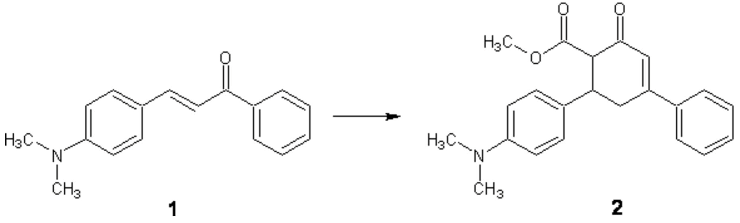
A mixture of 4-dimethylaminochalcone 1 (0.25 g, 1.00 mmol), methyl acetoacetate (0.17 g, 1.5 mmol) in methanol (5.00 mL) and sodium metoxide (0.25 mg) was stirred at room temperature for 24 hour to give a yellow precipitate. Then it was filtered and washed with water. Recrystalized of the crude substance from methanol afforded pure cyclohexenone 2 (2.27 g, 97.00 %).
Melting point: 134-136 °C.
IR (KBr, cm-1): 1740(CO); 1666 (CO).
1H-NMR (CDCl3, 270 MHz): d= 7.59 (d, 2H, J = 8.56 Hz), 7.33 (dd, 2H, J = 8.56, 8.56 Hz), 7.24 (dd, 2H, J = 8.50, 2.2 Hz) 6.58 (d, 1H J = 1.98 Hz), 6.60 (dd, 2H, J = 8.56, 8,56 Hz), 3.82 (dd, 1H, J = 5.4, 2.45 Hz), 3.58 (s, 3H, CO2CH3), 3.10 (dt, 1H, J = 12.5, 3.7, 1.98 Hz), 3.01 (d, 1H, J = 12.5 Hz), 2.98 (s, 6H, (CH3)2N).
13C NMR (CDCl3) (270 MHz) d (ppm): 190.0 (C=O), 174.8 (COO), 141.3, 128.6, 127.2, 126.8, 111.9 (aromatic carbons), 149.6, 120.3, 59.2, 52.5, 44,5, 39.2, 31.5.
MS (m/z, %): 351(M + 1, 10), 292 (351- CO2CH3,100).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Reference
- Rojas; Domínguez, José N.; Charris, Jaime E.; Lobo, Gricela; Payá, Miguel; Ferrándiz, M. Luisa. European Journal of Medicinal Chemistry, 2002; 37, 699.
- Sample Availability: Available from MDPI.
© 2005 MDPI. All rights reserved.