Astragalus Mongholicus Polysaccharides Alleviate Kidney Injury in Rats with Type 2 Diabetes Through Modulation of Oxidation, Inflammation, and Gut Microbiota
Abstract
1. Introduction
2. Results
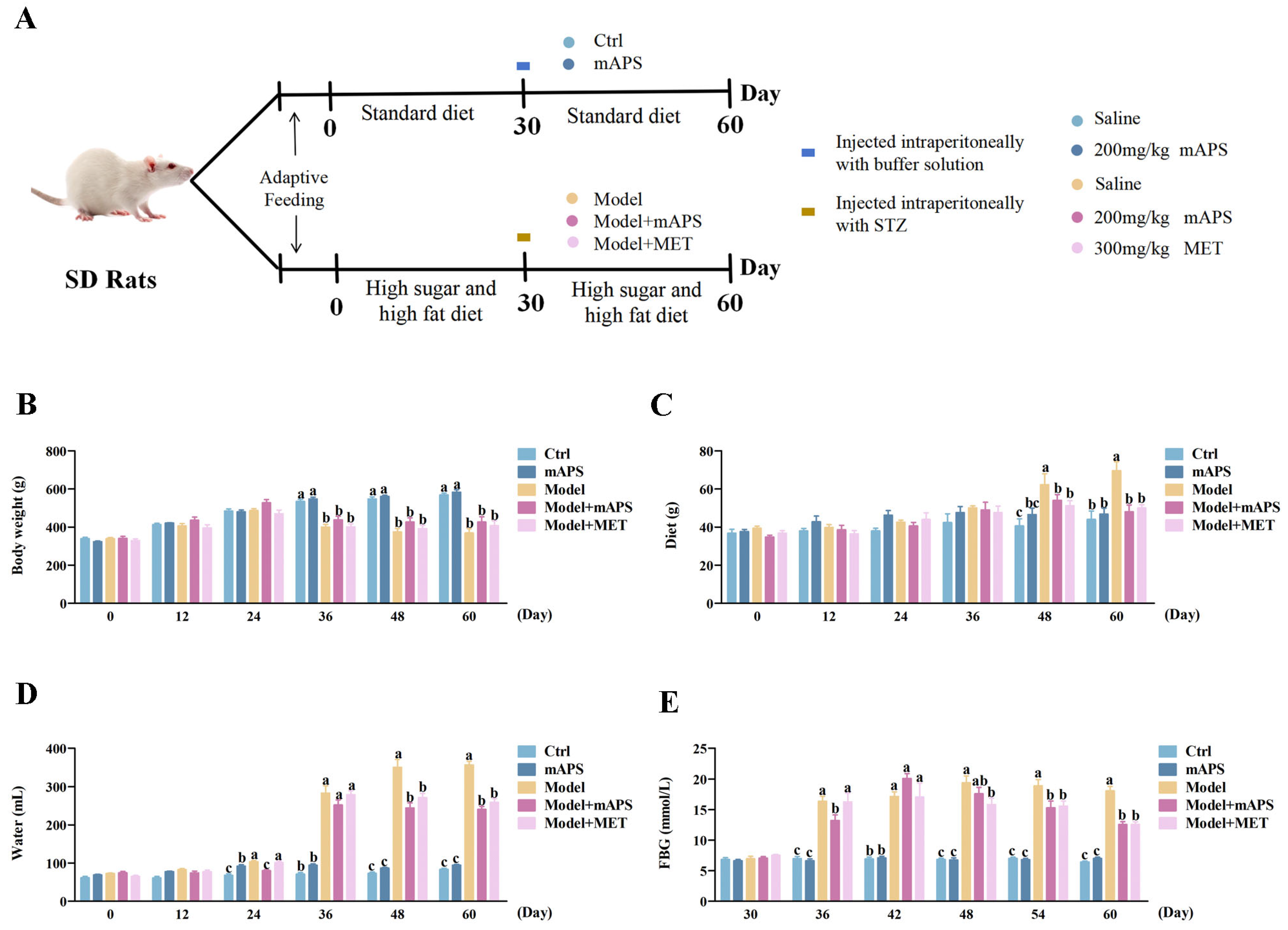
2.1. mAPS Supplementation Affects the General Changes in HSHFD/STZ-Induced Diabetic Rats
2.2. mAPS Ameliorated Renal Injury in HSHFD/STZ-Induced Diabetic Rats
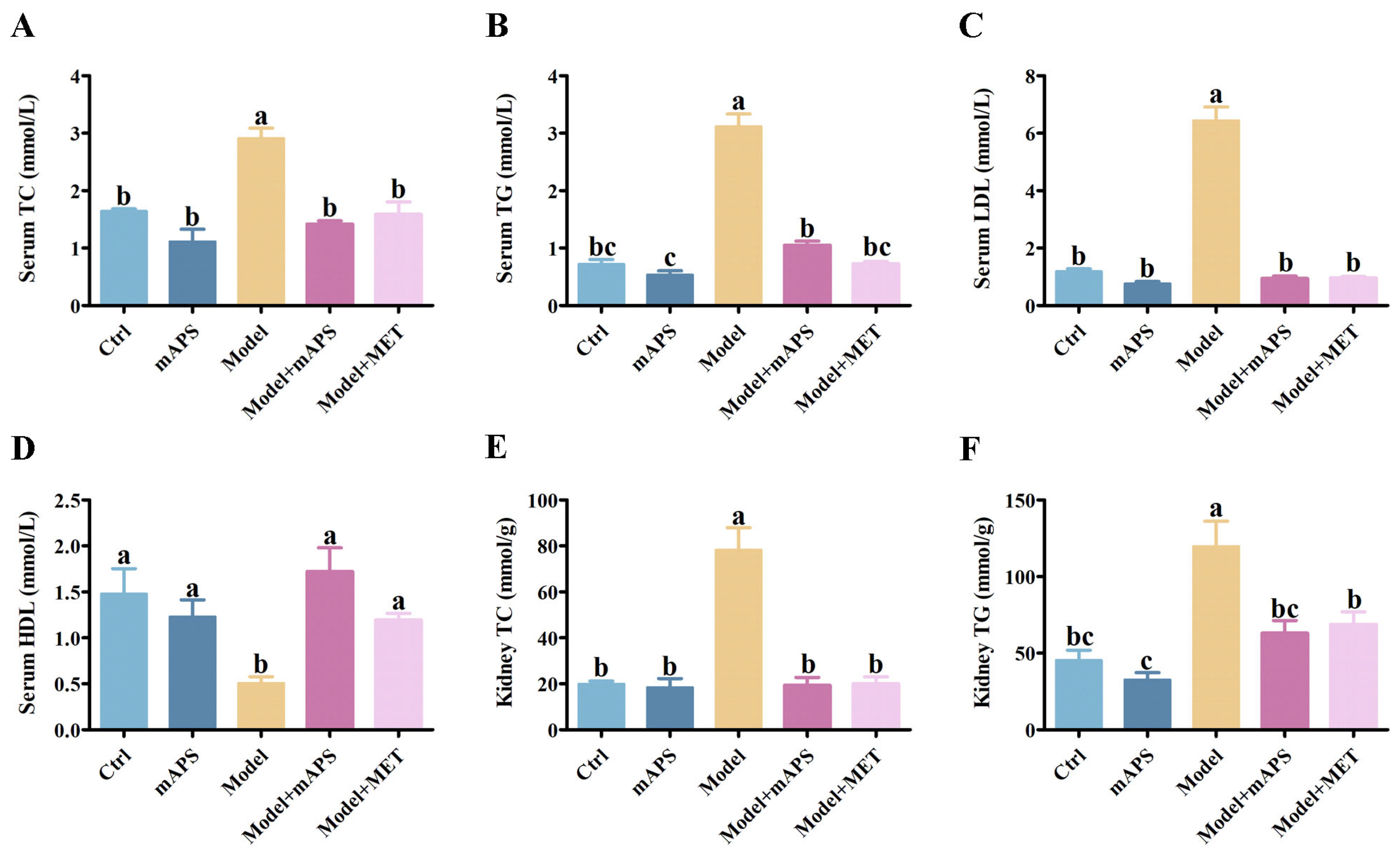
2.3. mAPS Ameliorated Dyslipidemia in HSHFD/STZ-Induced Diabetic Rats
2.4. Effect of mAPS on Colon and Kidney Pathological Injury
2.5. mAPS Mitigated Oxidative Stress in HSHFD/STZ-Induced Diabetic Rats
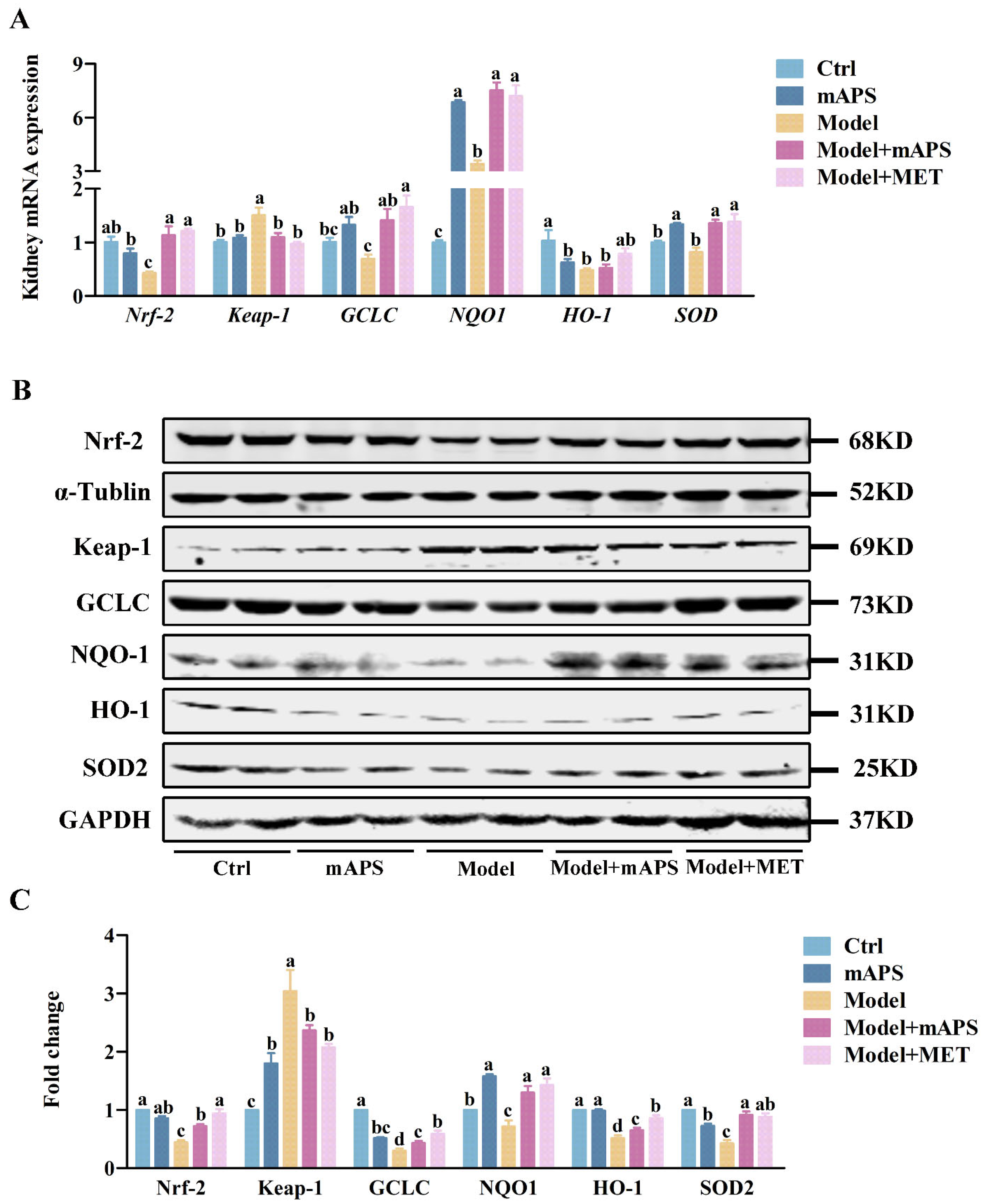
2.6. mAPS Activated the Renal Nrf-2/Keap1 Signaling Pathway in HSHFD/STZ-Induced Diabetic Rats
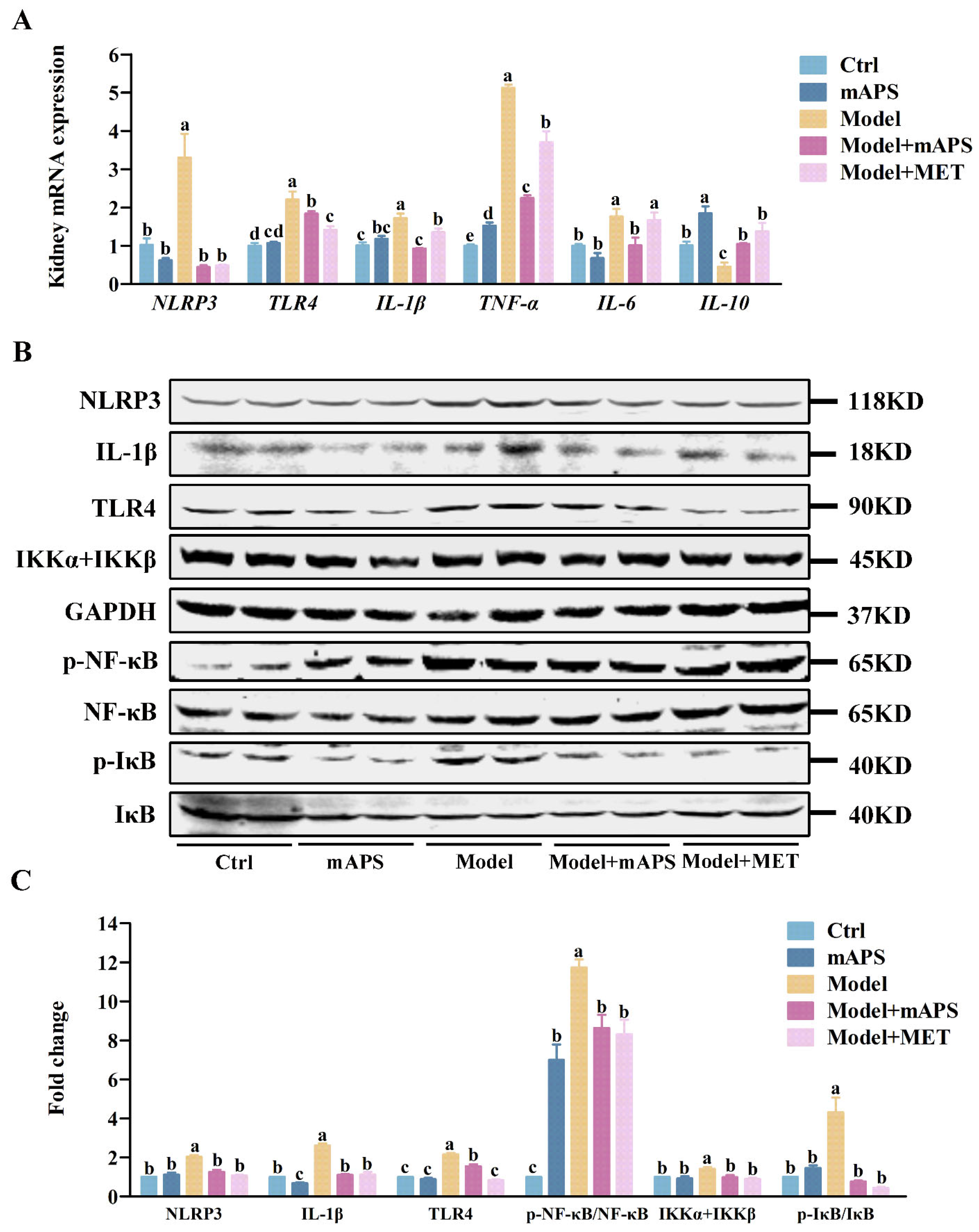
2.7. mAPS Inhibits Kidney TLR4/NF-κB Signaling Pathway in HSHFD/STZ-Induced Diabetic Rats
2.8. mAPS Improved the Renal Fibrosis in HSHFD/STZ-Induced Diabetic Rats
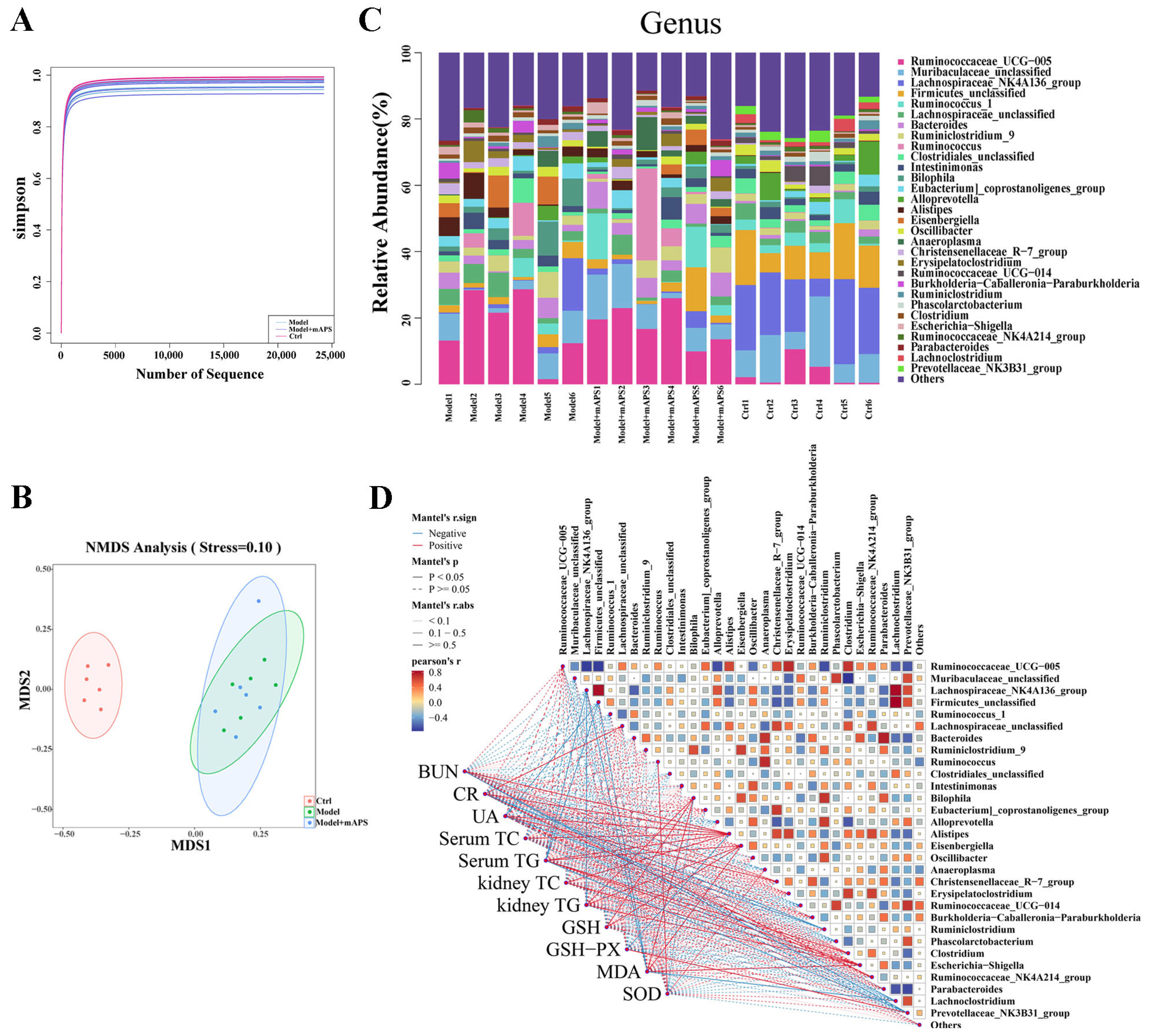
2.9. mAPS Reshaped Gut Microbiota in HSHFD/STZ-Induced Diabetic Rats
2.10. mAPS Improved Colon Injury in HSHFD/STZ-Induced Diabetic Rats
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Experimental Design
4.3. Biochemical Analysis
4.4. Histological Analysis
4.5. Quantitative PCR
4.6. Western Blotting
4.7. 16S rDNA Gene Sequencing
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BUN | Blood urea nitrogen |
| CR | Creatinine |
| DM | Diabetes mellitus |
| DN | Diabetic nephropathy |
| FBG | Fasting blood glucose |
| GCLC | Glutamate-cysteine ligase catalytic subunit |
| GSH | Glutathione |
| GSH-PX | Glutathione peroxidase |
| HDL | High-density lipoprotein |
| HE | Hematoxylin and eosin |
| HO-1 | Heme oxygenase 1 |
| HSHFD | High-sugar and high-fat diet |
| IκB | Inhibitor of kappa B |
| IKKα + IKKβ | IκB kinase alpha + IκB kinase beta |
| IL-10 | Interleukin-10 |
| IL-1β | Interleukin-1beta |
| IL-6 | Interleukin-6 |
| Keap1 | Kelch-like ECH-associated protein 1 |
| LDL | Low-density lipoprotein |
| LPS | Lipopolysaccharide |
| mAPS | Astragalus mongholicus polysaccharides |
| MDA | Malondialdehyde |
| MET | Metformin |
| NF-κB | Nuclear factor kappa-B |
| NLRP3 | NOD-like receptor protein 3 |
| NQO1 | NAD(P)H quinone dehydrogenase 1 |
| Nrf-2 | Nuclear factor erythroid 2-related factor 2 |
| PCR | Polymerase chain reaction |
| Smad4 | Smad family member 4 |
| SOD | Superoxide dismutase |
| STZ | Streptozotocin |
| TC | Total cholesterol |
| TG | Triglyceride |
| TGF-β | Transforming growth factor beta |
| TGFβR | Transforming growth factor beta-receptor |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor-alpha |
| UA | Uric acid |
| ZO-1 | Zona occludens 1 |
References
- Yuan, C.M.; Nee, R.; Ceckowski, K.A.; Knight, K.R.; Abbott, K.C. Diabetic nephropathy as the cause of end-stage kidney disease reported on the medical evidence form CMS2728 at a single center. Clin. Kidney J. 2016, 10, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Gui, D.; Zheng, L.; Zhai, R.; Wang, F.; Wang, N. Mechanistic Insight and Management of Diabetic Nephropathy: Recent Progress and Future Perspective. J. Diabetes Res. 2017, 2017, 1839809. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Biomed. Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.K.; Tesch, G.H. Inflammation in diabetic nephropathy. Mediat. Inflamm. 2012, 2012, 146154. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, T.; Qiao, Y.; Liu, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Peng, L.; Zhan, Y. Oxidative stress and inflammation in diabetic nephropathy: Role of polyphenols. Front. Immunol. 2023, 14, 1185317. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, N.; Haruna, Y.; Kondeti, V.K.; Kanwar, Y.S. Oxidative stress in diabetic nephropathy. Curr. Med. Chem. 2010, 17, 4256–4269. [Google Scholar] [CrossRef]
- Cheng, G.; Liu, Y.; Guo, R.; Wang, H.; Zhang, W.; Wang, Y. Molecular mechanisms of gut microbiota in diabetic nephropathy. Diabetes Res. Clin. Pract. 2024, 213, 111726. [Google Scholar] [CrossRef]
- Li, J.; Lv, J.L.; Cao, X.Y.; Zhang, H.P.; Tan, Y.J.; Chu, T.; Zhao, L.L.; Liu, Z.; Ren, Y.S. Gut microbiota dysbiosis as an inflammaging condition that regulates obesity-related retinopathy and nephropathy. Front. Microbiol. 2022, 13, 1040846. [Google Scholar] [CrossRef]
- Nagase, N.; Ikeda, Y.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Efficacy of probiotics on the modulation of gut microbiota in the treatment of diabetic nephropathy. World J. Diabetes 2022, 13, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.W.; Huang, Y.Y.; Tsai, S.Y.; Wang, J.Y.; Lin, J.H.; Syu, Z.J.; Wang, H.S.; Hsu, Y.C.; Chen, J.F.; Hsia, K.C.; et al. Probiotic Formula Ameliorates Renal Dysfunction Indicators, Glycemic Levels, and Blood Pressure in a Diabetic Nephropathy Mouse Model. Nutrients 2023, 15, 2803. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, Y.; Xu, D.; Wang, Q. Probiotics ameliorates glycemic control of patients with diabetic nephropathy: A randomized clinical study. J. Clin. Lab. Anal. 2021, 35, e23650. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, R.; Lotfi, H.; Hejazi, M.A.; Ghiasi, F.; Keyhanmanesh, R. The role of probiotics on microvascular complications of type-2 diabetes: Nephropathy and retinopathy. J. Cardiovasc. Thorac. Res. 2024, 16, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dong, H.; Wang, Y.; Jiang, Y.; Zhang, W.; Lu, Y.; Chen, Y.; Chen, L. Cordyceps cicadae polysaccharides ameliorated renal interstitial fibrosis in diabetic nephropathy rats by repressing inflammation and modulating gut microbiota dysbiosis. Int. J. Biol. Macromol. 2020, 163, 442–456. [Google Scholar] [CrossRef]
- Feng, Y.; Weng, H.; Ling, L.; Zeng, T.; Zhang, Y.; Chen, D.; Li, H. Modulating the gut microbiota and inflammation is involved in the effect of Bupleurum polysaccharides against diabetic nephropathy in mice. Int. J. Biol. Macromol. 2019, 132, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Neumiller, J.J.; Johnson, E.J.; Dieter, B.; Tuttle, K.R. Sodium–Glucose Cotransporter 2 Inhibition and Diabetic Kidney Disease. Diabetes 2019, 68, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Melchior, W.R.; Bindlish, V.; Jaber, L.A. Angiotensin-converting enzyme inhibitors in diabetic nephropathy. Ann. Pharmacother. 1993, 27, 344–350. [Google Scholar] [CrossRef]
- Greco, E.V.; Russo, G.; Giandalia, A.; Viazzi, F.; Pontremoli, R.; De Cosmo, S. GLP-1 Receptor Agonists and Kidney Protection. Medicina 2019, 55, 233. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, H.; Zhou, Q.; Wang, M.W.; Li, Z. A potentially serious adverse effect of GLP-1 receptor agonists. Acta Pharm. Sin. B 2023, 13, 2291–2293. [Google Scholar] [CrossRef]
- Na Takuathung, M.; Sakuludomkan, W.; Khatsri, R.; Dukaew, N.; Kraivisitkul, N.; Ahmadmusa, B.; Mahakkanukrauh, C.; Wangthaweesap, K.; Onin, J.; Srichai, S.; et al. Adverse Effects of Angiotensin-Converting Enzyme Inhibitors in Humans: A Systematic Review and Meta-Analysis of 378 Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 8373. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Wu, Y.; Wu, Q.; Zhao, G.; Cai, Z.; Pu, F.; Li, B. Efficacy and safety of Shenqi Jiangtang Granules plus oral hypoglycemic agent in patients with type 2 diabetes mellitus: A protocol for systematic review and meta-analysis of 15 RCTs. Medicine 2021, 100, e23578. [Google Scholar] [CrossRef]
- Shao, P.; Zhao, L.H.; Zhi, C.; Pan, J.P. Regulation on maturation and function of dendritic cells by Astragalus mongholicus polysaccharides. Int. Immunopharmacol. 2006, 6, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Lin, H.; Zheng, P.; Hou, L.; Zhang, M.; Lin, S.; Yin, K.; Zhao, G. WTAP mediates the anti-inflammatory effect of Astragalus mongholicus polysaccharide on THP-1 macrophages. Front. Pharmacol. 2022, 13, 1023878. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Xie, Y.; Sun, S.; Sun, X.; Ren, F.; Shi, Q.; Wang, S.; Zhang, W.; Li, X.; Zhang, J. Chemical analysis of Astragalus mongholicus polysaccharides and antioxidant activity of the polysaccharides. Carbohydr. Polym. 2010, 82, 636–640. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, G.; Liu, J.; Yan, Y.; Zhao, S.; Cai, F.; Yu, X.; Wang, Y.; Li, M. Astragalus mongholicus polysaccharides alleviate insulin resistance through modulation of PI3K/AKT, TLR4/NF-kB signaling pathway and microbiota in rats with Type 2 Diabetes Mellitus. J. Tradit. Complement. Med. 2024. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Mou, X.; Wang, H.; Xie, Z. Amygdalin alleviates renal injury by suppressing inflammation, oxidative stress and fibrosis in streptozotocin-induced diabetic rats. Life Sci. 2021, 265, 118835. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, C.; Fu, X. Hypoglycemic activity in vitro and vivo of a water-soluble polysaccharide from Astragalus membranaceus. Food Funct. 2022, 13, 11210–11222. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, Y.; Tang, L.; Yang, X.; Chen, Z.; Wei, Y.; Shao, X.; Shao, X.; Xin, Z.; Cai, B.; et al. Astragalus Polysaccharide Attenuates Cisplatin-Induced Acute Kidney Injury by Suppressing Oxidative Damage and Mitochondrial Dysfunction. BioMed Res. Int. 2020, 2020, 2851349. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-M.; Sun, X.-Y.; Ouyang, J.-M. Structural Characterization, Antioxidant Activity, and Biomedical Application of Astragalus Polysaccharide Degradation Products. Int. J. Polym. Sci. 2018, 2018, 5136185. [Google Scholar] [CrossRef]
- Yousuf, S.; Shabir, S.; Singh, M.P. Protection Against Drug-Induced Liver Injuries Through Nutraceuticals via Amelioration of Nrf-2 Signaling. J. Am. Nutr. Assoc. 2023, 42, 495–515. [Google Scholar] [CrossRef]
- Gupta, A.; Behl, T.; Sehgal, A.; Bhatia, S.; Jaglan, D.; Bungau, S. Therapeutic potential of Nrf-2 pathway in the treatment of diabetic neuropathy and nephropathy. Mol. Biol. Rep. 2021, 48, 2761–2774. [Google Scholar] [CrossRef] [PubMed]
- Basak, P.; Sadhukhan, P.; Sarkar, P.; Sil, P.C. Perspectives of the Nrf-2 signaling pathway in cancer progression and therapy. Toxicol. Rep. 2017, 4, 306–318. [Google Scholar] [CrossRef]
- Qi, J.H.; Dong, F.X. The relevant targets of anti-oxidative stress: A review. J. Drug Target. 2021, 29, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Li, S.; Zhang, C.; Chen, H.; Wang, N.; Feng, Y. Clinical efficacies, underlying mechanisms and molecular targets of Chinese medicines for diabetic nephropathy treatment and management. Acta Pharm. Sin. B 2021, 11, 2749–2767. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Hua, J.; Lin, S.-J.; Zheng, H.-T.; Wang, J.-J.; Li, W.; Ke, J.-J.; Cai, H.-B. Astragalus polysaccharide alleviates cognitive impairment and β-amyloid accumulation in APP/PS1 mice via Nrf2 pathway. Biochem. Biophys. Res. Commun. 2020, 531, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Rayego-Mateos, S.; Morgado-Pascual, J.L.; Opazo-Ríos, L.; Guerrero-Hue, M.; García-Caballero, C.; Vázquez-Carballo, C.; Mas, S.; Sanz, A.B.; Herencia, C.; Mezzano, S.; et al. Pathogenic Pathways and Therapeutic Approaches Targeting Inflammation in Diabetic Nephropathy. Int. J. Mol. Sci. 2020, 21, 3798. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, J.; Liu, M.; Shao, Y.; Dong, X. Schisandrin C attenuates renal damage in diabetic nephropathy by regulating macrophage polarization. Am. J. Transl. Res. 2021, 13, 210–222. [Google Scholar] [PubMed]
- Ke, G.; Chen, X.; Liao, R.; Xu, L.; Zhang, L.; Zhang, H.; Kuang, S.; Du, Y.; Hu, J.; Lian, Z.; et al. Receptor activator of NF-κB mediates podocyte injury in diabetic nephropathy. Kidney Int. 2021, 100, 377–390. [Google Scholar] [CrossRef]
- Chen, J.; Peng, H.; Chen, C.; Wang, Y.; Sang, T.; Cai, Z.; Zhao, Q.; Chen, S.; Lin, X.; Eling, T.; et al. NAG-1/GDF15 inhibits diabetic nephropathy via inhibiting AGE/RAGE-mediated inflammation signaling pathways in C57BL/6 mice and HK-2 cells. Life Sci. 2022, 311, 121142. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bai, L.; Chen, X.; Li, Y.; Qin, Y.; Meng, X.; Zhang, Q. 6-Shogaol ameliorates diabetic nephropathy through anti-inflammatory, hyperlipidemic, anti-oxidative activity in db/db mice. Biomed. Pharmacother. 2018, 97, 633–641. [Google Scholar] [CrossRef]
- Leaf, I.A.; Duffield, J.S. What can target kidney fibrosis? Nephrol. Dial. Transplant. 2017, 32, i89–i97. [Google Scholar] [CrossRef]
- Scharenberg, M.A.; Pippenger, B.E.; Sack, R.; Zingg, D.; Ferralli, J.; Schenk, S.; Martin, I.; Chiquet-Ehrismann, R. TGF-β-induced differentiation into myofibroblasts involves specific regulation of two MKL1 isoforms. J. Cell Sci. 2014, 127, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Sun, Q.; Zhang, Y.M.; Zou, L.; Zhao, Y.Y. TGF-β/Smad Signaling Pathway in Tubulointerstitial Fibrosis. Front. Pharmacol. 2022, 13, 860588. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, W. Protective effect of berberine on renal fibrosis caused by diabetic nephropathy. Mol. Med. Rep. 2017, 16, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.; Zhang, X.; Zhao, L.; Chu, J.; Li, H.; Sun, W.; Yang, C.; Wang, H.; Dai, W.; et al. Alterations of the Gut Microbiota in Patients with Diabetic Nephropathy. Microbiol. Spectr. 2022, 10, e0032422. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, C.E.; Liu, T.; Zhang, M.X.; Niu, Y.; Wang, M.; Yu, J. The roles of gut microbiota and its metabolites in diabetic nephropathy. Front. Microbiol. 2023, 14, 1207132. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zheng, L.; Nan, S.; Ke, L.; Fu, Z.; Jin, J. Enterorenal crosstalks in diabetic nephropathy and novel therapeutics targeting the gut microbiota. Acta Biochim. Biophys. Sin. 2022, 54, 1406–1420. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, M.; Guo, Y.; Wang, Z.; Liu, Q.; Yan, R.; Wang, Y.; Wu, Q.; Yuan, K.; Sun, W. The Profile and Function of Gut Microbiota in Diabetic Nephropathy. Diabetes Metab. Syndr. Obes. 2021, 14, 4283–4296. [Google Scholar] [CrossRef]
- Smith, B.J.; Miller, R.A.; Schmidt, T.M. Muribaculaceae Genomes Assembled from Metagenomes Suggest Genetic Drivers of Differential Response to Acarbose Treatment in Mice. mSphere 2021, 6, e0085121. [Google Scholar] [CrossRef]
- Chen, P.; Liu, L.; Cheng, Z.; Zhang, Y.; Zheng, B.; Hu, X.; Zeng, H. Structure elucidation and in vitro rat intestinal fermentation properties of a novel sulfated glucogalactan from Porphyra haitanensis. Food Sci. Hum. Wellness 2023, 12, 596–606. [Google Scholar] [CrossRef]
- Natividad, J.M.; Lamas, B.; Pham, H.P.; Michel, M.L.; Rainteau, D.; Bridonneau, C.; da Costa, G.; van Hylckama Vlieg, J.; Sovran, B.; Chamignon, C.; et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat. Commun. 2018, 9, 2802. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, D.; He, Y.; Li, Y.; Yang, Z.; Zhao, X.; Liu, Y.; Wang, Y.; Sun, J.; Feng, X.; et al. Discrepant gut microbiota markers for the classification of obesity-related metabolic abnormalities. Sci. Rep. 2019, 9, 13424. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, A.G.; Izquierdo-Lahuerta, A.; Valverde, Á.M.; Ni, L.; Flores-Salguero, E.; Coward, R.J.; Medina-Gómez, G. The protective role of peroxisome proliferator-activated receptor gamma in lipotoxic podocytes. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2023, 7, 159329. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Banks, A.S.; Kamenecka, T.M.; Busby, S.A.; Chalmers, M.J.; Kumar, N.; Kuruvilla, D.S.; Shin, Y.; He, Y.; Bruning, J.B.; et al. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature 2011, 477, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Jermendy, G. PPARγ agonists—Antidiabetic drugs with a potential role in the treatment of diseases other than diabetes. Diabetes Res. Clin. Pract. 2007, 78, S29–S39. [Google Scholar] [CrossRef]
- Zhong, M.; Yan, Y.; Yuan, H.; Rong, A.; Xu, G.; Cai, F.; Yang, Y.; Wang, Y.; Zhang, W. Astragalus mongholicus polysaccharides ameliorate hepatic lipid accumulation and inflammation as well as modulate gut microbiota in NAFLD rats. Food Funct. 2022, 13, 7287–7301. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Zhou, Z.; Tian, F.; Yang, H.; Yan, J. Combination of leflunomide and benazepril reduces renal injury of diabetic nephropathy rats and inhibits high-glucose induced cell apoptosis through regulation of NF-κB, TGF-β and TRPC6. Ren. Fail. 2019, 41, 899–906. [Google Scholar] [CrossRef]
- Willard-Mack, C.L.; Elmore, S.A.; Hall, W.C.; Harleman, J.; Kuper, C.F.; Losco, P.; Rehg, J.E.; Rühl-Fehlert, C.; Ward, J.M.; Weinstock, D.; et al. Nonproliferative and Proliferative Lesions of the Rat and Mouse Hematolymphoid System. Toxicol. Pathol. 2019, 6, 665–783. [Google Scholar] [CrossRef] [PubMed]





| The Name of The Primer | Primer Sequences |
|---|---|
| Nrf-2 sense | CCCAGCAGGACATGGATTTG |
| Nrf-2 antisense | TTTGGGAATGTGGGCAACCT |
| NQO-1 sense | CAGCGGCTCCATGTACT |
| NQO-1 antisense | GACCTGGAAGCCACAGAAG |
| SOD-2 sense | TTGGCTTCAATAAGGAGCAAG |
| SOD-2 antisense | ACACATCAATCCCCAGCAGT |
| Keap-1 sense | TAGCCTCCATGAAGCATCGC |
| Keap-1 antisense | GTCAAGCGGGTCACTTCACT |
| HO-1 sense | ACATTGAGCTGTTTGAGGAGC |
| HO-1 antisense | CTGAGTGTGAGGACCCATCG |
| IL-6 sense | CCACCAGGAACGAAAGTCAAC |
| IL-6 antisense | TTGCGGAGAGAAACTTCATAGCT |
| IL-10 sense | GCCCAGAAATCAAGGAGCATT |
| IL-10 antisense | CAGCTGTATCCAGAGGGTCTTCA |
| IL-1β sense | TGCTGTCTGACCCATGTGAG |
| IL-1β antisense | GTCGTTGCTTGTCTCTCCTTG |
| TNF-α sense | ATCGGTCCCAACAAGGAGGA |
| TNF-α antisense | TCCGCTTGGTGGTTTGCTAC |
| TLR4 sense | TGAGGACTGGGTGAGAAATGAGC |
| TLR4 antisense | CTGCCATGTTTTGAGCAATCTCAT |
| NLRP3 sense | GTGGAGATCCTAGGTTTCTCTG |
| NLRP3 antisense | CAGGATCTCATTCTCTTGGATC |
| TGF-β sense | CAACAATTCCTGGCGTTACCT |
| TGF-β antisense | AAAGCCCTGTATTCCGTCTCC |
| Actin sense | CGCGAGTACAACCTTCTTGC |
| Actin antisense | ATACCCACCATCACACCCTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Yuan, H.; Liu, J.; Wang, X.; Ma, L.; Wang, Y.; Dong, G. Astragalus Mongholicus Polysaccharides Alleviate Kidney Injury in Rats with Type 2 Diabetes Through Modulation of Oxidation, Inflammation, and Gut Microbiota. Int. J. Mol. Sci. 2025, 26, 1470. https://doi.org/10.3390/ijms26041470
Xu G, Yuan H, Liu J, Wang X, Ma L, Wang Y, Dong G. Astragalus Mongholicus Polysaccharides Alleviate Kidney Injury in Rats with Type 2 Diabetes Through Modulation of Oxidation, Inflammation, and Gut Microbiota. International Journal of Molecular Sciences. 2025; 26(4):1470. https://doi.org/10.3390/ijms26041470
Chicago/Turabian StyleXu, Guoquan, Haisheng Yuan, Jingran Liu, Xianjue Wang, Li Ma, Yuzhen Wang, and Guicheng Dong. 2025. "Astragalus Mongholicus Polysaccharides Alleviate Kidney Injury in Rats with Type 2 Diabetes Through Modulation of Oxidation, Inflammation, and Gut Microbiota" International Journal of Molecular Sciences 26, no. 4: 1470. https://doi.org/10.3390/ijms26041470
APA StyleXu, G., Yuan, H., Liu, J., Wang, X., Ma, L., Wang, Y., & Dong, G. (2025). Astragalus Mongholicus Polysaccharides Alleviate Kidney Injury in Rats with Type 2 Diabetes Through Modulation of Oxidation, Inflammation, and Gut Microbiota. International Journal of Molecular Sciences, 26(4), 1470. https://doi.org/10.3390/ijms26041470





