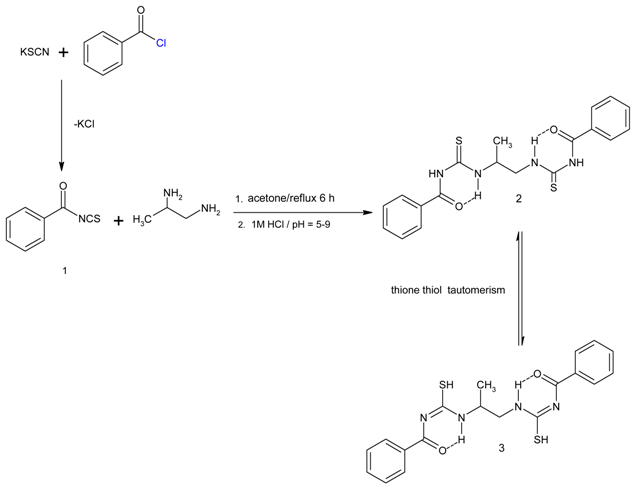
Benzoylthioureas have found some interest due to their biological activity [], spectroscopic and structural properties [,], or as synthetic building blocks []. Here, we report the convenient preparation of a new representative of this type of compounds. Benzoyl isothiocyanate (1) was prepared by known methods reported in the literature []. Benzoyl isothiocyanate (21 ml) was added to a solution of 1,2-diaminopropane (17 ml) in anhydrous acetone. The resulting mixture was refluxed for 6 h. Finally, the mixture was cooled in an ice bath and 1M HCl (250 ml) was added. The yellow precipitate was collected by filtration and it was washed with diethyl ether. The title compound 2 thus obtained was recrystallized from EtOH/CH2Cl2.
Color: yellow.
Mp 162-163°C.
Elemental analysis: Found: C, 57.5; H, 5.1; N, 13.9; S, 16.0. Calc. for C19H20N4S2O2: C, 57.0; H, 5.0; N, 14.0; S, 16.0.
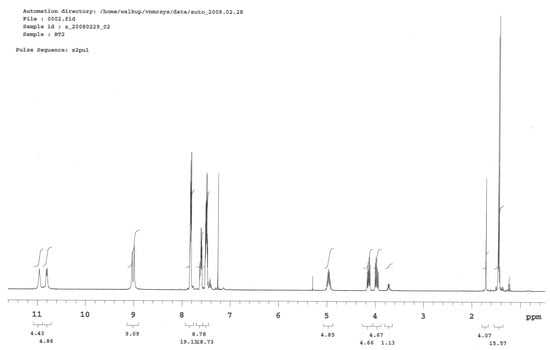
1H NMR: δ (CDCl3, 400.1 MHz): 11.00 (s, 1H, NH-CO); 10.96 (d, 1H, NH-CO); 9.04 (s, 1H, NH); 8.99 (s, 1H, NH); 7.84–7. 52 (m, 10H, PhH); 5.03–4.98 (m, 1H, CH); 4.19–3.99 (m, 2H, CH2); 1,46–1,44 (d, 3H, CH3).
13C NMR: δ (CDCl3, 100.0 MHz): 185.2 (C=S); 164.0 (C=O); 135.6–127.2 (C=Carom); 47.5 (CH); 46.9 (CH2); 18.7 (CH3).
IR (KBr) νmax/cm-1: 3350–3300 (N-H), 3161 (C-Haromatic), 3038 (CH3), 2935–2859 (C-Haliphatic), 1980–1835 (C=C), 1677 (C=O), 1540–1258 (C-N), 1189, 1162 (C=S).
UV-vis (CH2Cl2, abs): 240; 400.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Xu, X.Y.; Qian, X.H.; Li, Z.; Huang, Q.C.; Chen, G. J. Fluorine Chem. 2003, 121, 51–54. [CrossRef]
- Zhou, W.Q.; Yang, W.; Qiu, L.H.; Zhang, Y.; Yu, Z.F. J. Mol. Struct. 2005, 749, 89–95.
- Arslan, H.; Külcü, N.; Flörke, U. Spectrochim. Acta Pt. A 2006, 64, 1065–1071. [CrossRef] [PubMed]
- Kodomari, M.; Suzuki, M.; Tanigawa, K.; Aoyama, T. Tetrahedron Lett. 2005, 46, 5841–5843.
- Binzet, G.; Arslan, H.; Flörke, U.; Külcü, N.; Duran, N. J. Coordination Chem. 2006, 59, 1395–1406. [CrossRef]
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).