An AAV-Based Therapy Approach for Neurological Phenotypes of X-Linked Adrenoleukodystrophy
Abstract
1. Introduction
2. The ABCD1 Gene, Lipidomic Dysregulation, and Pathological Mechanisms in X-ALD
2.1. ABCD1: Gene, Coding Sequence, Protein Structure and Function
2.2. Lipidomics Associated with Defective ABCD1
2.3. Lipidomics Draws a Link Between the Pathological Processes of Neuroinflammation and Neurodegeneration in X-ALD
3. Determinants of the ABCD1 Expression Cassette for AAV-Based Gene Therapy
3.1. Defining Gene Therapy Targets in X-ALD
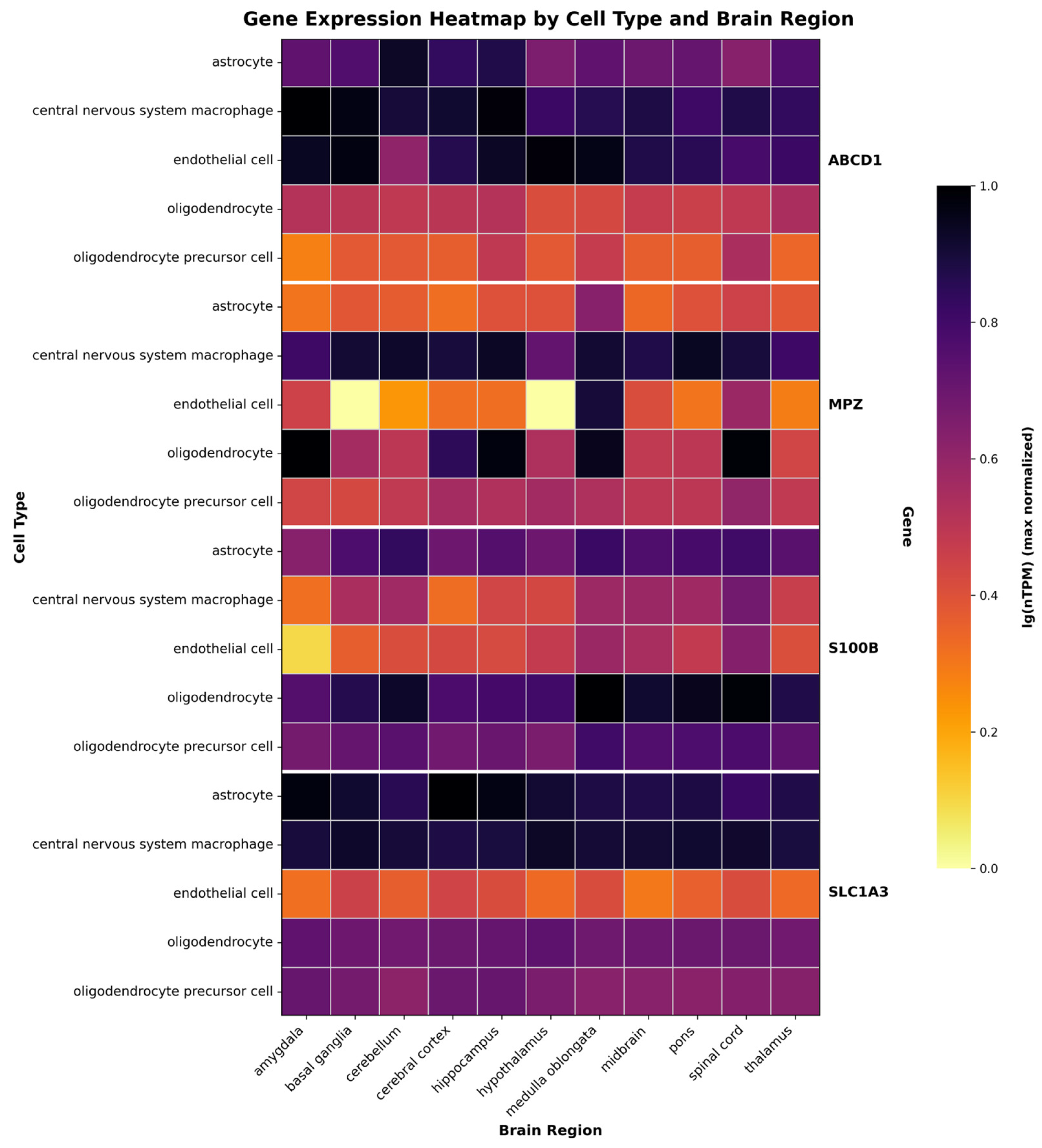
3.2. Determining Promoters for X-ALD Therapy Relying on a Gene Expression Patterns
3.3. Precision Gene Therapy via miRNA Control
4. Delivery Routes and Ramifications of AAV Delivery to the Nervous System Used in X-ALD
4.1. Direct Infusion of AAV Vectors to the CSF
4.1.1. Lumbar Intrathecal (IT) Delivery
4.1.2. Intracerebroventricular (ICV) Delivery
4.2. Intravenous (IV) Delivery
4.3. Addressing AAV Therapy Safety Concerns
5. Engineered AAV Capsids for Concurrent CNS and PNS Therapy in X-ALD
6. In Vitro Models Applied in X-ALD Gene Therapy
6.1. Fibroblasts as a Primary Model
6.2. Advancing X-ALD Research with Human iPSC Platforms
7. In Vivo Models Applied in X-ALD Gene Therapy
7.1. Late-Onset Neuropathy in an ALDP-Deficient Mouse Model with Exon 1 Deletion
7.2. Targeted Gene Delivery for AMN Model with Deletion of the First Exon
7.3. Double Knockout Abcd1−/Y; Abcd2−/− Mouse Model
7.4. SBT101 Gene Therapy in the Double Knockout Abcd1−/Y; Abcd2−/− Mouse Model
7.5. A Novel Abcd1 Exon 3–9 Deletion Model of X-ALD
7.6. Gene Therapy Applied in the Model with Δ3–9 Abcd1−/Y ALD
7.7. CPZ/EAE Abcd1−/Y Mouse Model
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogel, B.H.; Bradley, S.E.; Adams, D.J.; D’Aco, K.; Erbe, R.W.; Fong, C.; Iglesias, A.; Kronn, D.; Levy, P.; Morrissey, M.; et al. Newborn Screening for X-Linked Adrenoleukodystrophy in New York State: Diagnostic Protocol, Surveillance Protocol and Treatment Guidelines. Mol. Genet. Metab. 2015, 114, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Wiens, K.; Berry, S.A.; Choi, H.; Gaviglio, A.; Gupta, A.; Hietala, A.; Kenney-Jung, D.; Lund, T.; Miller, W.; Pierpont, E.I.; et al. A Report on State-wide Implementation of Newborn Screening for X-linked Adrenoleukodystrophy. Am. J. Med. Genet. A 2019, 179, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Moser, H.W.; Mahmood, A.; Raymond, G.V. X-Linked Adrenoleukodystrophy. Nat. Clin. Pract. Neurol. 2007, 3, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Van Geel, B.M.; Assies, J.; Wanders, R.J.A.; Barth, P.G. X Linked Adrenoleukodystrophy: Clinical Presentation, Diagnosis, and Therapy. J. Neurol. Neurosurg. Psychiatry 1997, 63, 4–14. [Google Scholar] [CrossRef]
- Moser, A.B.; Kreiter, N.; Bezman, L.; Lu, S.-E.; Raymond, G.V.; Naidu, S.; Moser, H.W. Plasma Very Long Chain Fatty Acids in 3000 Peroxisome Disease Patients and 29,000 Controls. Ann. Neurol. 1999, 45, 100–110. [Google Scholar] [CrossRef]
- Moser, H.W.; Moser, A.B. Very Long-chain Fatty Acids in Diagnosis, Pathogenesis, and Therapy of Peroxisomal Disorders. Lipids 1996, 31, S141–S144. [Google Scholar] [CrossRef]
- Aubourg, P.; Blanche, S.; Jambaqué, I.; Rocchiccioli, F.; Kalifa, G.; Naud-Saudreau, C.; Rolland, M.-O.; Debré, M.; Chaussain, J.-L.; Griscelli, C.; et al. Reversal of Early Neurologic and Neuroradiologic Manifestations of X-Linked Adrenoleukodystrophy by Bone Marrow Transplantation. N. Engl. J. Med. 1990, 322, 1860–1866. [Google Scholar] [CrossRef]
- Cartier, N.; Aubourg, P. Hematopoietic Stem Cell Transplantation and Hematopoietic Stem Cell Gene Therapy in X-Linked Adrenoleukodystrophy. Brain Pathol. 2010, 20, 857–862. [Google Scholar] [CrossRef]
- Cox, C.S.; Dubey, P.; Raymond, G.V.; Mahmood, A.; Moser, A.B.; Moser, H.W. Cognitive Evaluation of Neurologically Asymptomatic Boys With X-Linked Adrenoleukodystrophy. Arch. Neurol. 2006, 63, 69. [Google Scholar] [CrossRef]
- Videbæk, C.; Melgaard, L.; Lund, A.M.; Grønborg, S.W. Newborn Screening for Adrenoleukodystrophy: International Experiences and Challenges. Mol. Genet. Metab. 2023, 140, 107734. [Google Scholar] [CrossRef]
- Raymond, G.V.; Aubourg, P.; Paker, A.; Escolar, M.; Fischer, A.; Blanche, S.; Baruchel, A.; Dalle, J.-H.; Michel, G.; Prasad, V.; et al. Survival and Functional Outcomes in Boys with Cerebral Adrenoleukodystrophy with and without Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transpl. 2019, 25, 538–548. [Google Scholar] [CrossRef]
- Moser, H.W. Gene Therapy in Neurology. Eur. Neurol. 1994, 34, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Cartier, N.; Lopez, J.; Moullier, P.; Rocchiccioli, F.; Rolland, M.O.; Jorge, P.; Mosser, J.; Mandel, J.L.; Bougnères, P.F.; Danos, O. Retroviral-Mediated Gene Transfer Corrects Very-Long-Chain Fatty Acid Metabolism in Adrenoleukodystrophy Fibroblasts. Proc. Natl. Acad. Sci. USA 1995, 92, 1674–1678. [Google Scholar] [CrossRef] [PubMed]
- Schlimgen, R.; Howard, J.; Wooley, D.; Thompson, M.; Baden, L.R.; Yang, O.O.; Christiani, D.C.; Mostoslavsky, G.; Diamond, D.V.; Duane, E.G.; et al. Risks Associated with Lentiviral Vector Exposures and Prevention Strategies. J. Occup. Environ. Med. 2016, 58, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, G.; Qasim, W. Current Landscape of Vector Safety and Genotoxicity after Hematopoietic Stem or Immune Cell Gene Therapy. Leukemia 2025, 39, 1325–1333. [Google Scholar] [CrossRef]
- Dunbar, C.E. Weighing the Risks of Lentiviral Gene Therapy for Cerebral Adrenoleukodystrophy. N. Engl. J. Med. 2024, 391, 1358–1359. [Google Scholar] [CrossRef]
- Goyal, S.; Tisdale, J.; Schmidt, M.; Kanter, J.; Jaroscak, J.; Whitney, D.; Bitter, H.; Gregory, P.D.; Parsons, G.; Foos, M.; et al. Acute Myeloid Leukemia Case after Gene Therapy for Sickle Cell Disease. N. Engl. J. Med. 2022, 386, 138–147. [Google Scholar] [CrossRef]
- Brunson, A.; Keegan, T.H.M.; Bang, H.; Mahajan, A.; Paulukonis, S.; Wun, T. Increased Risk of Leukemia among Sickle Cell Disease Patients in California. Blood 2017, 130, 1597–1599. [Google Scholar] [CrossRef]
- Kohn, D.B.; Booth, C.; Naldini, L. Myelodysplasia after Lentiviral Gene Therapy. N. Engl. J. Med. 2024, 391, 2382–2384. [Google Scholar] [CrossRef]
- FDA Investigating Serious Risk of Hematologic Malignancy Following Skysona (Elivaldogene Autotemcel). FDA 2024. Available online: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-investigating-serious-risk-hematologic-malignancy-following-skysona-elivaldogene-autotemcel (accessed on 22 September 2025).
- FDA Approves Required Labeling Changes for Increased Risk of Hematologic Malignancy Following Treatment with Skysona (Elivaldogene Autotemcel). FDA 2025. Available online: https://www.fda.gov/vaccines-blood-biologics/fda-approves-required-labeling-changes-increased-risk-hematologic-malignancy-following-treatment (accessed on 22 September 2025).
- Fang, E.; He, G.; Chang, Y.; He, Q.; Chen, P.; Hu, K. Application Advances of Lentiviral Vectors: From Gene Therapy to Vaccine Development. Mol. Biotechnol. 2025. [Google Scholar] [CrossRef]
- Xu, L.; Yao, S.; Ding, Y.E.; Xie, M.; Feng, D.; Sha, P.; Tan, L.; Bei, F.; Yao, Y. Designing and Optimizing AAV-Mediated Gene Therapy for Neurodegenerative Diseases: From Bench to Bedside. J. Transl. Med. 2024, 22, 866. [Google Scholar] [CrossRef]
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC Transporters: The Power to Change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef]
- Wang, R.; Qin, Y.; Li, X. Structural Basis of Acyl-CoA Transport across the Peroxisomal Membrane by Human ABCD1. Cell Res. 2022, 32, 214–217. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Morita, M. ABC Transporter Subfamily D: Distinct Differences in Behavior between ABCD1–3 and ABCD4 in Subcellular Localization, Function, and Human Disease. BioMed Res. Int. 2016, 2016, 6786245. [Google Scholar] [CrossRef]
- Baker, A.; Carrier, D.J.; Schaedler, T.; Waterham, H.R.; Van Roermund, C.W.; Theodoulou, F.L. Peroxisomal ABC Transporters: Functions and Mechanism. Biochem. Soc. Trans. 2015, 43, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Geillon, F.; Gondcaille, C.; Charbonnier, S.; Van Roermund, C.W.; Lopez, T.E.; Dias, A.M.M.; Pais De Barros, J.-P.; Arnould, C.; Wanders, R.J.; Trompier, D.; et al. Structure-Function Analysis of Peroxisomal ATP-Binding Cassette Transporters Using Chimeric Dimers. J. Biol. Chem. 2014, 289, 24511–24520. [Google Scholar] [CrossRef] [PubMed]
- Roermund, C.W.T.; Visser, W.F.; IJlst, L.; Cruchten, A.; Boek, M.; Kulik, W.; Waterham, H.R.; Wanders, R.J.A. The Human Peroxisomal ABC Half Transporter ALDP Functions as a Homodimer and Accepts Acyl–CoA Esters. FASEB J. 2008, 22, 4201–4208. [Google Scholar] [CrossRef]
- Tawbeh, A.; Gondcaille, C.; Trompier, D.; Savary, S. Peroxisomal ABC Transporters: An Update. Int. J. Mol. Sci. 2021, 22, 6093. [Google Scholar] [CrossRef] [PubMed]
- Wanders, R.J.A.; Waterham, H.R. Biochemistry of Mammalian Peroxisomes Revisited. Annu. Rev. Biochem. 2006, 75, 295–332. [Google Scholar] [CrossRef]
- Kallabi, F.; Ellouz, E.; Tabebi, M.; Ben Salah, G.; Kaabechi, N.; Keskes, L.; Triki, C.; Kamoun, H. Phenotypic Variability in a Tunisian Family with X-Linked Adrenoleukodystrophy Caused by the p.Gln316Pro Novel Mutation. Clin. Chim. Acta 2016, 453, 141–146. [Google Scholar] [CrossRef]
- Johnson, A.B.; Schaumburg, H.H.; Powers, J.M. Histochemical Characteristics of the Striated Inclusions of Adrenoleukodystrophy. J. Histochem. Cytochem. 1976, 24, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.M. Adreno-Leukodystrophy: Similar Ultrastructural Changes in Adrenal Cortical and Schwann Cells. Arch. Neurol. 1974, 30, 406. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.M.; Schaumburg, H.H. Adreno-Leukodystrophy (Sex-Linked Schilder’s Disease). A Pathogenetic Hypothesis Based on Ultrastructural Lesions in Adrenal Cortex, Peripheral Nerve and Testis. Am. J. Pathol. 1974, 76, 481–491. [Google Scholar] [PubMed]
- Moskvitina, T.A.; Kamyshanskaya, N.S.; Kaverina, L.P.; Gorkin, V.Z. Qualitative Alteration in Substrate Specificity of Mitochondrial Monoamine Oxidase in Brain. J. Neurochem. 1976, 26, 209–210. [Google Scholar] [CrossRef]
- Chung, H.-L.; Ye, Q.; Park, Y.-J.; Zuo, Z.; Mok, J.-W.; Kanca, O.; Tattikota, S.G.; Lu, S.; Perrimon, N.; Lee, H.K.; et al. Very-Long-Chain Fatty Acids Induce Glial-Derived Sphingosine-1-Phosphate Synthesis, Secretion, and Neuroinflammation. Cell Metab. 2023, 35, 855–874.e5. [Google Scholar] [CrossRef]
- Ho, J.K.; Moser, H.; Kishimoto, Y.; Hamilton, J.A. Interactions of a Very Long Chain Fatty Acid with Model Membranes and Serum Albumin. Implications for the Pathogenesis of Adrenoleukodystrophy. J. Clin. Investig. 1995, 96, 1455–1463. [Google Scholar] [CrossRef]
- Fourcade, S.; Lopez-Erauskin, J.; Galino, J.; Duval, C.; Naudi, A.; Jove, M.; Kemp, S.; Villarroya, F.; Ferrer, I.; Pamplona, R.; et al. Early Oxidative Damage Underlying Neurodegeneration in X-Adrenoleukodystrophy. Hum. Mol. Genet. 2008, 17, 1762–1773. [Google Scholar] [CrossRef]
- Van De Beek, M.-C.; Ofman, R.; Dijkstra, I.; Wijburg, F.; Engelen, M.; Wanders, R.; Kemp, S. Lipid-Induced Endoplasmic Reticulum Stress in X-Linked Adrenoleukodystrophy. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 2255–2265. [Google Scholar] [CrossRef]
- Raas, Q.; Van De Beek, M.-C.; Forss-Petter, S.; Dijkstra, I.M.E.; Deschiffart, A.; Freshner, B.C.; Stevenson, T.J.; Jaspers, Y.R.J.; Nagtzaam, L.; Wanders, R.J.A.; et al. Metabolic Rerouting via SCD1 Induction Impacts X-Linked Adrenoleukodystrophy. J. Clin. Investig. 2021, 131, e142500. [Google Scholar] [CrossRef]
- Jang, J.; Park, S.; Jin Hur, H.; Cho, H.-J.; Hwang, I.; Pyo Kang, Y.; Im, I.; Lee, H.; Lee, E.; Yang, W.; et al. 25-Hydroxycholesterol Contributes to Cerebral Inflammation of X-Linked Adrenoleukodystrophy through Activation of the NLRP3 Inflammasome. Nat. Commun. 2016, 7, 13129. [Google Scholar] [CrossRef]
- Khan, M.; Singh, J.; Gilg, A.G.; Uto, T.; Singh, I. Very Long-Chain Fatty Acid Accumulation Causes Lipotoxic Response via 5-Lipoxygenase in Cerebral Adrenoleukodystrophy. J. Lipid Res. 2010, 51, 1685–1695. [Google Scholar] [CrossRef]
- Moser, H.W.; Moser, A.E.; Singh, I.; O’Neill, P. Adrenoleukodystrophy: Survey of 303 Cases: Biochemistry, Diagnosis, and Therapy. Ann. Neurol. 1984, 16, 628–641. [Google Scholar] [CrossRef]
- Schaumburg, H.H. Adreno-Leukodystrophy (Sex-Linked Schilder Disease): Ultrastructural Demonstration of Specific Cytoplasmic Inclusions in the Central Nervous System. Arch. Neurol. 1974, 31, 210. [Google Scholar] [CrossRef] [PubMed]
- Theda, C.; Moser, A.B.; Powers, J.M.; Moser, H.W. Phospholipids in X-Linked Adrenoleukodystrophy White Matter: Fatty Acid Abnormalities before the Onset of Demyelination. J. Neurol. Sci. 1992, 110, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.; Johnson, D.; Poulos, A. Molecular Species of Phosphatidylcholine Containing Very Long Chain Fatty Acids in Human Brain: Enrichment in X-Linked Adrenoleukodystrophy Brain and Diseases of Peroxisome Biogenesis Brain. J. Neurochem. 1991, 56, 30–37. [Google Scholar] [CrossRef]
- Eichler, F.S.; Ren, J.; Cossoy, M.; Rietsch, A.M.; Nagpal, S.; Moser, A.B.; Frosch, M.P.; Ransohoff, R.M. Is Microglial Apoptosis an Early Pathogenic Change in Cerebral X-linked Adrenoleukodystrophy? Ann. Neurol. 2008, 63, 729–742. [Google Scholar] [CrossRef]
- Jaspers, Y.R.J.; Yska, H.A.F.; Bergner, C.G.; Dijkstra, I.M.E.; Huffnagel, I.C.; Voermans, M.M.C.; Wever, E.; Salomons, G.S.; Vaz, F.M.; Jongejan, A.; et al. Lipidomic Biomarkers in Plasma Correlate with Disease Severity in Adrenoleukodystrophy. Commun. Med. 2024, 4, 175. [Google Scholar] [CrossRef]
- Knuplez, E.; Marsche, G. An Updated Review of Pro- and Anti-Inflammatory Properties of Plasma Lysophosphatidylcholines in the Vascular System. Int. J. Mol. Sci. 2020, 21, 4501. [Google Scholar] [CrossRef]
- Cas, M.D.; Roda, G.; Li, F.; Secundo, F. Functional Lipids in Autoimmune Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 3074. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Nagai, A.; Ryu, J.K.; McLarnon, J.G.; Kim, S.U.; Masuda, J. Lysophosphatidylcholine Induces Glial Cell Activation: Role of Rho Kinase. Glia 2009, 57, 898–907. [Google Scholar] [CrossRef]
- Scholz, H.; Eder, C. Lysophosphatidylcholine Activates Caspase-1 in Microglia via a Novel Pathway Involving Two Inflammasomes. J. Neuroimmunol. 2017, 310, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, E.; Srivastava, I.; Aguirre, A.; Yoseph, E.; Kaushal, E.; Awani, A.; Ryu, J.; Talebian, S.; Chu, P.; Steinman, L.; et al. A Novel Mouse Model of Cerebral Demyelination in X-Linked Adrenoleukodystrophy Highlights NLRP3 Activation in Lesion Pathogenesis (P11-4.004). Neurology 2024, 102, 5886. [Google Scholar] [CrossRef]
- Srivastava, I.; Aguirre, A.; Cayrol, R.; Vogel, H.; Lund, T.; Van Haren, K. NLPR3 Inflammasome Activation within Demyelinating Lesions in Cerebral Adrenoleukodystrophy (P4-4.001). Neurology 2022, 98, 1072. [Google Scholar] [CrossRef]
- Sádaba, M.C.; Rothhammer, V.; Muñoz, Ú.; Sebal, C.; Escudero, E.; Kivisäkk, P.; Garcia Sanchez, M.I.; Izquierdo, G.; Hauser, S.L.; Baranzini, S.E.; et al. Serum Antibodies to Phosphatidylcholine in MS. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e765. [Google Scholar] [CrossRef]
- Powers, J.M.; Deciero, D.P.; Cox, C.; Richfield, E.K.; Ito, M.; Moser, A.B.; Moser, H.W. The Dorsal Root Ganglia in Adrenomyeloneuropathy: Neuronal Atrophy and Abnormal Mitochondria. J. Neuropathol. Exp. Neurol. 2001, 60, 493–501. [Google Scholar] [CrossRef]
- Gong, Y.; Sasidharan, N.; Laheji, F.; Frosch, M.; Musolino, P.; Tanzi, R.; Kim, D.Y.; Biffi, A.; El Khoury, J.; Eichler, F. Microglial Dysfunction as a Key Pathological Change in Adrenomyeloneuropathy. Ann. Neurol. 2017, 82, 813–827. [Google Scholar] [CrossRef]
- Ingusci, S.; Verlengia, G.; Soukupova, M.; Zucchini, S.; Simonato, M. Gene Therapy Tools for Brain Diseases. Front. Pharmacol. 2019, 10, 724. [Google Scholar] [CrossRef]
- Yska, H.A.F.; Engelen, M.; Bugiani, M. The Pathology of X-Linked Adrenoleukodystrophy: Tissue Specific Changes as a Clue to Pathophysiology. Orphanet J. Rare Dis. 2024, 19, 138. [Google Scholar] [CrossRef]
- Siletti, K.; Hodge, R.; Mossi Albiach, A.; Lee, K.W.; Ding, S.-L.; Hu, L.; Lönnerberg, P.; Bakken, T.; Casper, T.; Clark, M.; et al. Transcriptomic Diversity of Cell Types across the Adult Human Brain. Science 2023, 382, eadd7046. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Bergner, C.G.; Van Der Meer, F.; Winkler, A.; Wrzos, C.; Türkmen, M.; Valizada, E.; Fitzner, D.; Hametner, S.; Hartmann, C.; Pfeifenbring, S.; et al. Microglia Damage Precedes Major Myelin Breakdown in X-linked Adrenoleukodystrophy and Metachromatic Leukodystrophy. Glia 2019, 67, 1196–1209. [Google Scholar] [CrossRef] [PubMed]
- Hasel, P.; Aisenberg, W.H.; Bennett, F.C.; Liddelow, S.A. Molecular and Metabolic Heterogeneity of Astrocytes and Microglia. Cell Metab. 2023, 35, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Panov, A.; Orynbayeva, Z.; Vavilin, V.; Lyakhovich, V. Fatty Acids in Energy Metabolism of the Central Nervous System. BioMed Res. Int. 2014, 2014, 472459. [Google Scholar] [CrossRef]
- Ioannou, M.S.; Jackson, J.; Sheu, S.-H.; Chang, C.-L.; Weigel, A.V.; Liu, H.; Pasolli, H.A.; Xu, C.S.; Pang, S.; Matthies, D.; et al. Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell 2019, 177, 1522–1535.e14. [Google Scholar] [CrossRef]
- Mi, Y.; Qi, G.; Vitali, F.; Shang, Y.; Raikes, A.C.; Wang, T.; Jin, Y.; Brinton, R.D.; Gu, H.; Yin, F. Loss of Fatty Acid Degradation by Astrocytic Mitochondria Triggers Neuroinflammation and Neurodegeneration. Nat. Metab. 2023, 5, 445–465. [Google Scholar] [CrossRef]
- Schönfeld, P.; Reiser, G. Why Does Brain Metabolism Not Favor Burning of Fatty Acids to Provide Energy?—Reflections on Disadvantages of the Use of Free Fatty Acids as Fuel for Brain. J. Cereb. Blood Flow Metab. 2013, 33, 1493–1499. [Google Scholar] [CrossRef]
- Camargo, N.; Goudriaan, A.; Van Deijk, A.-L.F.; Otte, W.M.; Brouwers, J.F.; Lodder, H.; Gutmann, D.H.; Nave, K.-A.; Dijkhuizen, R.M.; Mansvelder, H.D.; et al. Oligodendroglial Myelination Requires Astrocyte-Derived Lipids. PLOS Biol. 2017, 15, e1002605. [Google Scholar] [CrossRef]
- Van Deijk, A.F.; Camargo, N.; Timmerman, J.; Heistek, T.; Brouwers, J.F.; Mogavero, F.; Mansvelder, H.D.; Smit, A.B.; Verheijen, M.H.G. Astrocyte Lipid Metabolism Is Critical for Synapse Development and Function in Vivo. Glia 2017, 65, 670–682. [Google Scholar] [CrossRef]
- Vargas, C.R.; Wajner, M.; Sirtori, L.R.; Goulart, L.; Chiochetta, M.; Coelho, D.; Latini, A.; Llesuy, S.; Bello-Klein, A.; Giugliani, R.; et al. Evidence That Oxidative Stress Is Increased in Patients with X-Linked Adrenoleukodystrophy. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2004, 1688, 26–32. [Google Scholar] [CrossRef]
- Ferrer, R.M.; Jaspers, Y.R.J.; Dijkstra, I.M.E.; Breeuwsma, N.; Van Klinken, J.; Romero, C.; Engelen, M.; Kemp, S.; Heine, V.M. Altered Lipid Profile and Reduced Neuronal Support in human induced pluripotent stem cell-derived Astrocytes from Adrenoleukodystrophy Patients. J. Inherit. Metab. Dis. 2025, 48, e12832. [Google Scholar] [CrossRef]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef]
- Ferrer, I.; Aubourg, P.; Pujol, A. General Aspects and Neuropathology of X-Linked Adrenoleukodystrophy. Brain Pathol. 2010, 20, 817–830. [Google Scholar] [CrossRef]
- Lee, C.A.A.; Seo, H.S.; Armien, A.G.; Bates, F.S.; Tolar, J.; Azarin, S.M. Modeling and Rescue of Defective Blood–Brain Barrier Function of Induced Brain Microvascular Endothelial Cells from Childhood Cerebral Adrenoleukodystrophy Patients. Fluids Barriers CNS 2018, 15, 9. [Google Scholar] [CrossRef]
- Aubourg, P. Cerebral Adrenoleukodystrophy: A Demyelinating Disease That Leaves the Door Wide Open: Figure 1. Brain 2015, 138, 3133–3136. [Google Scholar] [CrossRef]
- Lauer, A.; Speroni, S.L.; Choi, M.; Da, X.; Duncan, C.; McCarthy, S.; Krishnan, V.; Lusk, C.A.; Rohde, D.; Hansen, M.B.; et al. Hematopoietic Stem-Cell Gene Therapy Is Associated with Restored White Matter Microvascular Function in Cerebral Adrenoleukodystrophy. Nat. Commun. 2023, 14, 1900. [Google Scholar] [CrossRef]
- O’Carroll, S.J.; Cook, W.H.; Young, D. AAV Targeting of Glial Cell Types in the Central and Peripheral Nervous System and Relevance to Human Gene Therapy. Front. Mol. Neurosci. 2021, 13, 618020. [Google Scholar] [CrossRef] [PubMed]
- Zwartkruis, M.M.; Groen, E.J. Promoting Expression in Gene Therapy: More Is Not Always Better. EMBO Mol. Med. 2024, 16, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Au, H.K.E.; Isalan, M.; Mielcarek, M. Gene Therapy Advances: A Meta-Analysis of AAV Usage in Clinical Settings. Front. Med. 2022, 8, 809118. [Google Scholar] [CrossRef] [PubMed]
- Kügler, S. Tissue-Specific Promoters in the CNS. In Gene Therapy for Neurological Disorders; Manfredsson, F.P., Ed.; Springer: New York, NY, USA, 2016; Volume 1382, pp. 81–91. ISBN 978-1-4939-3270-2. [Google Scholar]
- De Leeuw, C.N.; Dyka, F.M.; Boye, S.L.; Laprise, S.; Zhou, M.; Chou, A.Y.; Borretta, L.; McInerny, S.C.; Banks, K.G.; Portales-Casamar, E.; et al. Targeted CNS Delivery Using Human MiniPromoters and Demonstrated Compatibility with Adeno-Associated Viral Vectors. Mol. Ther.-Methods Clin. Dev. 2014, 1, 5. [Google Scholar] [CrossRef]
- Finneran, D.J.; Njoku, I.P.; Flores-Pazarin, D.; Ranabothu, M.R.; Nash, K.R.; Morgan, D.; Gordon, M.N. Toward Development of Neuron Specific Transduction After Systemic Delivery of Viral Vectors. Front. Neurol. 2021, 12, 685802. [Google Scholar] [CrossRef]
- Hollidge, B.S.; Carroll, H.B.; Qian, R.; Fuller, M.L.; Giles, A.R.; Mercer, A.C.; Danos, O.; Liu, Y.; Bruder, J.T.; Smith, J.B. Kinetics and Durability of Transgene Expression after Intrastriatal Injection of AAV9 Vectors. Front. Neurol. 2022, 13, 1051559. [Google Scholar] [CrossRef] [PubMed]
- Damdindorj, L.; Karnan, S.; Ota, A.; Hossain, E.; Konishi, Y.; Hosokawa, Y.; Konishi, H. A Comparative Analysis of Constitutive Promoters Located in Adeno-Associated Viral Vectors. PLoS ONE 2014, 9, e106472. [Google Scholar] [CrossRef]
- Gray, S.J.; Foti, S.B.; Schwartz, J.W.; Bachaboina, L.; Taylor-Blake, B.; Coleman, J.; Ehlers, M.D.; Zylka, M.J.; McCown, T.J.; Samulski, R.J. Optimizing Promoters for Recombinant Adeno-Associated Virus-Mediated Gene Expression in the Peripheral and Central Nervous System Using Self-Complementary Vectors. Hum. Gene Ther. 2011, 22, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Forss-Petter, S.; Eichler, F.S. Pathophysiology of X-Linked Adrenoleukodystrophy. Biochimie 2014, 98, 135–142. [Google Scholar] [CrossRef]
- Manor, J.; Jangam, S.V.; Chung, H.; Bhagwat, P.; Andrews, J.; Chester, H.; Kondo, S.; Srivastav, S.; Botas, J.; Moser, A.B.; et al. Genetic Analysis of the X-Linked Adrenoleukodystrophy ABCD1 Gene in Drosophila Uncovers a Role in Peroxisomal Dynamics. bioRxiv 2024. [Google Scholar] [CrossRef]
- Nieuwenhuis, B.; Haenzi, B.; Hilton, S.; Carnicer-Lombarte, A.; Hobo, B.; Verhaagen, J.; Fawcett, J.W. Optimization of Adeno-Associated Viral Vector-Mediated Transduction of the Corticospinal Tract: Comparison of Four Promoters. Gene Ther. 2021, 28, 56–74. [Google Scholar] [CrossRef]
- Gong, Y.; Mu, D.; Prabhakar, S.; Moser, A.; Musolino, P.; Ren, J.; Breakefield, X.O.; Maguire, C.A.; Eichler, F.S. Adenoassociated Virus Serotype 9-Mediated Gene Therapy for X-Linked Adrenoleukodystrophy. Mol. Ther. 2015, 23, 824–834. [Google Scholar] [CrossRef]
- Lunev, E.; Karan, A.; Egorova, T.; Bardina, M. Adeno-Associated Viruses for Modeling Neurological Diseases in Animals: Achievements and Prospects. Biomedicines 2022, 10, 1140. [Google Scholar] [CrossRef]
- Hinderer, C.; Bell, P.; Vite, C.H.; Louboutin, J.-P.; Grant, R.; Bote, E.; Yu, H.; Pukenas, B.; Hurst, R.; Wilson, J.M. Widespread Gene Transfer in the Central Nervous System of Cynomolgus Macaques Following Delivery of AAV9 into the Cisterna Magna. Mol. Ther.-Methods Clin. Dev. 2014, 1, 14051. [Google Scholar] [CrossRef]
- Buss, N.; Lanigan, L.; Zeller, J.; Cissell, D.; Metea, M.; Adams, E.; Higgins, M.; Kim, K.H.; Budzynski, E.; Yang, L.; et al. Characterization of AAV-Mediated Dorsal Root Ganglionopathy. Mol. Ther.-Methods Clin. Dev. 2022, 24, 342–354. [Google Scholar] [CrossRef]
- Hawley, Z.C.E.; Pardo, I.D.; Cao, S.; Zavodszky, M.I.; Casey, F.; Ferber, K.; Luo, Y.; Hana, S.; Chen, S.K.; Doherty, J.; et al. Dorsal Root Ganglion Toxicity after AAV Intra-CSF Delivery of a RNAi Expression Construct into Non-Human Primates and Mice. Mol. Ther. 2025, 33, 215–234. [Google Scholar] [CrossRef]
- Johnson, E.W.; Sutherland, J.J.; Meseck, E.; McElroy, C.; Chand, D.H.; Tukov, F.F.; Hudry, E.; Penraat, K. Neurofilament Light Chain and Dorsal Root Ganglia Injury after Adeno-Associated Virus 9 Gene Therapy in Nonhuman Primates. Mol. Ther.-Methods Clin. Dev. 2023, 28, 208–219. [Google Scholar] [CrossRef]
- Hordeaux, J.; Buza, E.L.; Jeffrey, B.; Song, C.; Jahan, T.; Yuan, Y.; Zhu, Y.; Bell, P.; Li, M.; Chichester, J.A.; et al. MicroRNA-Mediated Inhibition of Transgene Expression Reduces Dorsal Root Ganglion Toxicity by AAV Vectors in Primates. Sci. Transl. Med. 2020, 12, eaba9188. [Google Scholar] [CrossRef]
- Hinderer, C.; Katz, N.; Buza, E.L.; Dyer, C.; Goode, T.; Bell, P.; Richman, L.K.; Wilson, J.M. Severe Toxicity in Nonhuman Primates and Piglets Following High-Dose Intravenous Administration of an Adeno-Associated Virus Vector Expressing Human SMN. Hum. Gene Ther. 2018, 29, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Challis, R.C.; Ravindra Kumar, S.; Chen, X.; Goertsen, D.; Coughlin, G.M.; Hori, A.M.; Chuapoco, M.R.; Otis, T.S.; Miles, T.F.; Gradinaru, V. Adeno-Associated Virus Toolkit to Target Diverse Brain Cells. Annu. Rev. Neurosci. 2022, 45, 447–469. [Google Scholar] [CrossRef] [PubMed]
- Schiza, N.; Georgiou, E.; Kagiava, A.; Médard, J.-J.; Richter, J.; Tryfonos, C.; Sargiannidou, I.; Heslegrave, A.J.; Rossor, A.M.; Zetterberg, H.; et al. Gene Replacement Therapy in a Model of Charcot-Marie-Tooth 4C Neuropathy. Brain 2019, 142, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Raasakka, A.; Kursula, P. How Does Protein Zero Assemble Compact Myelin? Cells 2020, 9, 1832. [Google Scholar] [CrossRef]
- Jacob, C.; Christen, C.N.; Pereira, J.A.; Somandin, C.; Baggiolini, A.; Lötscher, P.; Özçelik, M.; Tricaud, N.; Meijer, D.; Yamaguchi, T.; et al. HDAC1 and HDAC2 Control the Transcriptional Program of Myelination and the Survival of Schwann Cells. Nat. Neurosci. 2011, 14, 429–436. [Google Scholar] [CrossRef]
- LeBlanc, S.E.; Jang, S.-W.; Ward, R.M.; Wrabetz, L.; Svaren, J. Direct Regulation of Myelin Protein Zero Expression by the Egr2 Transactivator. J. Biol. Chem. 2006, 281, 5453–5460. [Google Scholar] [CrossRef]
- Peirano, R.I.; Goerich, D.E.; Riethmacher, D.; Wegner, M. Protein Zero Gene Expression Is Regulated by the Glial Transcription Factor Sox10. Mol. Cell. Biol. 2000, 20, 3198–3209. [Google Scholar] [CrossRef]
- Michetti, F.; Clementi, M.E.; Di Liddo, R.; Valeriani, F.; Ria, F.; Rende, M.; Di Sante, G.; Romano Spica, V. The S100B Protein: A Multifaceted Pathogenic Factor More Than a Biomarker. Int. J. Mol. Sci. 2023, 24, 9605. [Google Scholar] [CrossRef] [PubMed]
- Marshak, D.R. Chapter 14 S100β as a Neurotrophic Factor. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 1990; Volume 86, pp. 169–181. ISBN 978-0-444-81121-9. [Google Scholar]
- Villarreal, A.; Aviles Reyes, R.X.; Angelo, M.F.; Reines, A.G.; Ramos, A.J. S100B Alters Neuronal Survival and Dendrite Extension via RAGE-mediated NF-κB Signaling. J. Neurochem. 2011, 117, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Castets, F.; Griffin, W.S.T.; Marks, A.; Van Eldik, L.J. Transcriptional Regulation of the Human S100β Gene. Mol. Brain Res. 1997, 46, 208–216. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, C.N.; Korecki, A.J.; Berry, G.E.; Hickmott, J.W.; Lam, S.L.; Lengyell, T.C.; Bonaguro, R.J.; Borretta, L.J.; Chopra, V.; Chou, A.Y.; et al. rAAV-Compatible MiniPromoters for Restricted Expression in the Brain and Eye. Mol. Brain 2016, 9, 52. [Google Scholar] [CrossRef]
- Parkin, G.M.; Udawela, M.; Gibbons, A.; Dean, B. Glutamate Transporters, EAAT1 and EAAT2, Are Potentially Important in the Pathophysiology and Treatment of Schizophrenia and Affective Disorders. World J. Psychiatry 2018, 8, 51–63. [Google Scholar] [CrossRef]
- Kim, S.; Choi, S.; Chao, W.; Volsky, D.J. Transcriptional Regulation of Human Excitatory Amino Acid Transporter 1 (EAAT1): Cloning of the EAAT1 Promoter and Characterization of Its Basal and Inducible Activity in Human Astrocytes. J. Neurochem. 2003, 87, 1485–1498. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Xie, J.; Xie, Q.; Zhang, H.; Ameres, S.L.; Hung, J.-H.; Su, Q.; He, R.; Mu, X.; Seher Ahmed, S.; Park, S.; et al. MicroRNA-Regulated, Systemically Delivered rAAV9: A Step Closer to CNS-Restricted Transgene Expression. Mol. Ther. 2011, 19, 526–535. [Google Scholar] [CrossRef]
- Geisler, A.; Fechner, H. MicroRNA-Regulated Viral Vectors for Gene Therapy. World J. Exp. Med. 2016, 6, 37. [Google Scholar] [CrossRef]
- Costa-Verdera, H.; Meneghini, V.; Fitzpatrick, Z.; Abou Alezz, M.; Fabyanic, E.; Huang, X.; Dzhashiashvili, Y.; Ahiya, A.; Mangiameli, E.; Valeri, E.; et al. AAV Vectors Trigger DNA Damage Response-Dependent pro-Inflammatory Signalling in Human iPSC-Derived CNS Models and Mouse Brain. Nat. Commun. 2025, 16, 3694. [Google Scholar] [CrossRef]
- Duan, D. Lethal Immunotoxicity in High-Dose Systemic AAV Therapy. Mol. Ther. 2023, 31, 3123–3126. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, A.; Markusic, D.; Muhuri, M.; Ou, L. Editorial: Immunogenicity and Toxicity of AAV Gene Therapy. Front. Immunol. 2023, 14, 1227231. [Google Scholar] [CrossRef] [PubMed]
- Assaf, B.T. Systemic Toxicity of Recombinant Adeno-Associated Virus Gene Therapy Vectors. Toxicol. Pathol. 2024, 52, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Hudry, E.; Vandenberghe, L.H. Therapeutic AAV Gene Transfer to the Nervous System: A Clinical Reality. Neuron 2019, 101, 839–862. [Google Scholar] [CrossRef]
- Daci, R.; Flotte, T.R. Delivery of Adeno-Associated Virus Vectors to the Central Nervous System for Correction of Single Gene Disorders. Int. J. Mol. Sci. 2024, 25, 1050. [Google Scholar] [CrossRef]
- Ye, D.; Chukwu, C.; Yang, Y.; Hu, Z.; Chen, H. Adeno-Associated Virus Vector Delivery to the Brain: Technology Advancements and Clinical Applications. Adv. Drug Deliv. Rev. 2024, 211, 115363. [Google Scholar] [CrossRef]
- Soytürk, H.; Yılmaz, M.; Önal, C.; Suveren, E.; Kılıç, Ü. Circulation of Cerebrospinal Fluid (CSF). In Cerebrospinal Fluid; Kuru Bektaşoğlu, P., Gürer, B., Eds.; IntechOpen: London, UK, 2022; ISBN 978-1-83969-695-4. [Google Scholar]
- Fahoum, F.; Eyal, S. Intracerebroventricular Administration for Delivery of Antiseizure Therapeutics: Challenges and Opportunities. Epilepsia 2023, 64, 1750–1765. [Google Scholar] [CrossRef]
- Taghian, T.; Marosfoi, M.G.; Puri, A.S.; Cataltepe, O.I.; King, R.M.; Diffie, E.B.; Maguire, A.S.; Martin, D.R.; Fernau, D.; Batista, A.R.; et al. A Safe and Reliable Technique for CNS Delivery of AAV Vectors in the Cisterna Magna. Mol. Ther. 2020, 28, 411–421. [Google Scholar] [CrossRef]
- Samaranch, L.; Bringas, J.; Pivirotto, P.; Sebastian, W.S.; Forsayeth, J.; Bankiewicz, K. Cerebellomedullary Cistern Delivery for AAV-Based Gene Therapy: A Technical Note for Nonhuman Primates. Hum. Gene Ther. Methods 2016, 27, 13–16. [Google Scholar] [CrossRef]
- Katz, N.; Goode, T.; Hinderer, C.; Hordeaux, J.; Wilson, J.M. Standardized Method for Intra-Cisterna Magna Delivery Under Fluoroscopic Guidance in Nonhuman Primates. Hum. Gene Ther. Methods 2018, 29, 212–219. [Google Scholar] [CrossRef]
- Rahman, M.D.; Lee, J.; Kim, Y.; Park, C.-K. Epidural and Intrathecal Drug Delivery in Rats and Mice for Experimental Research: Fundamental Concepts, Techniques, Precaution, and Application. Biomedicines 2023, 11, 1413. [Google Scholar] [CrossRef]
- Stavrou, M.; Georgiou, E.; Kleopa, K.A. Lumbar Intrathecal Injection in Adult and Neonatal Mice. Curr. Protoc. 2024, 4, e1091. [Google Scholar] [CrossRef]
- Gong, Y.; Berenson, A.; Laheji, F.; Gao, G.; Wang, D.; Ng, C.; Volak, A.; Kok, R.; Kreouzis, V.; Dijkstra, I.M.; et al. Intrathecal Adeno-Associated Viral Vector-Mediated Gene Delivery for Adrenomyeloneuropathy. Hum. Gene Ther. 2019, 30, 544–555. [Google Scholar] [CrossRef]
- Bey, K.; Ciron, C.; Dubreil, L.; Deniaud, J.; Ledevin, M.; Cristini, J.; Blouin, V.; Aubourg, P.; Colle, M.-A. Efficient CNS Targeting in Adult Mice by Intrathecal Infusion of Single-Stranded AAV9-GFP for Gene Therapy of Neurological Disorders. Gene Ther. 2017, 24, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, A.J. Intracerebroventricular Drug Administration. Transl. Clin. Pharmacol. 2017, 25, 117. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, D.K.; Chung, B.-R.; Kim, H.V.; Kim, Y. Intracerebroventricular Injection of Amyloid-β Peptides in Normal Mice to Acutely Induce Alzheimer-like Cognitive Deficits. J. Vis. Exp. 2016, 109, 53308. [Google Scholar] [CrossRef]
- Grieb, P. Intracerebroventricular Streptozotocin Injections as a Model of Alzheimer’s Disease: In Search of a Relevant Mechanism. Mol. Neurobiol. 2016, 53, 1741–1752. [Google Scholar] [CrossRef]
- Bey, K.; Deniaud, J.; Dubreil, L.; Joussemet, B.; Cristini, J.; Ciron, C.; Hordeaux, J.; Le Boulc’h, M.; Marche, K.; Maquigneau, M.; et al. Intra-CSF AAV9 and AAVrh10 Administration in Nonhuman Primates: Promising Routes and Vectors for Which Neurological Diseases? Mol. Ther.-Methods Clin. Dev. 2020, 17, 771–784. [Google Scholar] [CrossRef]
- Meijer, D.H.; Maguire, C.A.; LeRoy, S.G.; Sena-Esteves, M. Controlling Brain Tumor Growth by Intraventricular Administration of an AAV Vector Encoding IFN-β. Cancer Gene Ther. 2009, 16, 664–671. [Google Scholar] [CrossRef]
- Donsante, A.; McEachin, Z.; Riley, J.; Leung, C.H.; Kanz, L.; O’Connor, D.M.; Boulis, N.M. Intracerebroventricular Delivery of Self-Complementary Adeno-Associated Virus Serotype 9 to the Adult Rat Brain. Gene Ther. 2016, 23, 401–407. [Google Scholar] [CrossRef]
- Foust, K.D.; Nurre, E.; Montgomery, C.L.; Hernandez, A.; Chan, C.M.; Kaspar, B.K. Intravascular AAV9 Preferentially Targets Neonatal Neurons and Adult Astrocytes. Nat. Biotechnol. 2009, 27, 59–65. [Google Scholar] [CrossRef]
- Lin, Y.; Li, C.; Wang, W.; Li, J.; Huang, C.; Zheng, X.; Liu, Z.; Song, X.; Chen, Y.; Gao, J.; et al. Intravenous AAV9 Administration Results in Safe and Widespread Distribution of Transgene in the Brain of Mini-Pig. Front. Cell Dev. Biol. 2023, 10, 1115348. [Google Scholar] [CrossRef]
- Chauhan, M.; Daugherty, A.L.; Khadir, F.; Duzenli, O.F.; Hoffman, A.; Tinklenberg, J.A.; Kang, P.B.; Aslanidi, G.; Pacak, C.A. AAV-DJ Is Superior to AAV9 for Targeting Brain and Spinal Cord, and de-Targeting Liver across Multiple Delivery Routes in Mice. J. Transl. Med. 2024, 22, 824. [Google Scholar] [CrossRef]
- Chen, X.; Ravindra Kumar, S.; Adams, C.D.; Yang, D.; Wang, T.; Wolfe, D.A.; Arokiaraj, C.M.; Ngo, V.; Campos, L.J.; Griffiths, J.A.; et al. Engineered AAVs for Non-Invasive Gene Delivery to Rodent and Non-Human Primate Nervous Systems. Neuron 2022, 110, 2242–2257.e6. [Google Scholar] [CrossRef]
- Hudry, E.; Aihara, F.; Meseck, E.; Mansfield, K.; McElroy, C.; Chand, D.; Tukov, F.F.; Penraat, K. Liver Injury in Cynomolgus Monkeys Following Intravenous and Intrathecal scAAV9 Gene Therapy Delivery. Mol. Ther. 2023, 31, 2999–3014. [Google Scholar] [CrossRef]
- Chand, D.; Mohr, F.; McMillan, H.; Tukov, F.F.; Montgomery, K.; Kleyn, A.; Sun, R.; Tauscher-Wisniewski, S.; Kaufmann, P.; Kullak-Ublick, G. Hepatotoxicity Following Administration of Onasemnogene Abeparvovec (AVXS-101) for the Treatment of Spinal Muscular Atrophy. J. Hepatol. 2021, 74, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S. High-Dose AAV Gene Therapy Deaths. Nat. Biotechnol. 2020, 38, 910. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.A.; Wright, J.F. Challenges Posed by Immune Responses to AAV Vectors: Addressing Root Causes. Front. Immunol. 2021, 12, 675897. [Google Scholar] [CrossRef] [PubMed]
- Vasireddy, V.; Maguire, C.A.; Anderson, D.W.; Ng, C.; Gong, Y.; Eichler, F.; Fourcade, S.; Guilera, C.; Onieva, A.; Sanchez, A.; et al. An in Vitro and in Vivo Efficacy Evaluation of Gene Therapy Candidate SBT101 in Mouse Models of Adrenomyeloneuropathy and in NHPs. Mol. Ther.-Methods Clin. Dev. 2024, 32, 101354. [Google Scholar] [CrossRef]
- Salabarria, S.M.; Corti, M.; Coleman, K.E.; Wichman, M.B.; Berthy, J.A.; D’Souza, P.; Tifft, C.J.; Herzog, R.W.; Elder, M.E.; Shoemaker, L.R.; et al. Thrombotic Microangiopathy Following Systemic AAV Administration Is Dependent on Anti-Capsid Antibodies. J. Clin. Investig. 2024, 134, e173510. [Google Scholar] [CrossRef]
- Laforet, G.A. Thrombotic Microangiopathy Associated with Systemic Adeno-Associated Virus Gene Transfer: Review of Reported Cases. Hum. Gene Ther. 2025, 36, 64–76. [Google Scholar] [CrossRef]
- Chand, D.H.; Zaidman, C.; Arya, K.; Millner, R.; Farrar, M.A.; Mackie, F.E.; Goedeker, N.L.; Dharnidharka, V.R.; Dandamudi, R.; Reyna, S.P. Thrombotic Microangiopathy Following Onasemnogene Abeparvovec for Spinal Muscular Atrophy: A Case Series. J. Pediatr. 2021, 231, 265–268. [Google Scholar] [CrossRef]
- Hinderer, C.; Bell, P.; Katz, N.; Vite, C.H.; Louboutin, J.-P.; Bote, E.; Yu, H.; Zhu, Y.; Casal, M.L.; Bagel, J.; et al. Evaluation of Intrathecal Routes of Administration for Adeno-Associated Viral Vectors in Large Animals. Hum. Gene Ther. 2018, 29, 15–24. [Google Scholar] [CrossRef]
- Hordeaux, J.; Buza, E.L.; Dyer, C.; Goode, T.; Mitchell, T.W.; Richman, L.; Denton, N.; Hinderer, C.; Katz, N.; Schmid, R.; et al. Adeno-Associated Virus-Induced Dorsal Root Ganglion Pathology. Hum. Gene Ther. 2020, 31, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Fader, K.A.; Pardo, I.D.; Kovi, R.C.; Somps, C.J.; Wang, H.H.; Vaidya, V.S.; Ramaiah, S.K.; Sirivelu, M.P. Circulating Neurofilament Light Chain as a Promising Biomarker of AAV-Induced Dorsal Root Ganglia Toxicity in Nonclinical Toxicology Species. Mol. Ther.-Methods Clin. Dev. 2022, 25, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Astellas Announces FDA Update on the FORTIS Clinical Trial of AT845 in Adults with Late-Onset Pompe Disease. Available online: https://newsroom.astellas.com/2022-06-27-Astellas-Announces-FDA-Update-on-the-FORTIS-Clinical-Trial-of-AT845-in-Adults-with-Late-Onset-Pompe-Disease (accessed on 7 October 2025).
- Mueller, C.; Berry, J.D.; McKenna-Yasek, D.M.; Gernoux, G.; Owegi, M.A.; Pothier, L.M.; Douthwright, C.L.; Gelevski, D.; Luppino, S.D.; Blackwood, M.; et al. SOD1 Suppression with Adeno-Associated Virus and MicroRNA in Familial ALS. N. Engl. J. Med. 2020, 383, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kagiava, A.; Richter, J.; Tryfonos, C.; Leal-Julià, M.; Sargiannidou, I.; Christodoulou, C.; Bosch, A.; Kleopa, K.A. Efficacy of AAV Serotypes to Target Schwann Cells after Intrathecal and Intravenous Delivery. Sci. Rep. 2021, 11, 23358. [Google Scholar] [CrossRef]
- Gray, S.J.; Matagne, V.; Bachaboina, L.; Yadav, S.; Ojeda, S.R.; Samulski, R.J. Preclinical Differences of Intravascular AAV9 Delivery to Neurons and Glia: A Comparative Study of Adult Mice and Nonhuman Primates. Mol. Ther. 2011, 19, 1058–1069. [Google Scholar] [CrossRef]
- Yang, B.; Li, S.; Wang, H.; Guo, Y.; Gessler, D.J.; Cao, C.; Su, Q.; Kramer, J.; Zhong, L.; Ahmed, S.S.; et al. Global CNS Transduction of Adult Mice by Intravenously Delivered rAAVrh.8 and rAAVrh.10 and Nonhuman Primates by rAAVrh.10. Mol. Ther. 2014, 22, 1299–1309. [Google Scholar] [CrossRef]
- Miyake, N.; Miyake, K.; Yamamoto, M.; Hirai, Y.; Shimada, T. Global Gene Transfer into the CNS across the BBB after Neonatal Systemic Delivery of Single-Stranded AAV Vectors. Brain Res. 2011, 1389, 19–26. [Google Scholar] [CrossRef]
- Hoshino, Y.; Nishide, K.; Nagoshi, N.; Shibata, S.; Moritoki, N.; Kojima, K.; Tsuji, O.; Matsumoto, M.; Kohyama, J.; Nakamura, M.; et al. The Adeno-Associated Virus Rh10 Vector Is an Effective Gene Transfer System for Chronic Spinal Cord Injury. Sci. Rep. 2019, 9, 9844. [Google Scholar] [CrossRef] [PubMed]
- Ronzitti, G.; Gross, D.-A.; Mingozzi, F. Human Immune Responses to Adeno-Associated Virus (AAV) Vectors. Front. Immunol. 2020, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Costa Verdera, H.; Kuranda, K.; Mingozzi, F. AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer. Mol. Ther. 2020, 28, 723–746. [Google Scholar] [CrossRef] [PubMed]
- Mullagulova, A.; Shaimardanova, A.; Solovyeva, V.; Mukhamedshina, Y.; Chulpanova, D.; Kostennikov, A.; Issa, S.; Rizvanov, A. Safety and Efficacy of Intravenous and Intrathecal Delivery of AAV9-Mediated ARSA in Minipigs. Int. J. Mol. Sci. 2023, 24, 9204. [Google Scholar] [CrossRef]
- Jagadisan, B.; Dhawan, A. Hepatotoxicity in Adeno-Associated Viral Vector Gene Therapy. Curr. Hepatol. Rep. 2023, 22, 276–290. [Google Scholar] [CrossRef]
- Ghauri, M.S.; Ou, L. AAV Engineering for Improving Tropism to the Central Nervous System. Biology 2023, 12, 186. [Google Scholar] [CrossRef]
- Tan, F.; Dong, Y.; Qi, J.; Yu, W.; Chai, R. Artificial Intelligence-Based Approaches for AAV Vector Engineering. Adv. Sci. 2025, 12, 2411062. [Google Scholar] [CrossRef]
- Perabo, L.; Endell, J.; King, S.; Lux, K.; Goldnau, D.; Hallek, M.; Büning, H. Combinatorial Engineering of a Gene Therapy Vector: Directed Evolution of Adeno-associated Virus. J. Gene Med. 2006, 8, 155–162. [Google Scholar] [CrossRef]
- Boucas, J.; Lux, K.; Huber, A.; Schievenbusch, S.; Von Freyend, M.J.; Perabo, L.; Quadt-Humme, S.; Odenthal, M.; Hallek, M.; Büning, H. Engineering Adeno-associated Virus Serotype 2-based Targeting Vectors Using a New Insertion Site-position 453-and Single Point Mutations. J. Gene Med. 2009, 11, 1103–1113. [Google Scholar] [CrossRef]
- Adachi, K.; Enoki, T.; Kawano, Y.; Veraz, M.; Nakai, H. Drawing a High-Resolution Functional Map of Adeno-Associated Virus Capsid by Massively Parallel Sequencing. Nat. Commun. 2014, 5, 3075. [Google Scholar] [CrossRef]
- Girod, A.; Ried, M.; Wobus, C.; Lahm, H.; Leike, K.; Kleinschmidt, J.; Deléage, G.; Hallek, M. Genetic Capsid Modifications Allow Efficient Re-Targeting of Adeno-Associated Virus Type 2. Nat. Med. 1999, 5, 1052–1056. [Google Scholar] [CrossRef]
- Maheshri, N.; Koerber, J.T.; Kaspar, B.K.; Schaffer, D.V. Directed Evolution of Adeno-Associated Virus Yields Enhanced Gene Delivery Vectors. Nat. Biotechnol. 2006, 24, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Deverman, B.E.; Pravdo, P.L.; Simpson, B.P.; Kumar, S.R.; Chan, K.Y.; Banerjee, A.; Wu, W.-L.; Yang, B.; Huber, N.; Pasca, S.P.; et al. Cre-Dependent Selection Yields AAV Variants for Widespread Gene Transfer to the Adult Brain. Nat. Biotechnol. 2016, 34, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Jang, M.J.; Yoo, B.B.; Greenbaum, A.; Ravi, N.; Wu, W.-L.; Sánchez-Guardado, L.; Lois, C.; Mazmanian, S.K.; Deverman, B.E.; et al. Engineered AAVs for Efficient Noninvasive Gene Delivery to the Central and Peripheral Nervous Systems. Nat. Neurosci. 2017, 20, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Ravindra Kumar, S.; Miles, T.F.; Chen, X.; Brown, D.; Dobreva, T.; Huang, Q.; Ding, X.; Luo, Y.; Einarsson, P.H.; Greenbaum, A.; et al. Multiplexed Cre-Dependent Selection Yields Systemic AAVs for Targeting Distinct Brain Cell Types. Nat. Methods 2020, 17, 541–550. [Google Scholar] [CrossRef]
- Goertsen, D.; Flytzanis, N.C.; Goeden, N.; Chuapoco, M.R.; Cummins, A.; Chen, Y.; Fan, Y.; Zhang, Q.; Sharma, J.; Duan, Y.; et al. AAV Capsid Variants with Brain-Wide Transgene Expression and Decreased Liver Targeting after Intravenous Delivery in Mouse and Marmoset. Nat. Neurosci. 2022, 25, 106–115. [Google Scholar] [CrossRef]
- Liguore, W.A.; Domire, J.S.; Button, D.; Wang, Y.; Dufour, B.D.; Srinivasan, S.; McBride, J.L. AAV-PHP.B Administration Results in a Differential Pattern of CNS Biodistribution in Non-Human Primates Compared with Mice. Mol. Ther. 2019, 27, 2018–2037. [Google Scholar] [CrossRef]
- Phillips, K.A.; Bales, K.L.; Capitanio, J.P.; Conley, A.; Czoty, P.W.; T Hart, B.A.; Hopkins, W.D.; Hu, S.; Miller, L.A.; Nader, M.A.; et al. Why Primate Models Matter. Am. J. Primatol. 2014, 76, 801–827. [Google Scholar] [CrossRef]
- Campos, L.J.; Arokiaraj, C.M.; Chuapoco, M.R.; Chen, X.; Goeden, N.; Gradinaru, V.; Fox, A.S. Advances in AAV Technology for Delivering Genetically Encoded Cargo to the Nonhuman Primate Nervous System. Curr. Res. Neurobiol. 2023, 4, 100086. [Google Scholar] [CrossRef]
- Chuapoco, M.R.; Flytzanis, N.C.; Goeden, N.; Christopher Octeau, J.; Roxas, K.M.; Chan, K.Y.; Scherrer, J.; Winchester, J.; Blackburn, R.J.; Campos, L.J.; et al. Adeno-Associated Viral Vectors for Functional Intravenous Gene Transfer throughout the Non-Human Primate Brain. Nat. Nanotechnol. 2023, 18, 1241–1251. [Google Scholar] [CrossRef]
- Singh, I.; Moser, A.E.; Moser, H.W.; Kishimoto, Y. Adrenoleukodystrophy: Impaired Oxidation of Very Long Chain Fatty Acids in White Blood Cells, Cultured Skin Fibroblasts, and Amniocytes. Pediatr. Res. 1984, 18, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Moser, H.W.; Moser, A.B.; Kishimoto, Y. Adrenoleukodystrophy: Impaired Oxidation of Long Chain Fatty Acids in Cultured Skin Fibroblasts and Adrenal Cortex. Biochem. Biophys. Res. Commun. 1981, 102, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-M.; Yik, W.Y.; Zhang, P.; Lu, W.; Dranchak, P.K.; Shibata, D.; Steinberg, S.J.; Hacia, J.G. The Gene Expression Profiles of Induced Pluripotent Stem Cells from Individuals with Childhood Cerebral Adrenoleukodystrophy Are Consistent with Proposed Mechanisms of Pathogenesis. Stem Cell Res. Ther. 2012, 3, 39. [Google Scholar] [CrossRef]
- Jang, J.; Kang, H.; Kim, H.; Kim, J.Y.; Huh, Y.J.; Kim, D.; Yoo, J.; Lee, J.; Lim, B.; Lee, J.; et al. Induced Pluripotent Stem Cell Models from X-linked Adrenoleukodystrophy Patients. Ann. Neurol. 2011, 70, 402–409. [Google Scholar] [CrossRef]
- Parasar, P.; Kaur, N.; Singh, J. IPSC-Derived Astrocytes to Model Neuroinflammatory and Metabolic Responses in X-Linked Adrenoleukodystrophy. J. Biotechnol. Biomed. 2023, 6, 281. [Google Scholar] [CrossRef]
- Baarine, M.; Khan, M.; Singh, A.; Singh, I. Functional Characterization of IPSC-Derived Brain Cells as a Model for X-Linked Adrenoleukodystrophy. PLoS ONE 2015, 10, e0143238. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, J. Generation and Characterization of Human iPSC-Derived Astrocytes with Potential for Modeling X-Linked Adrenoleukodystrophy Phenotypes. Int. J. Mol. Sci. 2025, 26, 1576. [Google Scholar] [CrossRef]
- Asheuer, M.; Bieche, I.; Laurendeau, I.; Moser, A.; Hainque, B.; Vidaud, M.; Aubourg, P. Decreased Expression of ABCD4 and BG1 Genes Early in the Pathogenesis of X-Linked Adrenoleukodystrophy. Hum. Mol. Genet. 2005, 14, 1293–1303. [Google Scholar] [CrossRef]
- Xue, W.; Li, B.; Liu, H.; Xiao, Y.; Li, B.; Ren, L.; Li, H.; Shao, Z. Generation of Dorsoventral Human Spinal Cord Organoids via Functionalizing Composite Scaffold for Drug Testing. iScience 2023, 26, 105898. [Google Scholar] [CrossRef]
- Madhavan, M.; Nevin, Z.S.; Shick, H.E.; Garrison, E.; Clarkson-Paredes, C.; Karl, M.; Clayton, B.L.L.; Factor, D.C.; Allan, K.C.; Barbar, L.; et al. Induction of Myelinating Oligodendrocytes in Human Cortical Spheroids. Nat. Methods 2018, 15, 700–706. [Google Scholar] [CrossRef]
- Kim, H.; Xu, R.; Padmashri, R.; Dunaevsky, A.; Liu, Y.; Dreyfus, C.F.; Jiang, P. Pluripotent Stem Cell-Derived Cerebral Organoids Reveal Human Oligodendrogenesis with Dorsal and Ventral Origins. Stem Cell Rep. 2019, 12, 890–905. [Google Scholar] [CrossRef]
- Fagiani, F.; Pedrini, E.; Taverna, S.; Brambilla, E.; Murtaj, V.; Podini, P.; Ruffini, F.; Butti, E.; Braccia, C.; Andolfo, A.; et al. A Glia-Enriched Stem Cell 3D Model of the Human Brain Mimics the Glial-Immune Neurodegenerative Phenotypes of Multiple Sclerosis. Cell Rep. Med. 2024, 5, 101680. [Google Scholar] [CrossRef] [PubMed]
- Ormel, P.R.; Vieira De Sá, R.; Van Bodegraven, E.J.; Karst, H.; Harschnitz, O.; Sneeboer, M.A.M.; Johansen, L.E.; Van Dijk, R.E.; Scheefhals, N.; Berdenis Van Berlekom, A.; et al. Microglia Innately Develop within Cerebral Organoids. Nat. Commun. 2018, 9, 4167. [Google Scholar] [CrossRef] [PubMed]
- Schafer, S.T.; Mansour, A.A.; Schlachetzki, J.C.M.; Pena, M.; Ghassemzadeh, S.; Mitchell, L.; Mar, A.; Quang, D.; Stumpf, S.; Ortiz, I.S.; et al. An in Vivo Neuroimmune Organoid Model to Study Human Microglia Phenotypes. Cell 2023, 186, 2111–2126.e20. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-F.; Lawler, A.M.; Watkins, P.A.; Powers, J.M.; Moser, A.B.; Moser, H.W.; Smith, K.D. A Mouse Model for X-Linked Adrenoleukodystrophy. Proc. Natl. Acad. Sci. USA 1997, 94, 9366–9371. [Google Scholar] [CrossRef]
- Kobayashi, T.; Shinnoh, N.; Kondo, A.; Yamada, T. Adrenoleukodystrophy Protein-Deficient Mice Represent Abnormality of Very Long Chain Fatty Acid Metabolism. Biochem. Biophys. Res. Commun. 1997, 232, 631–636. [Google Scholar] [CrossRef]
- Pujol, A. Late Onset Neurological Phenotype of the X-ALD Gene Inactivation in Mice: A Mouse Model for Adrenomyeloneuropathy. Hum. Mol. Genet. 2002, 11, 499–505. [Google Scholar] [CrossRef]
- Pujol, A.; Ferrer, I.; Camps, C.; Metzger, E.; Hindelang, C.; Callizot, N.; Ruiz, M.; Pàmpols, T.; Giròs, M.; Mandel, J.L. Functional Overlap between ABCD1 (ALD) and ABCD2 (ALDR) Transporters: A Therapeutic Target for X-Adrenoleukodystrophy. Hum. Mol. Genet. 2004, 13, 2997–3006. [Google Scholar] [CrossRef]
- Gong, J.; Liu, Y.; Chung, T.-H.; Xu, L.; Lund, T.C.; Chang, L.-J. Intracerebral Lentiviral ABCD1 Gene Therapy in an Early Disease Onset ALD Mouse Model. Gene Ther. 2023, 30, 18–30. [Google Scholar] [CrossRef]
- Hashemi, E.; Srivastava, I.N.; Aguirre, A.; Yoseph, E.T.; Kaushal, E.; Awani, A.; Ryu, J.K.; Akassoglou, K.; Talebian, S.; Chu, P.; et al. A Novel Mouse Model of Cerebral Adrenoleukodystrophy Highlights NLRP3 Activity in Lesion Pathogenesis. bioRxiv 2023. [Google Scholar] [CrossRef]

| Model (Strain C57BL/6) | Model Generation Method | Key Biochemical Changes | Behavioral Changes/Neurological Manifestation | Tested Gene Therapy and Its Effect |
|---|---|---|---|---|
| Exon 1 deletion (Abcd1−/Y) model | Homologous recombination replaced the 5′ part of exon 1 with a neomycin-resistance cassette, completely abolishing ALDP synthesis | Hepatic and peripheral β-oxidation of VLCFA reduced to ~40% of normal; C26:0 rises by 70–240% in brain, spinal cord and adrenals glands during the early postnatal period | Motor coordination and spontaneous activity decline between 15 and 20 months; peripheral nerve-conduction velocity slows from ~15 months | Systemic or intraventricular AAV9-hABCD1 spreads broadly through the CNS, lowers the C26/C22 ratio by 20–35% and stabilizes motor performance |
| Double knockout Abcd1−/Y; Abcd2−/− model | Male Abcd1 knockouts were crossed with female Abcd2 knockouts, yielding offspring lacking both peroxisomal transporters | C26:0 in nervous tissue and plasma increases five- to six-fold; the C26/C22 ratio is about ten times higher by eight months | Axonopathy and motor deficit appear as early as 8–12 months of age; time on the rotarod is roughly three times shorter than in wild-type mice | Intrathecal AAV9-hABCD1 (SBT101) maintains expression for at least 11 months, decreases VLCFA and TNF-α, and doubles fore-limb grip strength |
| Δ 3–9 deletion (Abcd1 Δ3–9) model | CRISPR/Cas9 excised exons 3–9 (~8.7 kb) in zygotes; fusion of exon 2 to exon 10 was confirmed and ALDP expression is absent. | C26:0 is 31-fold higher; the C26/C22 ratio is 20-fold higher; free cholesterol and reactive oxygen species are 1.4-fold higher by 12 months. | Thigmotaxis in the open-field test is evident by 6 months; imbalance on the rotarod appears by 10 months. | Local lentiviral LV-hABCD1 restores ALDP expression where delivered, normalizes VLCFA and cholesterol, and prevents motor decline for at least 12 months. |
| Cuprizone and EAE “two-hit” Abcd1−/Y model | Mice receive 0.2% cuprizone for 14 days, followed on day 15 by MOG35–55 immunization with complete Freund’s adjuvant and pertussis toxin, combining oligodendrocyte stress with a myelin-directed immune response. | Baseline VLCFA excess is present; the challenge triggers a surge in IL-18, an increase in gp91-phox and deposition of fibrin around vessels. | By week 5, MRI detects T2-hyperintense, gadolinium-leaking lesions in the corpus callosum; tail and hind-limb paresis occurs on days 16–22. | No gene therapy has currently been tested; the model is used to evaluate anti-inflammatory and BBB-protective strategies. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gornostal, E.; Alsalloum, A.; Degtyarev, E.; Kuznetsova, E.; Levashova, A.; Mishina, D.; Mingaleva, N.; Mazloum, A.; Bogdanov, V.; Krupinova, J.; et al. An AAV-Based Therapy Approach for Neurological Phenotypes of X-Linked Adrenoleukodystrophy. Int. J. Mol. Sci. 2025, 26, 11645. https://doi.org/10.3390/ijms262311645
Gornostal E, Alsalloum A, Degtyarev E, Kuznetsova E, Levashova A, Mishina D, Mingaleva N, Mazloum A, Bogdanov V, Krupinova J, et al. An AAV-Based Therapy Approach for Neurological Phenotypes of X-Linked Adrenoleukodystrophy. International Journal of Molecular Sciences. 2025; 26(23):11645. https://doi.org/10.3390/ijms262311645
Chicago/Turabian StyleGornostal, Ekaterina, Almaqdad Alsalloum, Egor Degtyarev, Ekaterina Kuznetsova, Aygun Levashova, Daria Mishina, Natalia Mingaleva, Ali Mazloum, Viktor Bogdanov, Julia Krupinova, and et al. 2025. "An AAV-Based Therapy Approach for Neurological Phenotypes of X-Linked Adrenoleukodystrophy" International Journal of Molecular Sciences 26, no. 23: 11645. https://doi.org/10.3390/ijms262311645
APA StyleGornostal, E., Alsalloum, A., Degtyarev, E., Kuznetsova, E., Levashova, A., Mishina, D., Mingaleva, N., Mazloum, A., Bogdanov, V., Krupinova, J., Mikhalkov, S., Rybkina, I., Mityaeva, O., & Volchkov, P. (2025). An AAV-Based Therapy Approach for Neurological Phenotypes of X-Linked Adrenoleukodystrophy. International Journal of Molecular Sciences, 26(23), 11645. https://doi.org/10.3390/ijms262311645






