Anti-Müllerian Hormone as a Biomarker for Predicting Testicular Sperm Extraction Outcomes in Azoospermic Patients: A Comprehensive Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
- ‘AMH’ [MeSH Terms] AND (‘non-obstructive azoospermia’ [All Fields] OR ‘NOA’ [All Fields]) AND (‘testicular sperm extraction’ [All Fields]) AND (‘sperm parameters’ [MeSH Terms] OR ‘TESE’ [All Fields])
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
2.4. Statistical Analysis
2.5. Quality Assessment
2.6. Registration
3. Results
3.1. Study Selection
3.2. Study Characteristics
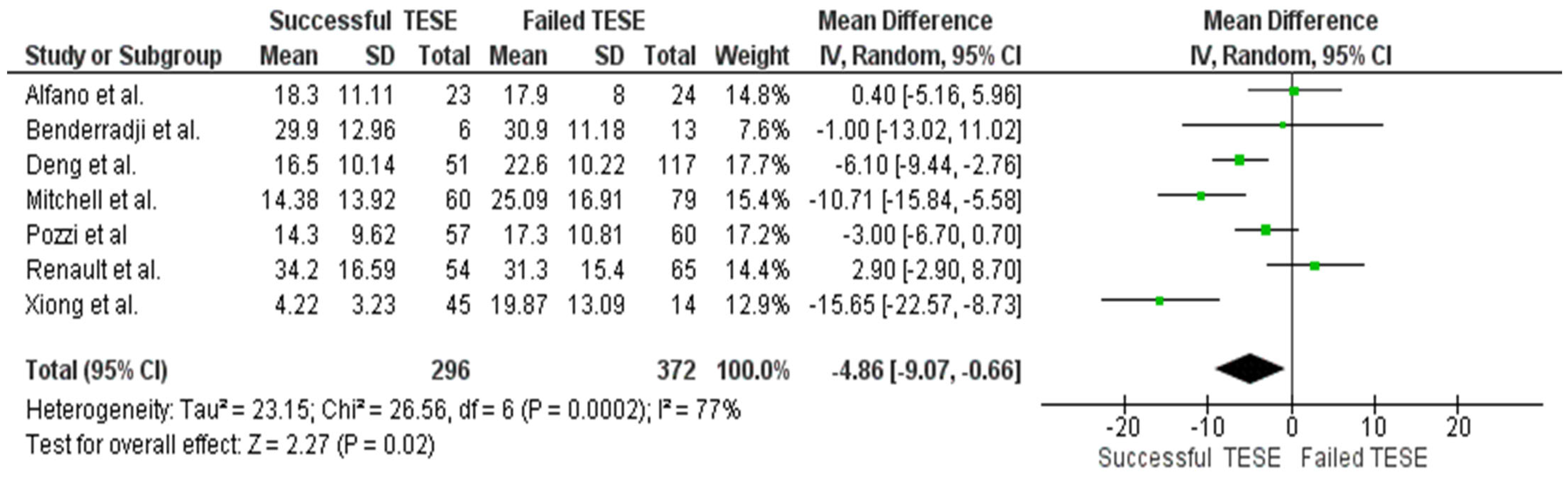
3.3. Serum AMH and Sperm Retrieval Outcomes
3.4. Seminal AMH and Sperm Retrieval Outcomes
3.5. FSH Levels and TESE Outcomes
3.6. Inhibin and Total Testosterone Levels
3.7. Combined Biomarker Models
3.8. Quality Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMH | Anti-Müllerian Hormone |
| TESE | testicular sperm extraction |
| NOA | non-obstructive azoospermia |
| FSH | follicle-stimulating hormone |
| LH | luteinizing hormone |
| ICSI | intracytoplasmic sperm injection |
| SRR | sperm retrieval rates |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PICO | Populations, Intervention, Comparison, and Outcome |
References
- Benderradji, H.; Prasivoravong, J.; Marcelli, F.; Barbotin, A.L.; Catteau-Jonard, S.; Marchetti, C.; Guittard, C.; Puech, P.; Mitchell, V.; Rigot, J.M.; et al. Contribution of serum anti-Müllerian hormone in the management of azoospermia and the prediction of testicular sperm retrieval outcomes: A study of 155 adult men. Basic Clin. Androl. 2021, 31, 15. [Google Scholar] [CrossRef] [PubMed]
- Olumide, O.B.; Godwin, A.I.; Titilayo, J.O.; Christian, I.O.; Etukudoh, N.S.; Uchejeso, O.M.; Temitope, S.T.; Dutta, S.; Sengupta, P. Assessment of serum anti-Müllerian hormone (AMH) as an independent marker for oligozoospermia and non-obstructive azoospermia in infertile Nigerian men. Biomed. Pharmacol. J. 2023, 16, 35–42. [Google Scholar] [CrossRef]
- Holt, R.; Yahyavi, S.K.; Kooij, I.; Andreassen, C.H.; Andersson, A.M.; Juul, A.; Jørgensen, N.; Blomberg Jensen, M. Low serum anti-Müllerian hormone is associated with semen quality in infertile men and not influenced by vitamin D supplementation. BMC Med. 2023, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Toulis, K.A.; Iliadou, P.K.; Venetis, C.A.; Tsametis, C.; Tarlatzis, B.C.; Papadimas, I.; Goulis, D.G. Inhibin B and anti-Müllerian hormone as markers of persistent spermatogenesis in men with non-obstructive azoospermia: A meta-analysis of diagnostic accuracy studies. Hum. Reprod. Update 2010, 16, 713–724. [Google Scholar] [CrossRef]
- Pozzi, E.; Raffo, M.; Negri, F.; Boeri, L.; Saccà, A.; Belladelli, F.; Cilio, S.; Ventimiglia, E.; d’Arma, A.; Pagliardini, L.; et al. Anti-Müllerian hormone predicts positive sperm retrieval in men with idiopathic non-obstructive azoospermia: Findings from a multi-centric cross-sectional study. Hum. Reprod. 2023, 38, 1464–1472. [Google Scholar] [CrossRef]
- Kaltsas, A.; Stavros, S.; Kratiras, Z.; Zikopoulos, A.; Machairiotis, N.; Potiris, A.; Dimitriadis, F.; Sofikitis, N.; Chrisofos, M.; Zachariou, A. Predictors of successful testicular sperm extraction: A new era for men with non-obstructive azoospermia. Biomedicines 2024, 12, 2679. [Google Scholar] [CrossRef]
- Al-Zubi, M.; Al-Khawaldeh, S.; Mallak, M.; Al-Dghaim, M.; Zytoon, R.; Abbas, R.; Alhabahbeh, A.; Alfadel, M.; Abuorouq, S.; Al-Magableh, M.R.; et al. Can we predict the outcome of micro testicular sperm extraction in non-obstructive azoospermia from preoperative hormonal profile, testicular volume, and patient health factors: A retrospective cross-sectional study. Am. J. Mens Health 2025, 19, 15579883251320017. [Google Scholar] [CrossRef]
- Pozzi, E.; Corsini, C.; Negri, F.; Lucianò, R.; Pontillo, M.; Montorsi, F.; Alfano, M.; Salonia, A. Serum anti-Müllerian hormone levels in primary infertile men with/without non-obstructive azoospermia versus fertile controls: Findings from a real-life cross-sectional study. J. Urol. 2025, 213 (Suppl. S5), e369. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- JAMA. Systeme International (SI) Conversion Factors for Selected Laboratory Components. JAMA. 2001. Available online: http://jama.ama-assn.org/content/vol290/issue1/images/data/125/DC6/auinst_si.dtl (accessed on 24 February 2024).
- Aboukhshaba, A.; Punjani, N.; Doukakis, S.; Schlegel, P.N. Anti-Müllerian hormone level as a predictor of sperm retrieval with microdissection testicular sperm extraction in non-obstructive azoospermia. Andrologia 2021, 53, e14220. [Google Scholar] [CrossRef]
- Alfano, M.; Ventimiglia, E.; Locatelli, I.; Capogrosso, P.; Cazzaniga, W.; Pederzoli, F.; Frego, N.; Matloob, R.; Saccà, A.; Pagliardini, L.; et al. Anti-Müllerian hormone-to-testosterone ratio is predictive of positive sperm retrieval in men with idiopathic non-obstructive azoospermia. Sci. Rep. 2017, 7, 17638. [Google Scholar] [CrossRef]
- Deng, C.; Liu, D.; Zhao, L.; Lin, H.; Mao, J.; Zhang, Z.; Yang, Y.; Zhang, H.; Xu, H.; Hong, K.; et al. Inhibin B-to-anti-Müllerian hormone ratio as noninvasive predictors of positive sperm retrieval in idiopathic non-obstructive azoospermia. J. Clin. Med. 2023, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Duvilla, E.; Lejeune, H.; Trombert-Paviot, B.; Gentil-Perret, A.; Tostain, J.; Levy, R. Significance of inhibin B and anti-Müllerian hormone in seminal plasma: A preliminary study. Fertil. Steril. 2008, 89, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Isikoglu, M.; Ozgur, K.; Oehninger, S.; Ozdem, S.; Seleker, M. Serum anti-Müllerian hormone levels do not predict the efficiency of testicular sperm retrieval in men with non-obstructive azoospermia. Gynecol. Endocrinol. 2006, 22, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, V.; Boitrelle, F.; Pigny, P.; Robin, G.; Marchetti, C.; Marcelli, F.; Rigot, J.M. Seminal plasma levels of anti-Müllerian hormone and inhibin B are not predictive of testicular sperm retrieval in non-obstructive azoospermia: A study of 139 men. Fertil. Steril. 2010, 94, 2147–2150. [Google Scholar] [CrossRef]
- Mostafa, T.; Amer, M.K.; Abdel-Malak, G.; Nsser, T.A.; Zohdy, W.; Ashour, S.; El-Gayar, D.; Awad, H.H. Seminal plasma anti-Müllerian hormone level correlates with semen parameters but does not predict success of testicular sperm extraction. Asian J. Androl. 2007, 9, 265–270. [Google Scholar] [CrossRef]
- Renault, L.; Labrune, E.; Giscard d’Estaing, S.; Cuzin, B.; Lapoirie, M.; Benchaib, M.; Lornage, J.; Soignon, G.; de Souza, A.; Dijoud, F.; et al. Delaying testicular sperm extraction in 47,XXY Klinefelter patients does not impair the sperm retrieval rate, and AMH levels are higher when TESE is positive. Hum. Reprod. 2022, 37, 2518–2531. [Google Scholar] [CrossRef]
- Xiong, Z.; Ye, Z.; Tu, J.; Meng, T.Q.; Ren, N. Serum anti-Müllerian hormone level for differential diagnosis of obstructive and non-obstructive azoospermia. Zhonghua Nan Ke Xue 2019, 25, 823–827. [Google Scholar]
- Zhang, Y.X.; Yao, C.C.; Huang, Y.H.; Li, P.; Zhi, E.L.; Zhu, Z.J.; Zhang, J.X.; Zhao, F.J.; Li, Z.; Tian, R.H. Efficacy of stepwise mini-incision microdissection testicular sperm extraction for non-obstructive azoospermia with varied etiologies. Asian J. Androl. 2023, 25, 621–626. [Google Scholar] [CrossRef]
- Layih, O.A.; Saleh, B.O. The role of serum and seminal fluid anti-Müllerian hormone in differentiating subtypes of male infertility. J. Fac. Med. Baghdad 2025, 64, 252–259. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, G.; Zheng, Y.; Jiang, X. From biological marker to clinical application: The role of anti-Müllerian hormone for delayed puberty and idiopathic non-obstructive azoospermia in males. Endocr. Connect. 2025, 14, e240630. [Google Scholar] [CrossRef]
- Turhan, G.; Çil, N.; Kabukçu, C.; Turan, T.; Fenkçi, I.V.; Mete, G.A. Relationship of seminal plasma anti-Müllerian hormone concentration with sperm morphology and sperm DNA damage. J. Urol. Surg. 2022, 9, 272–280. [Google Scholar] [CrossRef]
- Plotton, I.; Garby, L.; Morel, Y.; Lejeune, H. Decrease of anti-Müllerian hormone in genetic spermatogenic failure. Andrologia 2012, 44, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, T.; Dekalo, S.; Azem, F.; Hauser, R.; Barda, S. The predictive value of AMH, FSH, and inhibin B for sperm retrieval in azoospermic men. J. Sex. Med. 2024, 21 (Suppl. S2), qdae002.164. [Google Scholar] [CrossRef]
- Glina, S.; Vieira, M. Prognostic factors for sperm retrieval in non-obstructive azoospermia. Clinics 2013, 68 (Suppl. S1), 121–124. [Google Scholar] [CrossRef] [PubMed]
- Puia, D.; Miron, A.; Radavoi, D.; Jinga, V.; Pricop, C. Follicle-stimulating hormone and inhibin B as predictors of successful sperm retrieval in men undergoing testicular sperm extraction: A review of 44 cases and meta-analysis. Afr. J. Reprod. Health 2023, 27, 51–59. [Google Scholar]
- Schwarzkopf, V.; Wistuba, J.; Sandhowe-Klaverkamp, R.; Kliesch, S.; Gromoll, J.; Schubert, M. Unraveling a subgroup of men with unexplained male infertility: Men with normogonadotropic non-obstructive azoospermia. J. Clin. Endocrinol. Metab. 2025, 110, 3400–3411. [Google Scholar] [CrossRef]
- Xiao, H.; Ding, Y.L.; Yang, P.; Chen, Q.; Huang, H.L.; Chen, X.; Zhou, H.L.; Tang, S.X. Association between anti-Müllerian hormone concentrations and sperm retrieval outcomes in patients with idiopathic non-obstructive azoospermia: A systematic review and meta-analysis. Asian J. Androl. 2024, 26, 522–527. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, X.; Wang, Y.; Yang, C.; Wang, W.; Qin, C. An integrative prediction model of successful sperm retrieval for men with non-obstructive azoospermia. Rev. Int. Androl. 2024, 22, 48–56. [Google Scholar]
- Mahdy, B.; La Croce, G.; Roscigno, M.; Manica, M.; Da Pozzo, L.; Saccà, A. Microsurgical testicular sperm extraction: Predictive factors and outcomes for men with non-obstructive azoospermia. Andrologia 2024, 56, 6380023. [Google Scholar] [CrossRef]
- Lantsberg, D.; Mizrachi, Y.; Katz, D.J. Micro-testicular sperm extraction outcomes for non-obstructive azoospermia in a single large clinic in Victoria. Aust. N. Zeal. J. Obstet. Gynaecol. 2022, 62, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, A.A.; Greear, G.M.; Chen, T.; Ball, D.; McClure, R.D.; Ostrowski, K.A.; Nicholson, T.M.; Crisostomo-Wynne, T.; Hehemann, M.C.; Walsh, T.J. Testicular mapping-guided sperm retrieval vs. upfront microTESE in non-obstructive azoospermia: A comparison of sperm retrieval, pregnancy and live-birth rates. Transl. Androl. Urol. 2024, 13, 2672–2680. [Google Scholar] [CrossRef] [PubMed]
- Raffo, M.; Boeri, L.; Iafrate, M.; Negri, F.; Birolini, G.; Ramadani, R.; Falcone, M.; Pozzi, E.; Preto, M.; Parolin, V.; et al. Serum total testosterone and testicular histology help predicting positive sperm retrieval in nonmosaic Klinefelter patients undergoing testicular sperm extraction: Real-life data from a large multicenter cohort. Fertil. Steril. 2025; in press. [Google Scholar] [CrossRef]
- Dasgupta, S.; Le, T.S.; Rambhatla, A.; Shah, R.; Agarwal, A. Medical treatment prior to micro-TESE. Asian J. Androl. 2025, 27, 342–354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falcone, M.; Boeri, L.; Timpano, M.; Cirigliano, L.; Preto, M.; Russo, G.I.; Peretti, F.; Ferro, I.; Plamadeala, N.; Gontero, P. Combined Trifocal and Microsurgical Testicular Sperm Extraction Enhances Sperm Retrieval Rate in Low-Chance Retrieval Non-Obstructive Azoospermia. J. Clin. Med. 2022, 11, 4058. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Preto, M.; Boeri, L.; Cirigliano, L.; Falcone, M.; Parolin, V.; Peretti, F.; Ferro, I.; Plamadeala, N.; Scavone, M.; Zupo, E.; et al. Preliminary Results of Microsurgical Sperm Retrieval in Azoospermic Patients: A Randomized Controlled Trial Comparing Operating Microscope vs. Surgical Loupes. J. Clin. Med. 2025, 14, 970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shu, J.; Lu, Z.; Xia, J.; Xiang, M. Serum anti-Müllerian hormone in azoospermia approach. Farmacia 2023, 71, 280–287. [Google Scholar] [CrossRef]
- Goulis, D.G.; Tsametis, C.; Iliadou, P.K.; Polychronou, P.; Kantartzi, P.D.; Tarlatzis, B.C.; Bontis, I.N.; Papadimas, I. Serum inhibin B and anti-Müllerian hormone are not superior to follicle-stimulating hormone as predictors of the presence of sperm in testicular fine-needle aspiration in men with azoospermia. Fertil. Steril. 2009, 91, 1279–1284. [Google Scholar] [CrossRef]
- Tian, R.; Zhang, J.; Xu, Y.; Zhao, F.; Li, P.; Huang, Y.; Wang, H.; Liu, D. Predicting micro-TESE among heterogeneous non-obstructive azoospermic patients: The impact on surgical decision and ICSI. Andrologia 2023, 55, 4825062. [Google Scholar] [CrossRef]
- Puia, D.; Ivanuta, M.; Pricop, C. Effect of bariatric surgery on male infertility: An updated meta-analysis and literature review. World J. Mens Health 2025, 43, 807–817. [Google Scholar] [CrossRef]
- Weghofer, A.; Dietrich, W.; Barad, D.H.; Gleicher, N. Live birth chances in women with extremely low-serum anti-Müllerian hormone levels. Hum. Reprod. 2011, 26, 1905–1909. [Google Scholar] [CrossRef]



| Author | Year | Patients (n) | Method of Sperm Retrieval | SRR (%) | NOS Score |
|---|---|---|---|---|---|
| Aboukhshaba et al. [11] | 2021 | 46 | mTESE | 45.65 | 7 |
| Alfano et al. [12] | 2017 | 47 | mTESE | 48.93 | 7 |
| Benderradji et al. [1] | 2021 | 19 | TESE | 31.57 | 4 |
| Deng et al. [13] | 2023 | 168 | mTESE | 30.35 | 7 |
| Duvilla et al. [14] | 2008 | 26 | TESE | 52.3 | 7 |
| Isikoglu et al. [15] | 2006 | 24 | TESE | 54.00 | 7 |
| Mitchell et al. [16] | 2010 | 139 | TESE | 54.16 | 8 |
| Mostafa et al. [17] | 2007 | 40 | TESE | 52.50 | 8 |
| Pozzi et al. [5] | 2023 | 117 | mTESE | 48.71 | 8 |
| Renault et al. [18] | 2022 | 119 | TESE | 45.37 | 6 |
| Xiong et al. [19] | 2019 | 59 | NA | 76.27 | 4 |
| Zhang et al. [20] | 2023 | 502 | mTESE | 53.78 | 7 |
| Study | Combined Markers | Model Type | Performance | Key Findings |
|---|---|---|---|---|
| Alfano et al. [12] | AMH/testosterone ratio | Logistic regression | AUC 95% | AMH/tT slightly better than AMH alone |
| Duvilla et al. [14] | Serum FSH + seminal Inhibin B + seminal AMH | Logistic regression | AUC 0.985 | Combined markers are superior to individual models |
| Mitchell et al. [16] | Seminal AMH + seminal Inhibin B + serum FSH | Not specified | No improvement | The combination did not improve predictive value |
| Pozzi et al. [5] | AMH + age + testicular volume + FSH + estradiol | Not specified | AUC 70.3% | AMH remained significant after adjustment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puia, D.; Ivănuță, M.; Corlade-Andrei, M.; Bîcă, O.D.; Doroftei, B.; Pricop, C. Anti-Müllerian Hormone as a Biomarker for Predicting Testicular Sperm Extraction Outcomes in Azoospermic Patients: A Comprehensive Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 11643. https://doi.org/10.3390/ijms262311643
Puia D, Ivănuță M, Corlade-Andrei M, Bîcă OD, Doroftei B, Pricop C. Anti-Müllerian Hormone as a Biomarker for Predicting Testicular Sperm Extraction Outcomes in Azoospermic Patients: A Comprehensive Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2025; 26(23):11643. https://doi.org/10.3390/ijms262311643
Chicago/Turabian StylePuia, Dragoș, Marius Ivănuță, Mihaela Corlade-Andrei, Ovidiu Daniel Bîcă, Bogdan Doroftei, and Cătălin Pricop. 2025. "Anti-Müllerian Hormone as a Biomarker for Predicting Testicular Sperm Extraction Outcomes in Azoospermic Patients: A Comprehensive Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 26, no. 23: 11643. https://doi.org/10.3390/ijms262311643
APA StylePuia, D., Ivănuță, M., Corlade-Andrei, M., Bîcă, O. D., Doroftei, B., & Pricop, C. (2025). Anti-Müllerian Hormone as a Biomarker for Predicting Testicular Sperm Extraction Outcomes in Azoospermic Patients: A Comprehensive Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 26(23), 11643. https://doi.org/10.3390/ijms262311643