Development and Application of Visible-Light-Responsive Perylene Diimide Functionalized Silk Fibroin/Polylactic Acid Antibacterial Nanofibrous Membranes
Abstract
1. Introduction
2. Results and Discussion
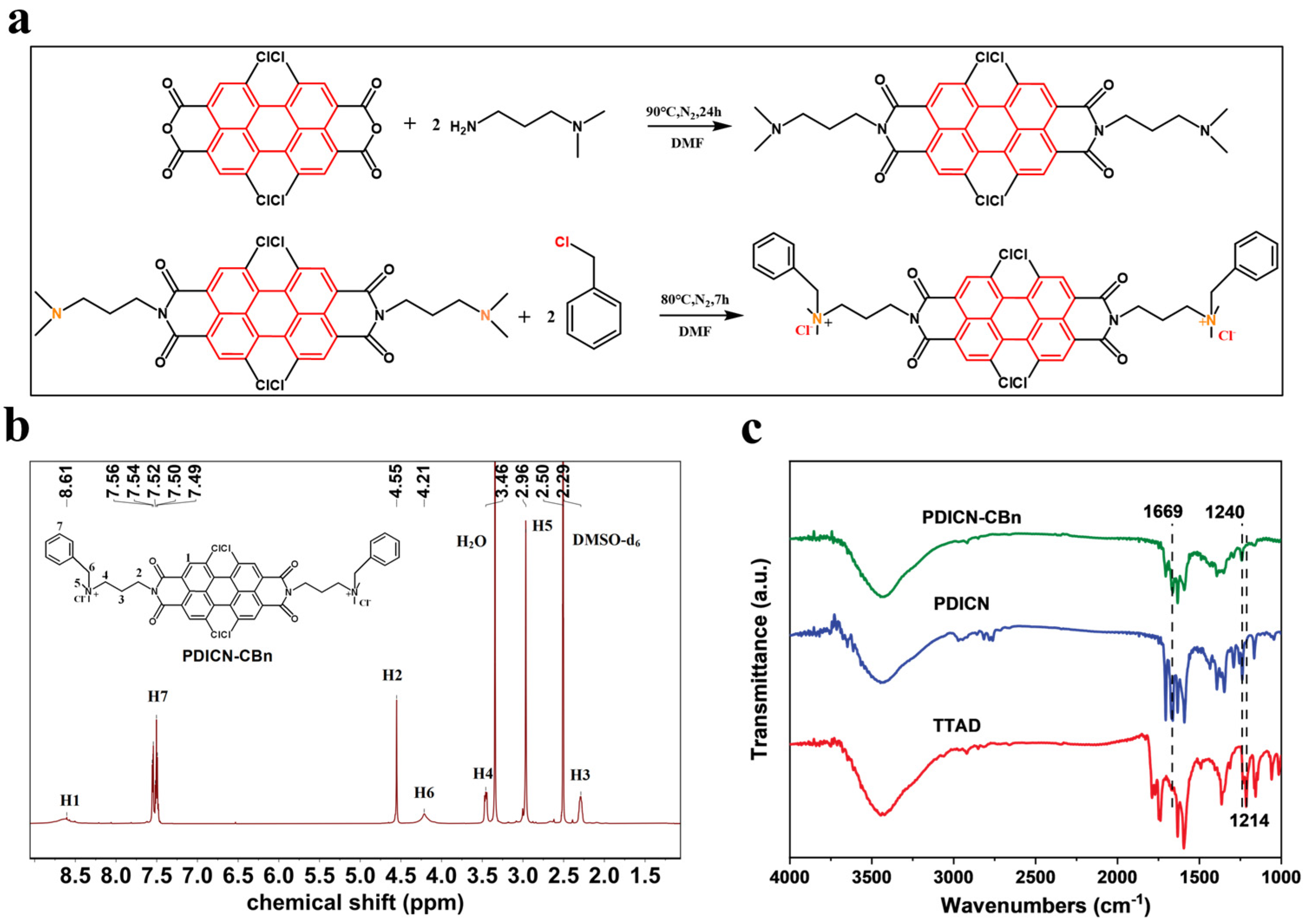
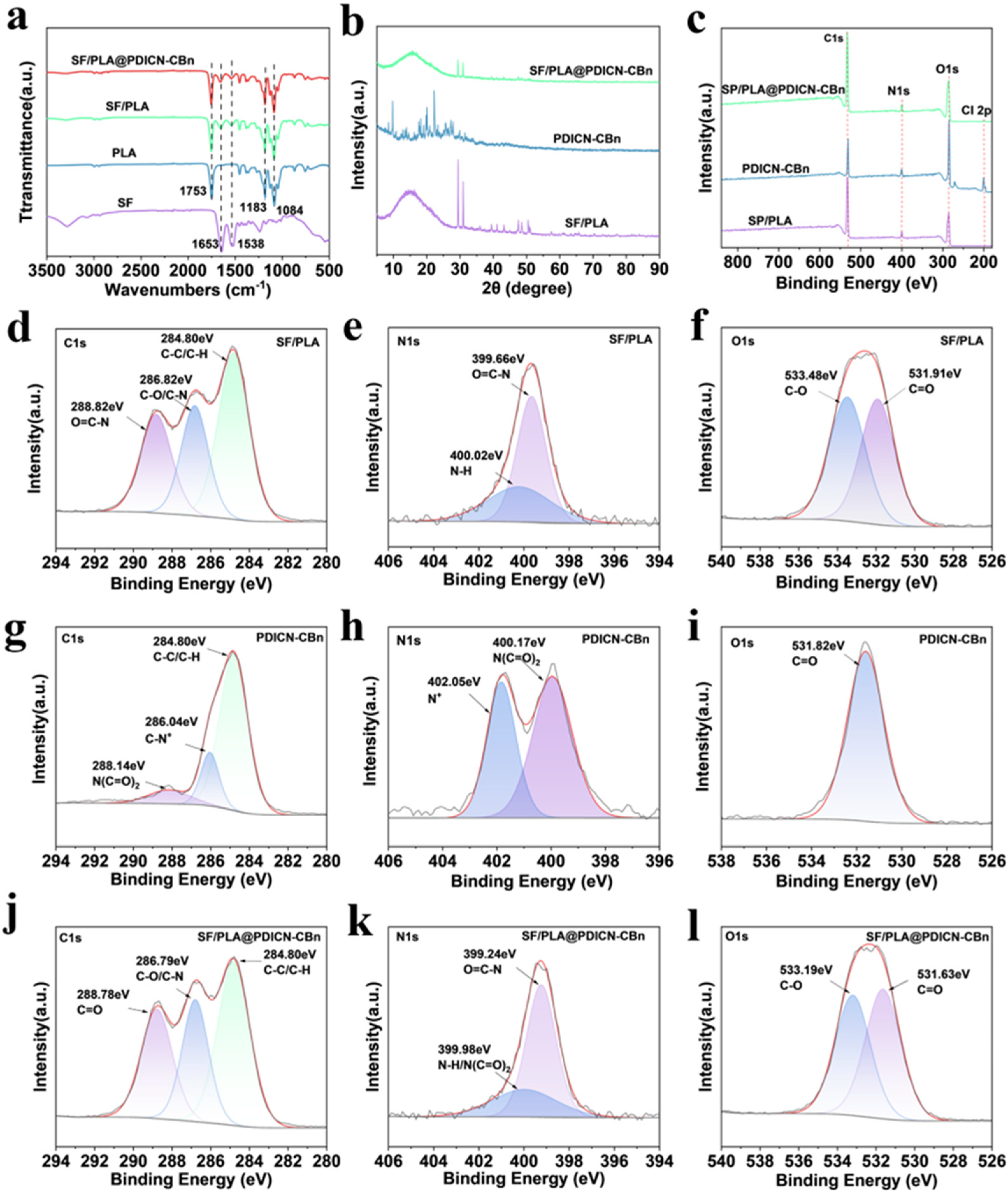
2.1. Chemical Characterization of SF/PLA@PDICN-CBn
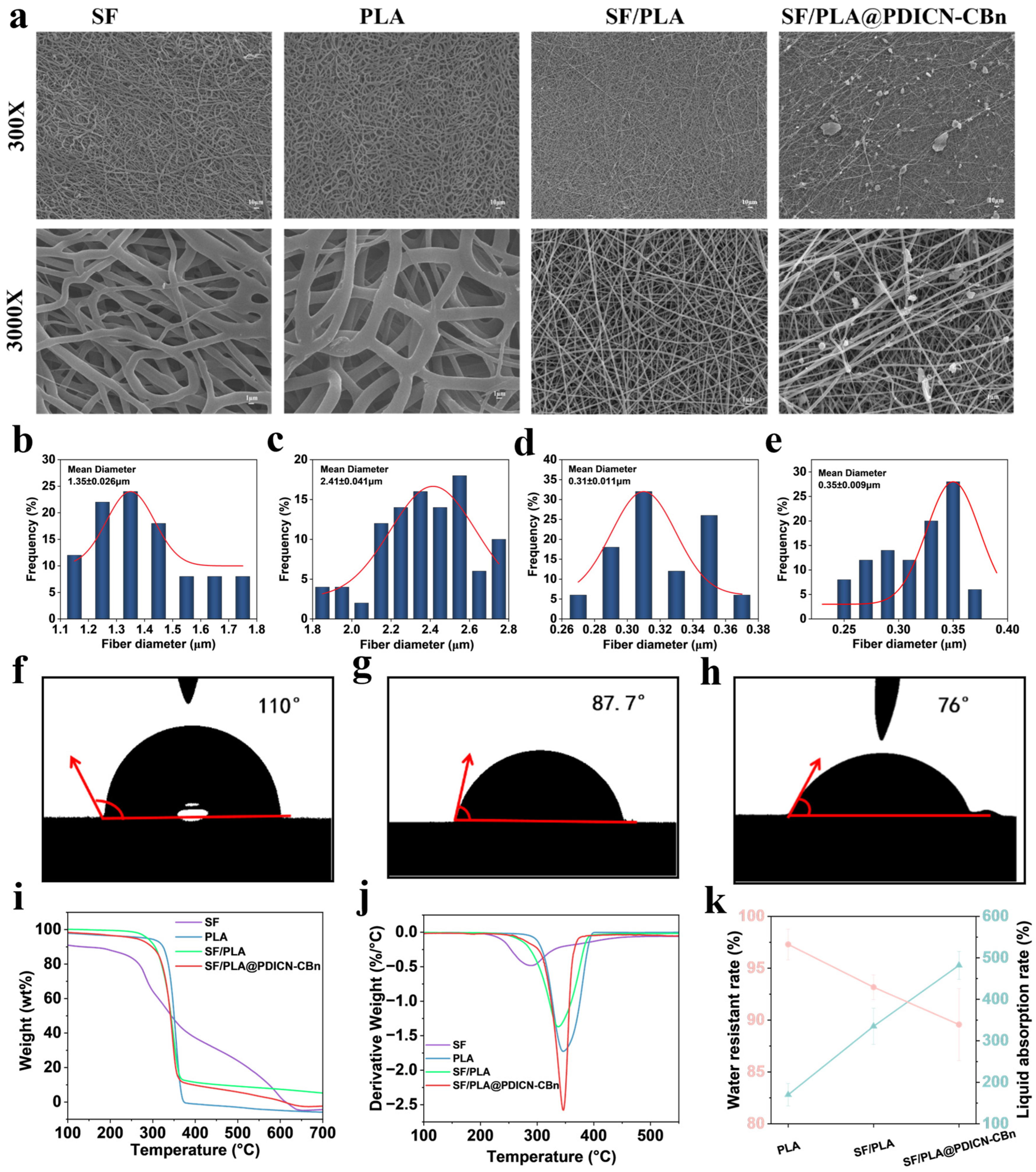
2.2. Surface Morphology and Physical Tests of SF/PLA@PDICN-CBn
2.3. Optical Properties and ROS Generation of PDICN-CBn
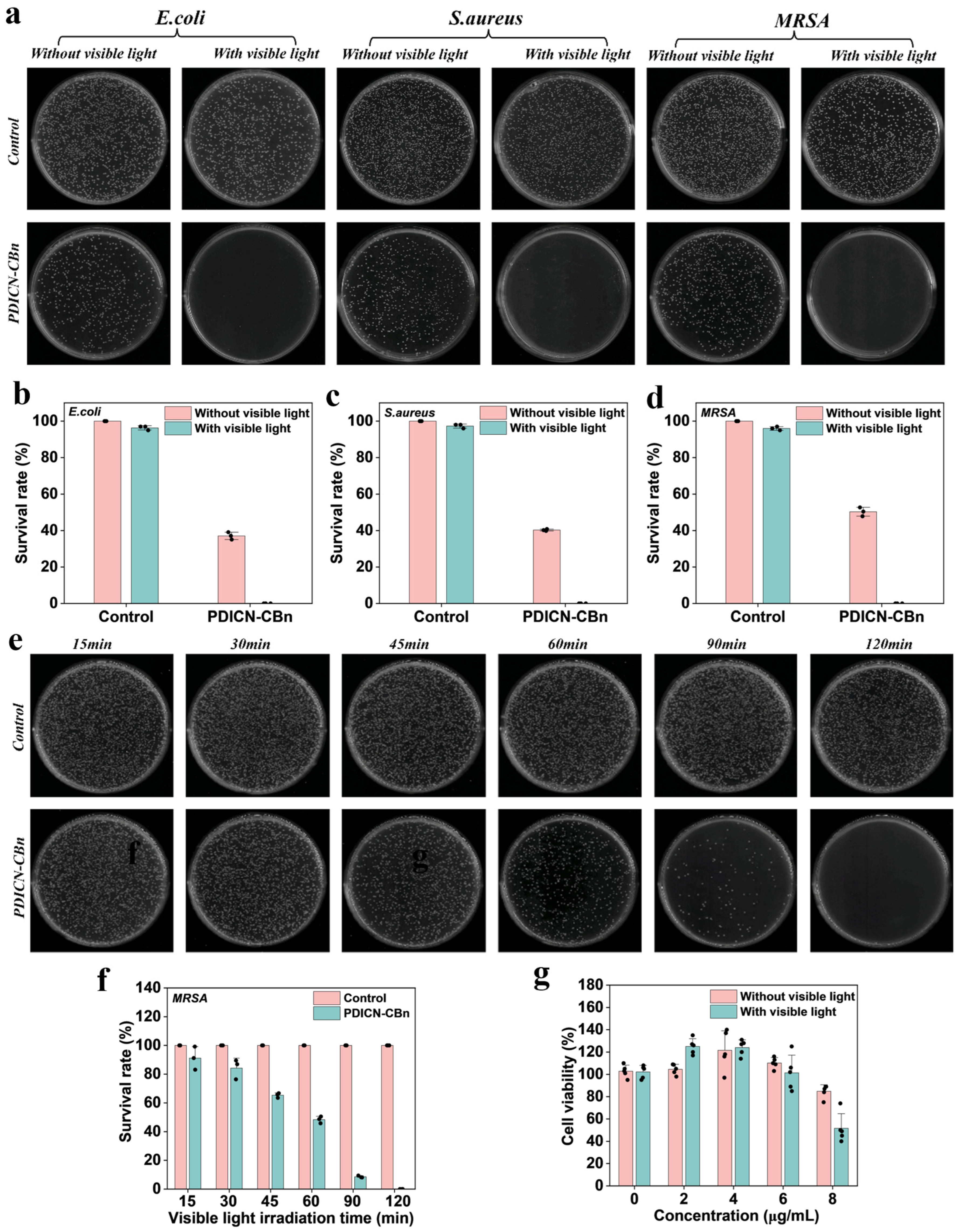
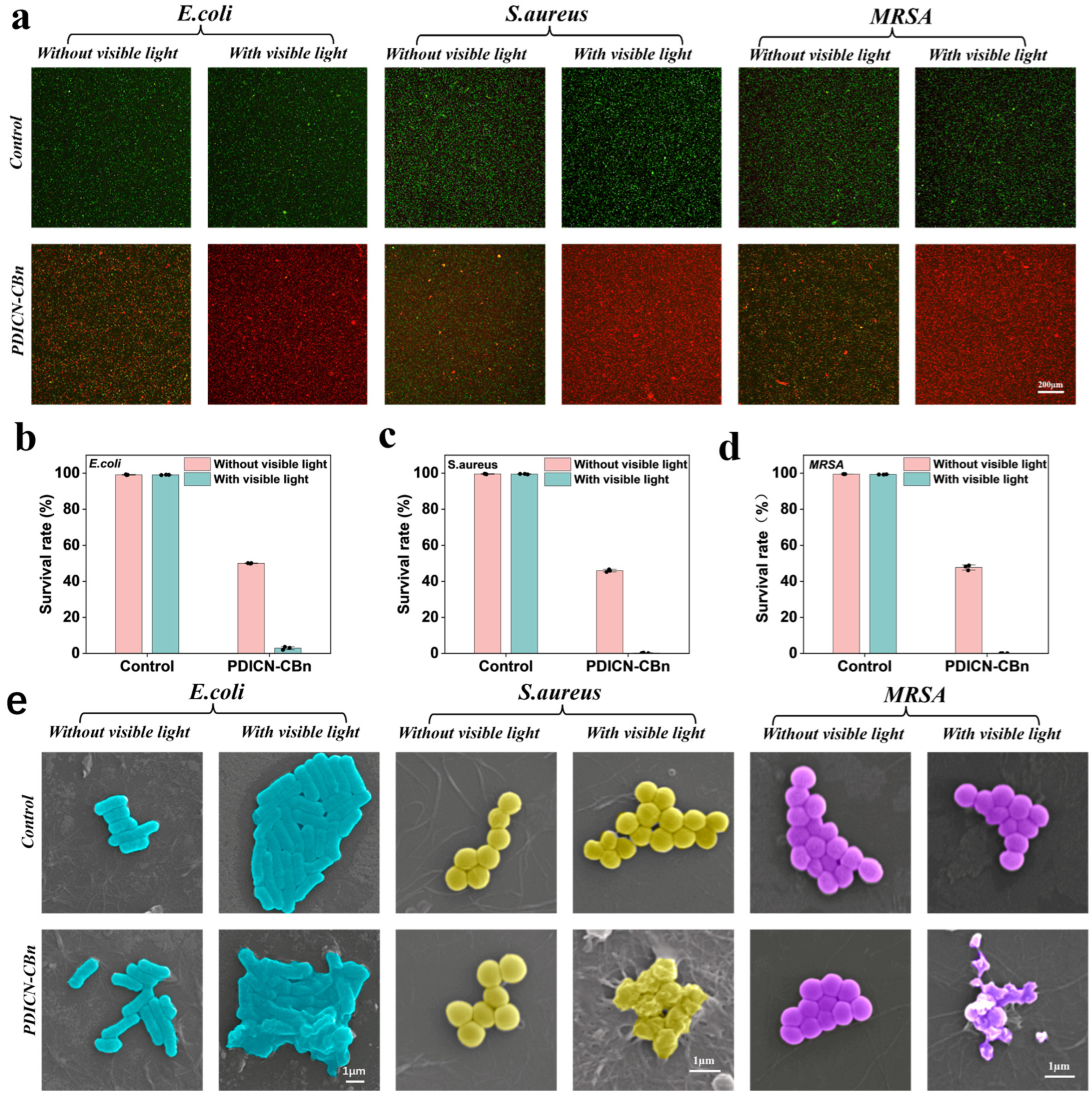
2.4. Cytotoxicity and Antibacterial Performance of PDICN-CBn
2.5. Recyclability Test of SF/PLA@PDICN-CBn
3. Materials and Methods
3.1. Synthesis of PDICN-CBn
3.2. Synthesis of SF/PLA @PDICN-CBn
3.3. Surface Morphology and Chemical Characterization of SF/PLA @PDICN-CBn
3.4. Optical Properties and ROS Generation Tests of PDICN-CBn
3.5. Cytotoxicity of PDICN-CBn
3.6. Antibacterial Performance of PDICN-CBn
3.7. Recyclability Test of SF/PLA@PDICN-CBn
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baran, A.; Kwiatkowska, A.; Potocki, L. Antibiotics and Bacterial Resistance—A Short Story of an Endless Arms Race. Int. J. Mol. Sci. 2023, 24, 5777. [Google Scholar] [CrossRef]
- He, L.; Di, D.; Chu, X.; Liu, X.; Wang, Z.; Lu, J.; Wang, S.; Zhao, Q. Photothermal Antibacterial Materials to Promote Wound Healing. J. Control. Release 2023, 363, 180–200. [Google Scholar] [CrossRef]
- Tarin-Pello, A.; Suay-Garcia, B.; Perez-Gracia, M.-T. Antibiotic Resistant Bacteria: Current Situation and Treatment Options to Accelerate the Development of a New Antimicrobial Arsenal. Expert Rev. Anti. Infect. Ther. 2022, 20, 1095–1108. [Google Scholar]
- Hu, X.; Huang, Y.-Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef]
- Thomas-Moore, B.A.; Del Valle, C.A.; Field, R.A.; Marín, M.J. Recent Advances in Nanoparticle-Based Targeting Tactics for Antibacterial Photodynamic Therapy. Photochem. Photobiol. Sci. 2022, 21, 1111–1131. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Wang, Q. Positive Effects of Low-dose Photodynamic Therapy with Aminolevulinic Acid or Its Methyl Ester in Skin Rejuvenation and Wound Healing: An Update. J. Biophotonics 2023, 16, e202200293. [Google Scholar]
- Oyama, J.; Ramos-Milaré, Á.C.F.H.; Lera-Nonose, D.S.S.L.; Nesi-Reis, V.; Demarchi, I.G.; Aristides, S.M.A.; Teixeira, J.J.V.; Silveira, T.G.V.; Lonardoni, M.V.C. Photodynamic Therapy in Wound Healing In Vivo: A Systematic Review. Photodiagnosis Photodyn. Ther. 2020, 30, 101682. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-N.; Zhao, Z.; Tang, B.Z.; Yoon, J. Organic Photosensitizers for Antimicrobial Phototherapy. Chem. Soc. Rev. 2022, 51, 3324–3340. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Yao, Y.; Feng, X.; Tian, Y. Recent Advances in Developing Bioorthogonally Activatable Photosensitizers for Photodynamic Therapy. Eur. J. Med. Chem. 2025, 291, 117672. [Google Scholar] [CrossRef]
- Aires-Fernandes, M.; Botelho Costa, R.; Rochetti do Amaral, S.; Mussagy, C.U.; Santos-Ebinuma, V.C.; Primo, F.L. Development of Biotechnological Photosensitizers for Photodynamic Therapy: Cancer Research and Treatment—From Benchtop to Clinical Practice. Molecules 2022, 27, 6848. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Hung, C.H.; Chan, K.H.; Kong, W.P. A Water-Soluble Aggregation-Induced Emission Photosensitizer with Intrinsic Antibacterial Activity as an Antiplanktonic and Antibiofilm Therapeutic Agent. J. Med. Chem. 2025, 68, 8768–8785. [Google Scholar]
- Park, J.; Lee, Y.-K.; Park, I.-K.; Hwang, S.R. Current Limitations and Recent Progress in Nanomedicine for Clinically Available Photodynamic Therapy. Biomedicines 2021, 9, 85. [Google Scholar] [CrossRef]
- Oskolkova, T.O.; Matiushkina, A.A.; Borodina, L.; Smirnova, E.S.; Dadadzhanova, A.I.; Sewid, F.A.; Veniaminov, A.V.; Moiseeva, E.O.; Orlova, A.O. FRET-Amplified Singlet Oxygen Generation by Nanocomposites Comprising Ternary AgInS2/ZnS Quantum Dots and Molecular Photosensitizers. ChemNanoMat 2024, 10, e202300469. [Google Scholar]
- Zhou, W.; Zhang, K.; Liu, N.; Li, Y.; Han, W.; Zhou, W.; Li, M.; Zhang, S.; Huang, H.; Yu, C. A Perylene Diimide Probe for NIR-II Fluorescence Imaging Guided Photothermal and Type I/Type II Photodynamic Synergistic Therapy. Biosens. Bioelectron. 2024, 259, 116424. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, F.; Lu, Y.; Hu, J.; Feng, J.; Shang, H.; Sun, B.; Jiang, W. Molecular Design of Perylene Diimide Derivatives for Photocatalysis. ACS Catal. 2025, 15, 1829–1840. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, N.; Wang, Y.; Ling, G.; Zhang, P. Perylene Diimide-Based Treatment and Diagnosis of Diseases. J. Mater. Chem. B 2021, 9, 8937–8950. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, B.; Liu, G.; Xu, C.; Ji, Q.; Xiang, W.; Sun, D.; Zhong, Q.; He, H.; Yazi, L. Perylene Diimide Supermolecule (PDI) as a Novel and Highly Efficient Cocatalyst for Photocatalytic Degradation of Tetracycline in Water: A Case Study of PDI Decorated Graphitic Carbon Nitride/Bismuth Tungstate Composite. J. Colloid Interface Sci. 2022, 615, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhang, J.; Song, Y.; Liu, J.; Xu, H.-J. Two Asymmetrical Perylene Diimide Derivatives: Synthesis, Optical-Electrochemical Properties and Morphologies of Self-Assembly. J. Solid State Chem. 2022, 305, 122665. [Google Scholar] [CrossRef]
- Yang, F.; Li, R.; Wei, W.; Ding, X.; Xu, Z.; Wang, P.; Wang, G.; Xu, Y.; Fu, H.; Zhao, Y. Water-Soluble Doubly-Strapped Isolated Perylene Diimide Chromophore. Angew. Chemie Int. Ed. 2022, 61, e202202491. [Google Scholar]
- Sun, M.; Müllen, K.; Yin, M. Water-Soluble Perylenediimides: Design Concepts and Biological Applications. Chem. Soc. Rev. 2016, 45, 1513–1528. [Google Scholar] [CrossRef]
- Özçil, F.; Yükrük, F. Evaluation of Singlet Oxygen Generators of Novel Water-Soluble Perylene Diimide Photosensitizers. RSC Adv. 2023, 13, 15416–15420. [Google Scholar] [CrossRef]
- Li, M.; Wen, H.; Li, H.; Yan, Z.-C.; Li, Y.; Wang, L.; Wang, D.; Tang, B.Z. AIEgen-Loaded Nanofibrous Membrane as Photodynamic/Photothermal Antimicrobial Surface for Sunlight-Triggered Bioprotection. Biomaterials 2021, 276, 121007. [Google Scholar]
- Wang, S.; Xu, X.; Zhu, X.; Tan, X.; Xie, B. Electrospun Carvacrol-Loaded Polyacrylonitrile/Poly (Ethylene Oxide) Nanofibrous Films as Wound Dressings. ACS Omega 2024, 9, 39472–39483. [Google Scholar]
- Ilyas, R.A.; Zuhri, M.Y.M.; Aisyah, H.A.; Asyraf, M.R.M.; Hassan, S.A.; Zainudin, E.S.; Sapuan, S.M.; Sharma, S.; Bangar, S.P.; Jumaidin, R. Natural Fiber-Reinforced Polylactic Acid, Polylactic Acid Blends and Their Composites for Advanced Applications. Polymers 2022, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chen, Z.; Murillo, L.L.; Tang, D.; Meng, C.; Zhong, X.; Wang, T.; Li, J. Hierarchical Porous Silk Fibroin/Poly (L-Lactic Acid) Fibrous Membranes towards Vascular Scaffolds. Int. J. Biol. Macromol. 2021, 166, 1111–1120. [Google Scholar] [CrossRef]
- Pesaranhajiabbas, E.; Misra, M.; Mohanty, A.K. Recent Progress on Biodegradable Polylactic Acid Based Blends and Their Biocomposites: A Comprehensive Review. Int. J. Biol. Macromol. 2023, 253, 126231. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Z.; Jin, Y.; Huang, Y.; Zhou, S.; Tian, H.; Wu, H. Electrospun High Hydrophilicity Antimicrobial Poly (Lactic Acid)/Silk Fibroin Nanofiber Membrane for Wound Dressings. Int. J. Biol. Macromol. 2024, 277, 133905. [Google Scholar] [CrossRef]
- Rabiej, M. Application of Immune and Genetic Algorithms to the Identification of a Polymer Based on Its X-Ray Diffraction Curve. J. Appl. Crystallogr. 2013, 46, 1136–1144. [Google Scholar] [CrossRef]
- Tan, X.; Jiang, Y.; Puchalski, M.; Peng, Q.; Hu, S.; Xiong, W.; Saskova, J.; Wiener, J.; Venkataraman, M.; Militky, J. The Multifunctional Flexible Conductive Viscose Fabric Prepared by Thiol Modification Followed by Copper Plating. Cellulose 2024, 31, 3169–3184. [Google Scholar] [CrossRef]


| Sample | C 1s Group Content (at%) | N 1s Group Content (at%) | O 1s Group Content (at%) | |||||
|---|---|---|---|---|---|---|---|---|
| C-C/C-H (284.8 eV) | C-O/C-N (286 eV) | C=O (288 eV) | O=C-N (399 eV) | N-H (400 eV) | N+ (402 eV) | C=O (531 eV) | C-O (533 eV) | |
| SF/PLA | 45.87 | 27.26 | 26.87 | 61.82 | 38.18 | / | 48.74 | 51.26 |
| PDICN-CBn | 73.88 | 16.50 | 9.63 | / | 58.24 | 41.76 | 100 | / |
| SF/PLA@PDICN-CBn | 44.25 | 27.11 | 28.64 | 69.01 | 30.99 | / | 52.14 | 47.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Lin, H.; Lin, Y.; Peng, Q.; Song, Y.; Tan, X.; Yang, X.; Yi, S. Development and Application of Visible-Light-Responsive Perylene Diimide Functionalized Silk Fibroin/Polylactic Acid Antibacterial Nanofibrous Membranes. Int. J. Mol. Sci. 2025, 26, 11533. https://doi.org/10.3390/ijms262311533
Lv S, Lin H, Lin Y, Peng Q, Song Y, Tan X, Yang X, Yi S. Development and Application of Visible-Light-Responsive Perylene Diimide Functionalized Silk Fibroin/Polylactic Acid Antibacterial Nanofibrous Membranes. International Journal of Molecular Sciences. 2025; 26(23):11533. https://doi.org/10.3390/ijms262311533
Chicago/Turabian StyleLv, Sheng, Hongyu Lin, Ying Lin, Qingyan Peng, Yuyang Song, Xiaodong Tan, Xiao Yang, and Shixiong Yi. 2025. "Development and Application of Visible-Light-Responsive Perylene Diimide Functionalized Silk Fibroin/Polylactic Acid Antibacterial Nanofibrous Membranes" International Journal of Molecular Sciences 26, no. 23: 11533. https://doi.org/10.3390/ijms262311533
APA StyleLv, S., Lin, H., Lin, Y., Peng, Q., Song, Y., Tan, X., Yang, X., & Yi, S. (2025). Development and Application of Visible-Light-Responsive Perylene Diimide Functionalized Silk Fibroin/Polylactic Acid Antibacterial Nanofibrous Membranes. International Journal of Molecular Sciences, 26(23), 11533. https://doi.org/10.3390/ijms262311533





