Analysis of Normalizers and Neurodevelopment-Related microRNAs in the Prefrontal Cortex and in the Sperm of SHR Rats
Abstract
1. Introduction
2. Results
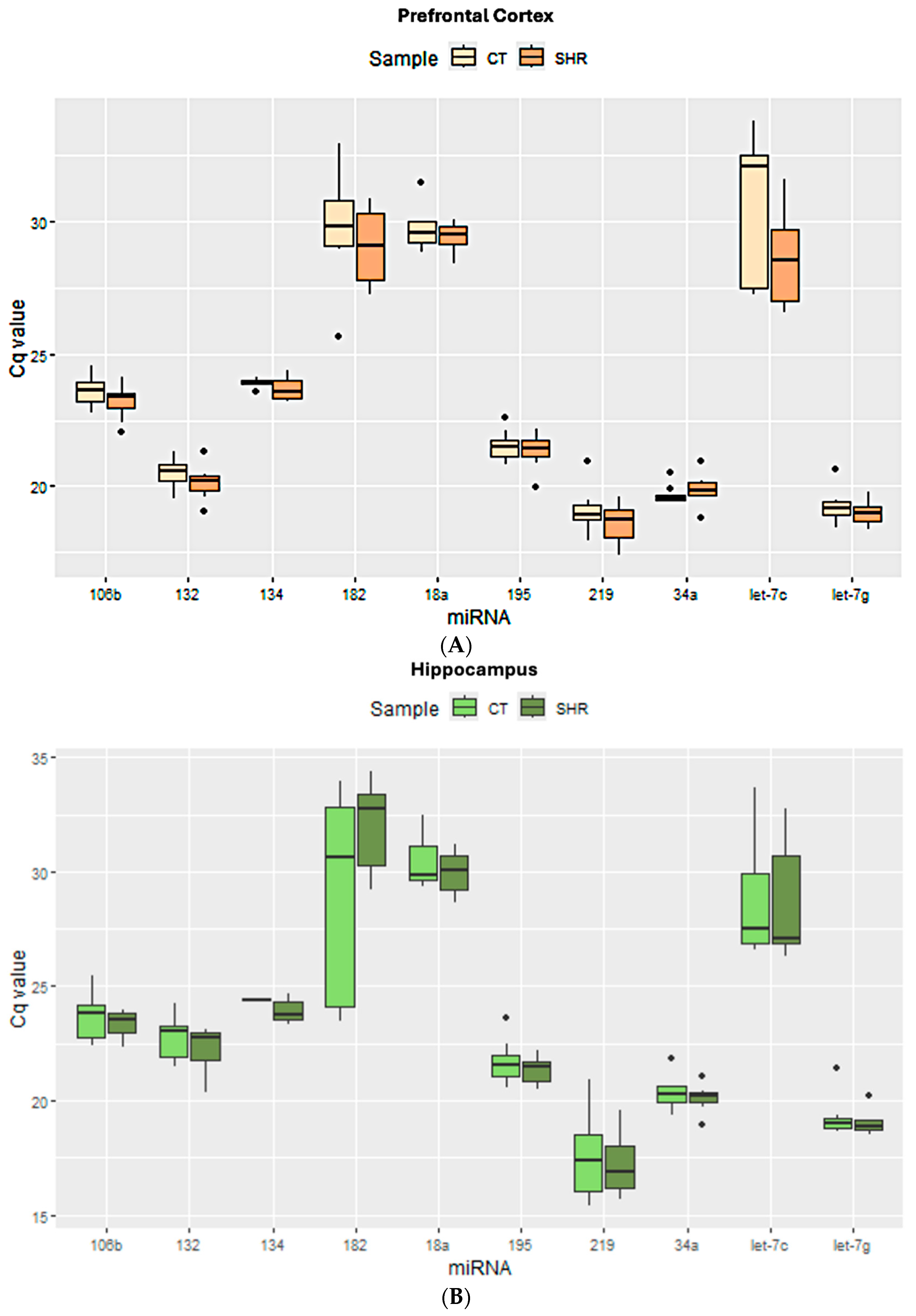
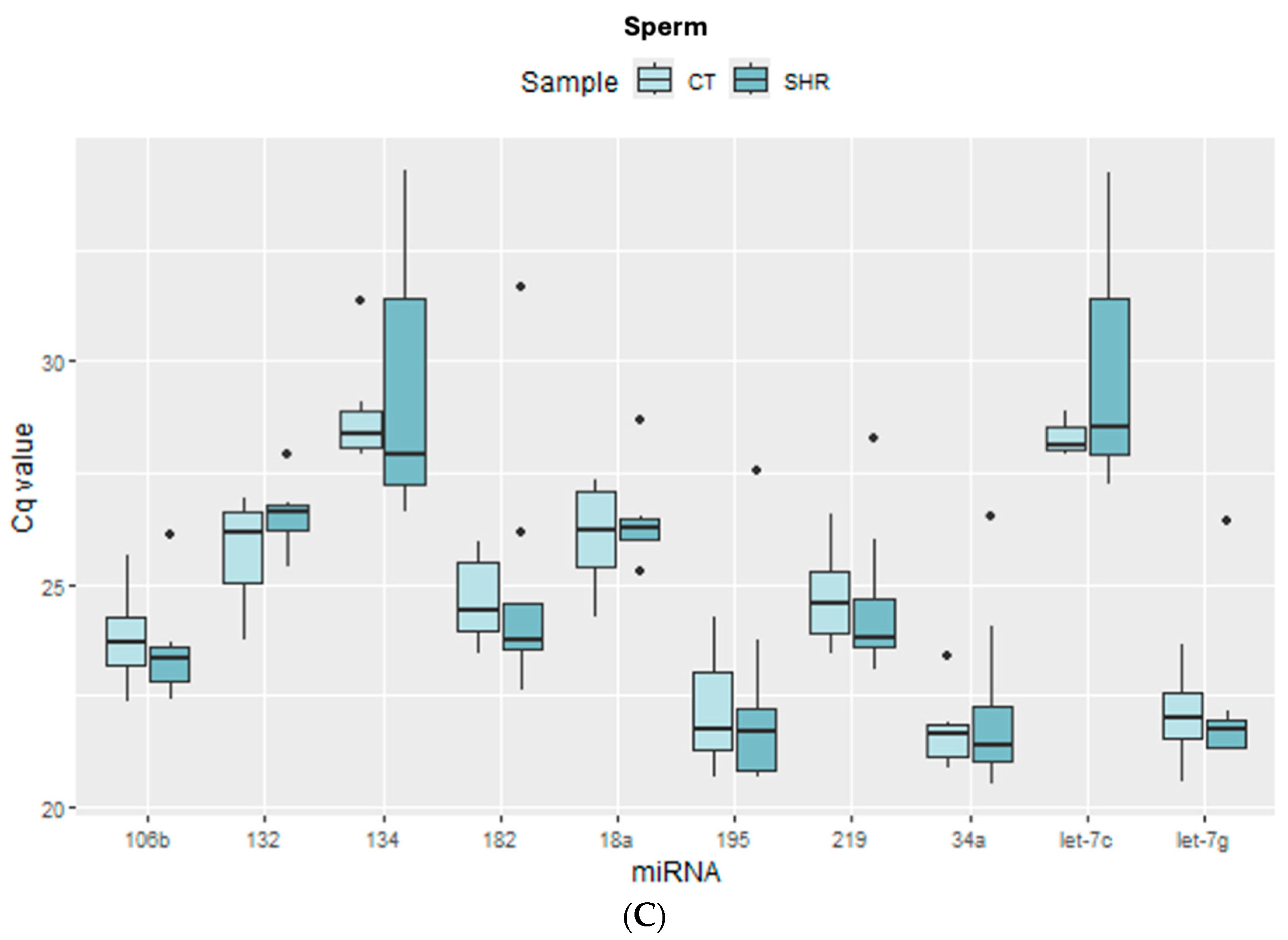
2.1. Expression of the Candidate Reference Genes in the Brain and Sperm Cells
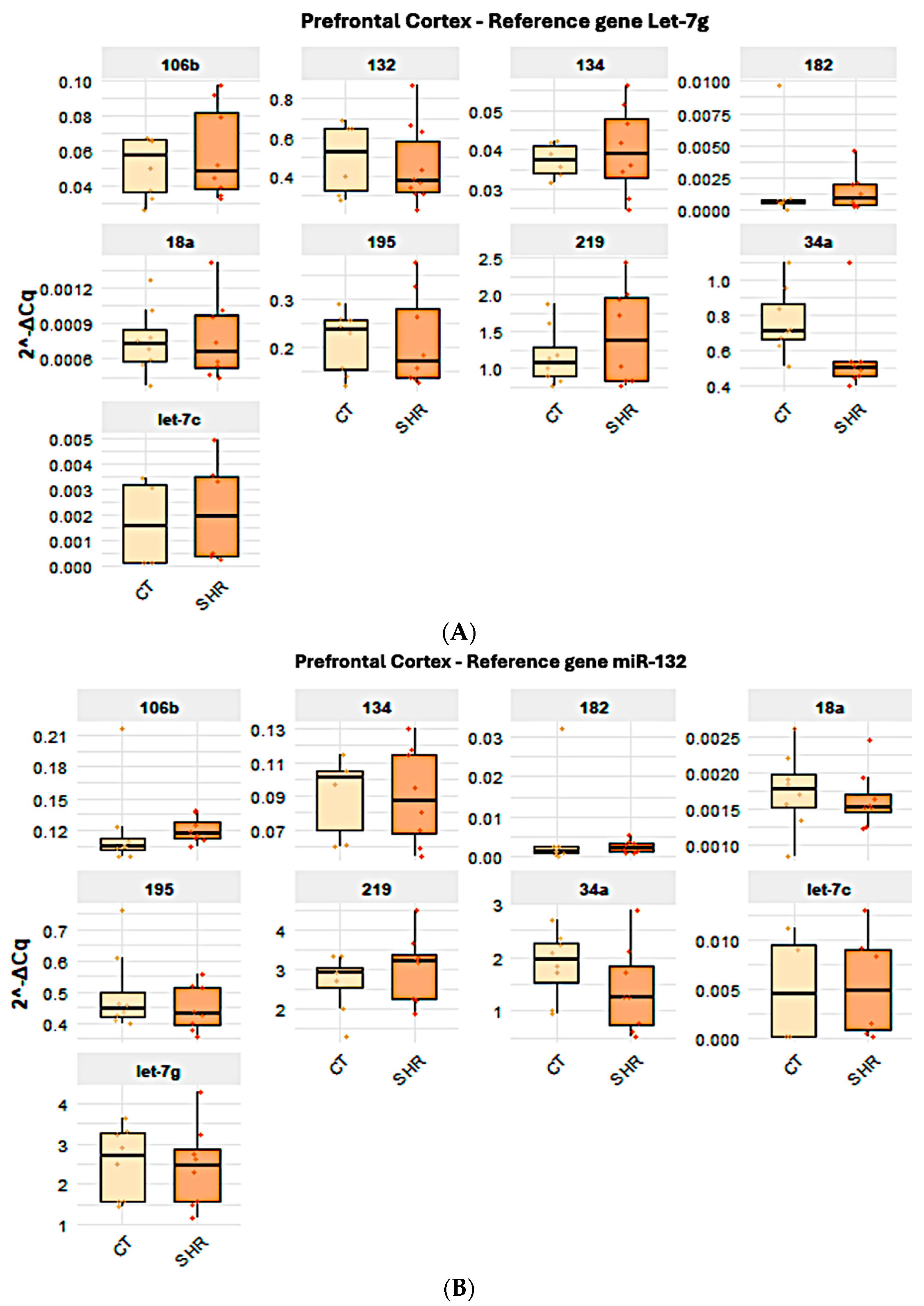
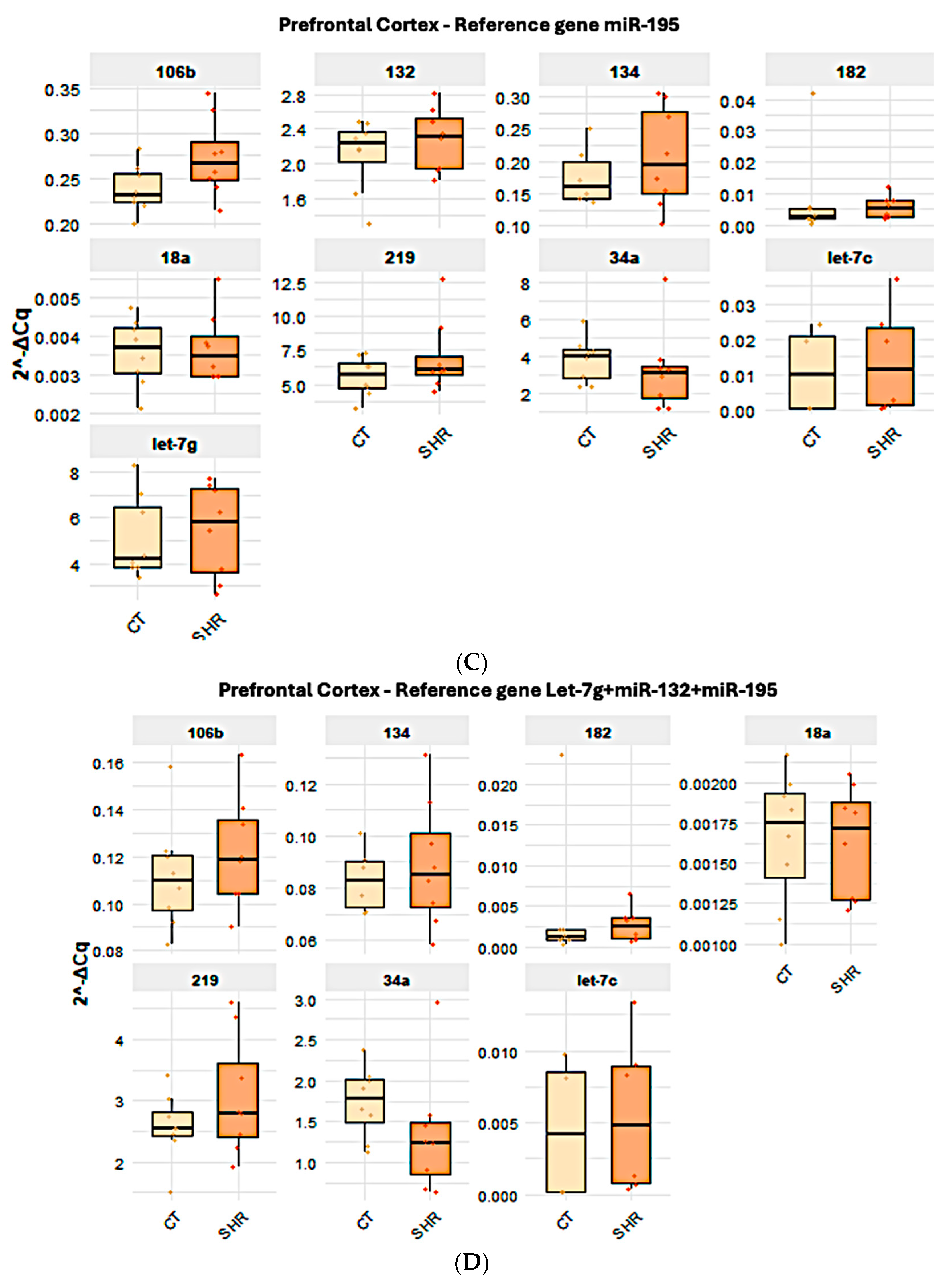
2.2. Reference Gene Analysis
2.3. miRNA Expression in Sperm, Prefrontal Cortex, and Hippocampus in Control and SHR Rats
2.4. Effect of the Reference Gene Choice in the Analysis of the Expression of Target miRNA in Control and SHR Rats
3. Discussion
4. Materials and Methods
4.1. Animals and Sample Collection
4.2. MicroRNA Extraction, cDNA Synthesis, and qPCR
4.3. Reference miRNA Analysis for qPCR Normalization
4.4. RT-qPCR
4.5. Analysis of Predicted Target mRNAs
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCZ | Schizophrenia |
| SHR | Spontaneously hypertensive rats |
| miRNA | microRNA |
| RT-qPCR | Reverse transcription—quantitative polymerase chain reaction |
| LINE-1 | Long Interspersed Nuclear Element-1 |
| sncRNA | Short non-coding RNA |
| piRNA | PIWI-interacting RNA |
| siRNA | Small interfering RNA |
| Cq | Cycle quantification |
References
- Kneeland, R.E.; Fatemi, S.H. Viral infection, inflammation and schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 42, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Fineberg, A.M.; Ellman, L.M.; Schaefer, C.A.; Maxwell, S.D.; Shen, L.; Chaudhury, N.H.; Cook, A.L.; Bresnahan, M.A.; Susser, E.S.; Brown, A.S. Fetal exposure to maternal stress and risk for schizophrenia spectrum disorders among offspring: Differential influences of fetal sex. Psychiatry Res. 2016, 236, 91–97. [Google Scholar] [CrossRef]
- Torrey, E.F.; Yolken, R.H. Schizophrenia and infections: The eyes have it. Schizophr. Bull. 2017, 43, 247–252. [Google Scholar] [CrossRef]
- Cruz-Rizzolo, R.J.; Limieri, L.L.; de Paiva, I.R.; Ribeiro, J.O.B.; Pimenta, T.F.; Pinato, L.; Ervolino, E.; Casatti, C.A.; Campos, L.M.G.; Liberti, E.A. Protein malnutrition during gestation and early life decreases neuronal size in the medial prefrontal cortex of post-pubertal rats. IBRO Rep. 2017, 3, 65–71. [Google Scholar] [CrossRef]
- He, P.; Chen, G.; Guo, C.; Wen, X.; Song, X.; Zheng, X. Long-term effect of prenatal exposure to malnutrition on risk of schizophrenia in adulthood: Evidence from the Chinese famine of 1959–1961. Eur. Psychiatry 2018, 51, 42–47. [Google Scholar] [CrossRef]
- Bailey, T.; Alvarez-Jimenez, M.; Garcia-Sanchez, A.M.; Hulbert, C.; Barlow, E.; Bendall, S. Childhood trauma is associated with severity of hallucinations and delusions in psychotic disorders: A systematic review and meta-analysis. Schizophr. Bull. 2018, 44, 1111–1122. [Google Scholar] [CrossRef]
- Asmal, L.; Kilian, S.; du Plessis, S.; Scheffler, F.; Chiliza, B.; Fouche, J.-P.; Seedat, S.; Dazzan, P.; Emsley, R. Childhood trauma associated white matter abnormalities in first-episode schizophrenia. Schizophr. Bull. 2019, 45, 369–376. [Google Scholar] [CrossRef]
- Guffanti, G.; Gaudi, S.; Klengel, T.; Fallon, J.H.; Mangalam, H.; Madduri, R.; Rodriguez, A.; DeCrescenzo, P.; Glovienka, E.; Sobell, J.; et al. LINE1 insertions as a genomic risk factor for schizophrenia: Preliminary evidence from an affected family. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2016, 171, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Doyle, G.A.; Crist, R.C.; Karatas, E.T.; Hammond, M.J.; Ewing, A.D.; Ferraro, T.N.; Hahn, C.-G.; Berrettini, W.H. Analysis of LINE-1 elements in DNA from postmortem brains of individuals with schizophrenia. Neuropsychopharmacology 2017, 42, 2602–2611. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.V.; Chan, S.W.; Jacobsen, S.E.; Looney, D.J. Small interfering RNA-induced transcriptional gene silencing in human cells. Science 2004, 305, 1289–1292. [Google Scholar] [CrossRef]
- Issler, O.; Chen, A. Determining the role of microRNAs in psychiatric disorders. Nat. Rev. Neurosci. 2015, 16, 201–212. [Google Scholar] [CrossRef]
- Fineberg, S.K.; Kosik, K.S.; Davidson, B.L. MicroRNAs potentiate neural development. Neuron 2009, 64, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, N.J.; Cairns, M.J. MicroRNA dysregulation in schizophrenia. Neurobiol. Dis. 2012, 46, 263–271. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Yang, J.; Huang, Y. MicroRNAs as novel biomarkers of schizophrenia (Review). Exp. Ther. Med. 2014, 8, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- del Río, C.; Oliveras, I.; Cañete, T.; Blázquez, G.; Tobeña, A.; Fernández-Teruel, A. Genetic rat models of schizophrenia-relevant symptoms. World J. Neurosci. 2014, 4, 261–278. [Google Scholar] [CrossRef]
- Peres, F.F.; Eufrásio, R.Á.; Gouvêa, D.A.; Diana, M.C.; Santos, C.M.; Swardfager, W.; Abílio, V.C.; Cogo-Moreira, H. A schizophrenia-like behavioral trait in the SHR model: Applying confirmatory factor analysis as a new statistical tool. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 85, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Calzavara, M.B.; Medrano, W.A.; Levin, R.; Suiama, M.A.; Abílio, V.C.; Frussa-Filho, R. Neuroleptic drugs revert the contextual fear conditioning deficit presented by spontaneously hypertensive rats: A potential animal model of emotional context processing in schizophrenia? Schizophr. Bull. 2009, 35, 748–759. [Google Scholar] [CrossRef]
- Calzavara, M.B.; Medrano, W.A.; Levin, R.; Suiama, M.A.; Abílio, V.C.; Frussa-Filho, R. Effects of antipsychotics and amphetamine on social behaviors in spontaneously hypertensive rats. Behav. Brain Res. 2011, 225, 15–22. [Google Scholar] [CrossRef]
- Levin, R.; Calzavara, M.B.; Santos, C.M.; Medrano, W.A.; Niigaki, S.T.; Abílio, V.C. Spontaneously hypertensive rats (SHR) present deficits in prepulse inhibition of startle specifically reverted by clozapine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1748–1752. [Google Scholar] [CrossRef]
- Levin, R.; Peres, F.F.; Almeida, V.; Calzavara, M.B.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.S.; Abílio, V.C. Effects of cannabinoid drugs on the deficit of prepulse inhibition of startle in an animal model of schizophrenia: The SHR strain. Front. Pharmacol. 2014, 5, 10. [Google Scholar] [CrossRef]
- Ramos, A.C.; de Mattos Hungria, F.; Camerini, B.A.; Suiama, M.A.; Calzavara, M.B. Potential beneficial effects of caffeine administration in the neonatal period of an animal model of schizophrenia. Behav. Brain Res. 2020, 388, 112674. [Google Scholar] [CrossRef]
- Tendilla-Beltrán, H.; Garcés-Ramírez, L.; Martínez-Vásquez, E.; Nakakawa, A.; Gómez-Villalobos, M.d.J.; Flores, G. Differential effects of neonatal ventral hippocampus lesion on behavior and corticolimbic plasticity in Wistar-Kyoto and spontaneously hypertensive rats. Neurochem. Res. 2024, 49, 959–979. [Google Scholar] [CrossRef] [PubMed]
- Nani, J.V.; Ushirohira, J.M.; Bradshaw, N.J.; Machado-De-Sousa, J.P.; Hallak, J.E.C.; Hayashi, M.A.F. Sodium nitroprusside as an adjunctive treatment for schizophrenia reduces Ndel1 oligopeptidase activity. Braz. J. Psychiatry 2024, 46, e20233315. [Google Scholar] [CrossRef] [PubMed]
- Niigaki, S.T.; Peres, F.F.; Ferreira, L.; Libanio, T.; Gouvea, D.A.; Levin, R.; Almeida, V.; Silva, N.D.; Diana, M.C.; Suiama, M.A.; et al. Young spontaneously hypertensive rats (SHRs) display prodromal schizophrenia-like behavioral abnormalities. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 90, 169–176. [Google Scholar] [CrossRef]
- Peres, F.F.; Levin, R.; Almeida, V.; Zuardi, A.W.; Hallak, J.E.; Crippa, J.A.; Abilio, V.C. Cannabidiol, among Other Cannabinoid Drugs, Modulates Prepulse Inhibition of Startle in the SHR Animal Model: Implications for Schizophrenia Pharmacotherapy. Front. Pharmacol. 2016, 7, 303. [Google Scholar] [CrossRef]
- Kendler, K.S. Overview: A current perspective on twin studies of schizophrenia. Am. J. Psychiatry 1983, 140, 1413–1425. [Google Scholar] [CrossRef]
- Lichtenstein, P.; Yip, B.H.; Björk, C.; Pawitan, Y.; Cannon, T.D.; Sullivan, P.F.; Hultman, C.M. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: A population-based study. Lancet 2009, 373, 234–239. [Google Scholar] [CrossRef]
- Varela, R.B.; Cararo, J.H.; Tye, S.J.; Carvalho, A.F.; Valvassori, S.S.; Fries, G.R.; Quevedo, J. Contributions of epigenetic inheritance to the predisposition of major psychiatric disorders: Theoretical framework, evidence, and implications. Neurosci. Biobehav. Rev. 2022, 140, 1049. [Google Scholar] [CrossRef] [PubMed]
- Monaco, A.P. An epigenetic, transgenerational model of increased mental health disorders in children, adolescents and young adults. Eur. J. Hum. Genet. 2021, 29, 387–395. [Google Scholar] [CrossRef]
- Franklin, T.B.; Russig, H.; Weiss, I.C.; Gräff, J.; Linder, N.; Michalon, A.; Vizi, S.; Mansuy, I.M. Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry 2010, 68, 408–415. [Google Scholar] [CrossRef]
- Gapp, K.; Jawaid, A.; Sarkies, P.; Bohacek, J.; Pelczar, P.; Prados, J.; Farinelli, L.; Miska, E.; Mansuy, I.M. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014, 17, 667–669. [Google Scholar] [CrossRef]
- Lehrner, A.; Yehuda, R. Cultural trauma and epigenetic inheritance. Dev. Psychopathol. 2018, 30, 1763–1777. [Google Scholar] [CrossRef]
- Bale, T.L. Lifetime stress experience: Transgenerational epigenetics and germ cell programming. Dialogues Clin. Neurosci. 2014, 16, 297–305. [Google Scholar] [CrossRef]
- Bohacek, J.; Farinelli, M.; Mirante, O.; Steiner, G.; Gapp, K.; Coiret, G.; Ebeling, M.; Durán-Pacheco, G.; Iniguez, A.L.; Manuella, F.; et al. Pathological brain plasticity and cognition in the offspring of males subjected to postnatal traumatic stress. Mol. Psychiatry 2015, 20, 621–631. [Google Scholar] [CrossRef]
- Rando, O.J. Intergenerational Transfer of Epigenetic Information in Sperm. Cold Spring Harb. Perspect. Med. 2016, 6, a022988. [Google Scholar] [CrossRef]
- Stenz, L.; Schechter, D.S.; Serpa, S.R.; Paoloni-Giacobino, A. Intergenerational Transmission of DNA Methylation Signatures Associated with Early Life Stress. Curr. Genom. 2018, 19, 665–675. [Google Scholar] [CrossRef]
- Greeson, K.W.; Crow, K.M.S.; Edenfield, R.C.; Easley, C.A. Inheritance of paternal lifestyles and exposures through sperm DNA methylation. Nat. Rev. Urol. 2023, 20, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A.B.; Morgan, C.P.; Leu, N.A.; Bale, T.L. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl. Acad. Sci. USA 2015, 112, 13699–13704. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S.; Conine, C.C. The Transmission of Intergenerational Epigenetic Information by Sperm microRNAs. Epigenomes 2022, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Mawaribuchi, S.; Aiki, Y.; Ikeda, N.; Ito, Y. mRNA and miRNA expression profiles in an ectoderm-biased substate of human pluripotent stem cells. Sci. Rep. 2019, 9, 11910. [Google Scholar] [CrossRef]
- Matos, B.; Publicover, S.J.; Castro, L.F.C.; Esteves, P.J.; Fardilha, M. Brain and testis: More alike than previously thought? Open Biol. 2023, 13, 230032. [Google Scholar] [CrossRef]
- Ferro, M.H.S.; Morante, I.; Nishino, F.A.; Estevam, C.; Amaral, F.G.; Cipolla-Neto, J.; Stumpp, T. Melatonin influence on miRNA expression in sperm, hypothalamus, prefrontal cortex and cerebellum of Wistar rats. PLoS ONE 2025, 20, e0312403. [Google Scholar] [CrossRef]
- Friocourt, G.; Liu, J.S.; Antypa, M.; Rakić, S.; Walsh, C.A.; Parnavelas, J.G. Both doublecortin and doublecortin-like kinase play a role in cortical interneuron migration. J. Neurosci. 2007, 27, 3875–3883. [Google Scholar] [CrossRef]
- Cohen, D.; Segal, M.; Reiner, O. Doublecortin supports the development of dendritic arbors in primary hippocampal neurons. Dev. Neurosci. 2008, 30, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.H.; Reimers, M.; Maher, B.; Williamson, V.; McMichael, O.; McClay, J.L.; Oord, E.J.v.D.; Riley, B.P.; Kendler, K.S.; Vladimirov, V.I. MicroRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders. Schizophr. Res. 2010, 124, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Mollinari, C.; Racaniello, M.; Berry, A.; Pieri, M.; de Stefano, M.C.; Cardinale, A.; Zona, C.; Cirulli, F.; Garaci, E.; Merlo, D. miR-34a regulates cell proliferation, morphology and function of newborn neurons resulting in improved behavioural outcomes. Cell Death Dis. 2015, 6, e1622. [Google Scholar] [CrossRef]
- Lai, C.-Y.; Yu, S.-L.; Hsieh, M.H.; Chen, C.-H.; Chen, H.-Y.; Wen, C.-C.; Huang, Y.-H.; Hsiao, P.-C.; Hsiao, C.K.; Liu, C.-M.; et al. MicroRNA expression aberration as potential peripheral blood biomarkers for schizophrenia. PLoS ONE 2011, 6, e21635. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, F.; Wang, X.; Shugart, Y.Y.; Zhao, Y.; Li, X.; Liu, Z.; Sun, N.; Yang, C.; Zhang, K.; et al. Diagnostic value of blood-derived microRNAs for schizophrenia: Results of a meta-analysis and validation. Sci. Rep. 2017, 7, 15328. [Google Scholar] [CrossRef]
- Shi, W.; Du, J.; Qi, Y.; Liang, G.; Wang, T.; Li, S.; Xie, S.; Zeshan, B.; Xiao, Z. Aberrant expression of serum miRNAs in schizophrenia. J. Psychiatr. Res. 2012, 46, 198–204. [Google Scholar] [CrossRef]
- Rizos, E.; Siafakas, N.; Katsantoni, E.; Skourti, E.; Salpeas, V.; Rizos, I.; Tsoporis, J.N.; Kastania, A.; Filippopoulou, A.; Xiros, N.; et al. Correction: Let-7, Mir-98 and Mir-181 as Biomarkers for Cancer and Schizophrenia. PLoS ONE 2015, 10, e0135863. [Google Scholar] [CrossRef]
- Guo, W.; Xie, B.; Xiong, S.; Liang, X.; Gui, J.-F.; Mei, J. miR-34a Regulates Sperm Motility in Zebrafish. Int. J. Mol. Sci. 2017, 18, 2676. [Google Scholar] [CrossRef]
- Momeni, A.; Najafipour, R.; Hamta, A.; Jahani, S.; Moghbelinejad, S. Expression and methylation pattern of hsa-miR-34 family in sperm samples of infertile men. Reprod. Sci. 2020, 27, 301–308. [Google Scholar] [CrossRef]
- Akbarian, S. Epigenetic mechanisms in schizophrenia. Dialogues Clin. Neurosci. 2014, 16, 405–417. [Google Scholar] [CrossRef]
- Nishioka, M.; Bundo, M.; Kasai, K.; Iwamoto, K. DNA methylation in schizophrenia: Progress and challenges of epigenetic studies. Genome Med. 2012, 4, 96. [Google Scholar] [CrossRef]
- Roth, T.L.; Lubin, F.D.; Sodhi, M.; Kleinman, J.E. Epigenetic mechanisms in schizophrenia. Biochim. Biophys. Acta 2009, 1790, 869–877. [Google Scholar] [CrossRef]
- Dapunt, J.; Kluge, U.; Heinz, A. Risk of psychosis in refugees: A literature review. Transl. Psychiatry 2017, 7, e1149. [Google Scholar] [CrossRef]
- Campagnoli, G.; Cantão, I.H.; Marins, M.M.; Mendes, A.R.; Nogueira, B.R.; Martin, R.P.; Pesquero, J.B.; Stumpp, T. miRNA profile in hippocampus, prefrontal cortex and sperm of spontaneously hypertensive rats (SHR), a model for schizophrenia. J. Genet. Eng. Biotechnol. Res. 2022, 4, 111–126. [Google Scholar] [CrossRef]
- Martins, H.C.; Schratt, G. MicroRNA-dependent control of neuroplasticity in affective disorders. Transl. Psychiatry 2021, 11, 263. [Google Scholar] [CrossRef]
- Chen, B.Y.; Lin, J.J.; Lu, M.K.; Tan, H.P.; Jang, F.L.; Lin, S.H. Neurodevelopment regulators miR-137 and miR-34 family as biomarkers for early and adult onset schizophrenia. npj Schizophr. 2021, 7, 35. [Google Scholar] [CrossRef]
- Tonk, O.; Tokgun, P.E.; Yılmaz, Ö.S.; Tokgun, O.; Inci, K.; Çelikkaya, B.; Altintas, N. An In Vitro Study for the Role of Schizophrenia-Related Potential miRNAs in the Regulation of COMT Gene. Mol. Neurobiol. 2024, 61, 7680–7690, Erratum in Mol. Neurobiol. 2024, 61, 7691. [Google Scholar] [CrossRef]
- He, K.; Guo, C.; Guo, M.; Tong, S.; Zhang, Q.; Sun, H.; He, L.; Shi, Y. Identification of serum microRNAs as diagnostic biomarkers for schizophrenia. Hereditas 2019, 156, 23. [Google Scholar] [CrossRef]
- Rodrigues, A.L.d.S.; Sant’Anna, C.d.C.; Alcantara, D.D.F.Á.; Cohen-Paes, A.; Imbiriba, M.M.B.G.; Burbano, R.M.R. Evaluation of Circulating MicroRNAs in Schizophrenia: From Epigenomic Dysregulation to Potential Biomarkers. Psychiatry Int. 2024, 5, 1026–1035. [Google Scholar] [CrossRef]
- Camkurt, M.A.; Güneş, S.; Coşkun, S.; Fındıklı, E. Peripheral Signatures of Psychiatric Disorders: MicroRNAs. Clin. Psychopharmacol. Neurosci. 2017, 15, 313–319. [Google Scholar] [CrossRef]
- Camkurt, M.A.; Karababa, F.; Erdal, M.E.; Bayazıt, H.; Kandemir, S.B.; Ay, M.E.; Kandemir, H.; Ay, Ö.I.; Çiçek, E.; Selek, S.; et al. Investigation of Dysregulation of Several MicroRNAs in Peripheral Blood of Schizophrenia Patients. Clin. Psychopharmacol. Neurosci. 2016, 14, 256–260. [Google Scholar] [CrossRef]
- Shboul, M.; Domi, A.B.; Abu Zahra, A.; Khasawneh, A.G.; Darweesh, R. Plasma miRNAs as potential biomarkers for schizophrenia in a Jordanian cohort. Noncoding RNA Res. 2024, 9, 350–358. [Google Scholar] [CrossRef]
- Perkins, D.O.; Jeffries, C.D.; Jarskog, L.F.; Thomson, J.M.; Woods, K.; Newman, M.A.; Parker, J.S.; Jin, J.; Hammond, S.M. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007, 8, R27. [Google Scholar] [CrossRef]
- Miller, B.H.; Zeier, Z.; Xi, L.; Lanz, T.A.; Deng, S.; Strathmann, J.; Willoughby, D.; Kenny, P.J.; Elsworth, J.D.; Lawrence, M.S.; et al. MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc. Natl. Acad. Sci. USA 2012, 109, 3125–3130. [Google Scholar] [CrossRef]
- Santarelli, D.M.; Beveridge, N.J.; Tooney, P.A.; Cairns, M.J. Upregulation of dicer and microRNA expression in the dorsolateral prefrontal cortex Brodmann area 46 in schizophrenia. Biol. Psychiatry 2011, 69, 180–187. [Google Scholar] [CrossRef]
- Gardiner, E.; Beveridge, N.J.; Wu, J.Q.; Carr, V.; Scott, R.J.; Tooney, P.A.; Cairns, M.J. Imprinted DLK1-DIO3 region of 14q32 defines a schizophrenia-associated miRNA signature in peripheral blood mononuclear cells. Mol. Psychiatry 2012, 17, 827–840. [Google Scholar] [CrossRef]
- Wang, Z.; Su, L.; Wu, T.; Sun, L.; Sun, Z.; Wang, Y.; Li, P.; Cui, G. Inhibition of MicroRNA-182/183 Cluster Ameliorates Schizophrenia by Activating the Axon Guidance Pathway and Upregulating DCC. Oxidative Med. Cell. Longev. 2022, 2022, 9411276. [Google Scholar] [CrossRef]
- Li, K.; Zhu, L.; Lv, H.; Bai, Y.; Guo, C.; He, K. The Role of microRNA in Schizophrenia: A Scoping Review. Int. J. Mol. Sci. 2024, 25, 7673. [Google Scholar] [CrossRef]
- Lim, M.; Carollo, A.; Neoh, M.J.Y.; Esposito, G. Mapping miRNA Research in Schizophrenia: A Scientometric Review. Int. J. Mol. Sci. 2023, 24, 436. [Google Scholar] [CrossRef] [PubMed]
- Hass, J.; Walton, E.; Wright, C.; Beyer, A.; Scholz, M.; Turner, J.; Liu, J.; Smolka, M.N.; Roessner, V.; Sponheim, S.R.; et al. Associations between DNA methylation and schizophrenia-related intermediate phenotypes—A gene set enrichment analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 59, 31–39. [Google Scholar] [CrossRef]
- Bradshaw, N.J.; Ukkola-Vuoti, L.; Pankakoski, M.; Zheutlin, A.B.; Ortega-Alonso, A.; Torniainen-Holm, M.; Sinha, V.; Therman, S.; Paunio, T.; Suvisaari, J.; et al. The NDE1 genomic locus can affect treatment of psychiatric illness through gene expression changes related to microRNA-484. Open Biol. 2017, 7, 170153. [Google Scholar] [CrossRef]
- Warnica, W.; Merico, D.; Costain, G.; Alfred, S.E.; Wei, J.; Marshall, C.R.; Scherer, S.W.; Bassett, A.S. Copy number variable microRNAs in schizophrenia and their neurodevelopmental gene targets. Biol. Psychiatry 2015, 77, 158–166. [Google Scholar] [CrossRef]
- Roush, S.; Slack, F.J. The let-7 family of microRNAs. Trends Cell Biol. 2008, 18, 505–516. [Google Scholar] [CrossRef]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Anauate, A.C.; Leal, M.F.; Wisnieski, F.; Santos, L.C.; Gigek, C.O.; Chen, E.S.; Geraldis, J.C.; Calcagno, D.Q.; Assumpção, P.P.; Demachki, S.; et al. Identification of suitable reference genes for miRNA expression normalization in gastric cancer. Gene 2017, 621, 59–68. [Google Scholar] [CrossRef]
- Fung, S.J.; Joshi, D.; Allen, K.M.; Sivagnanasundaram, S.; Rothmond, D.A.; Saunders, R.; Noble, P.L.; Webster, M.J.; Weickert, C.S.; Manzoni, O.J. Developmental patterns of doublecortin expression and white matter neuron density in the postnatal primate prefrontal cortex and schizophrenia. PLoS ONE 2011, 6, e25194. [Google Scholar] [CrossRef]

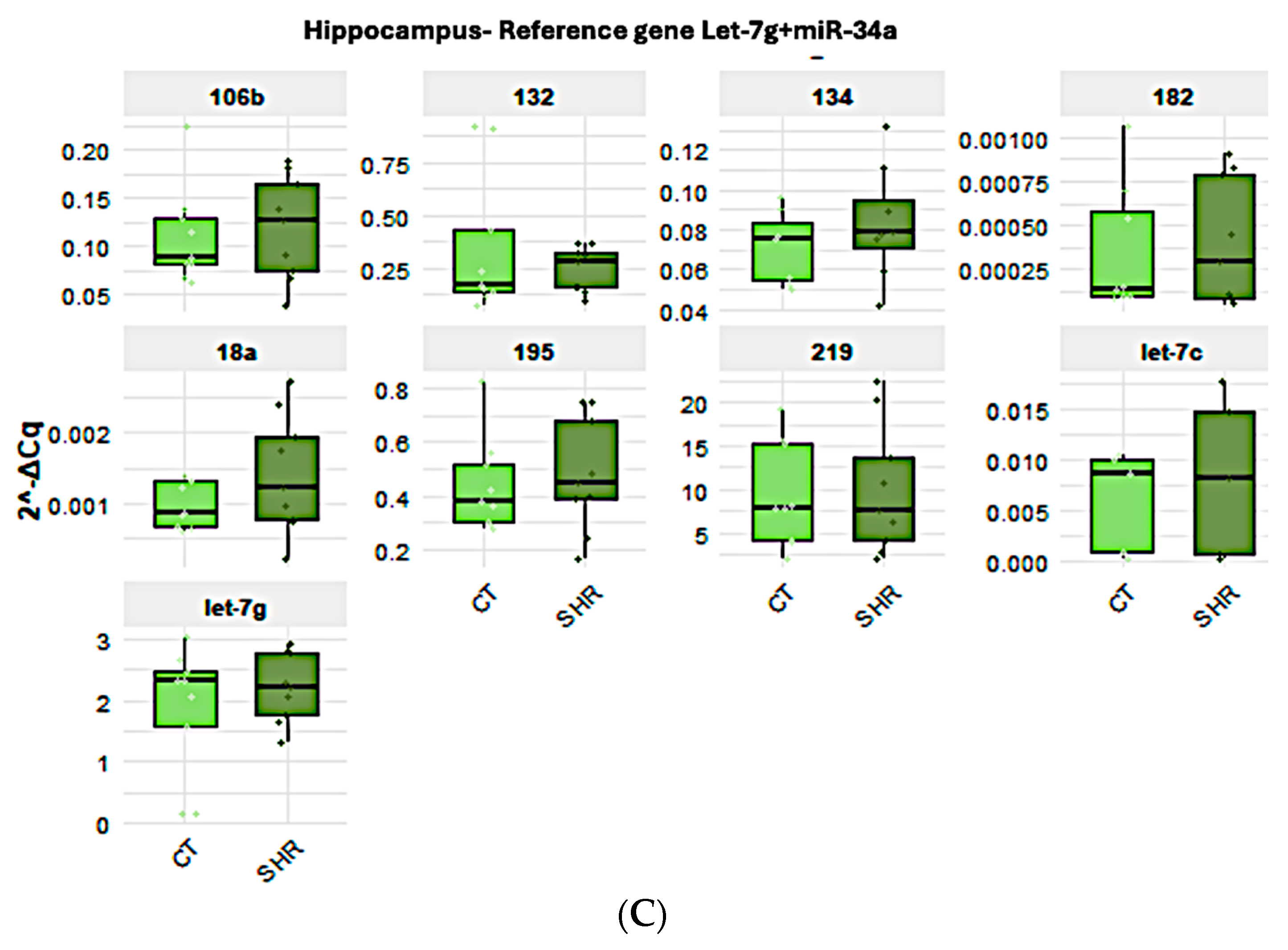

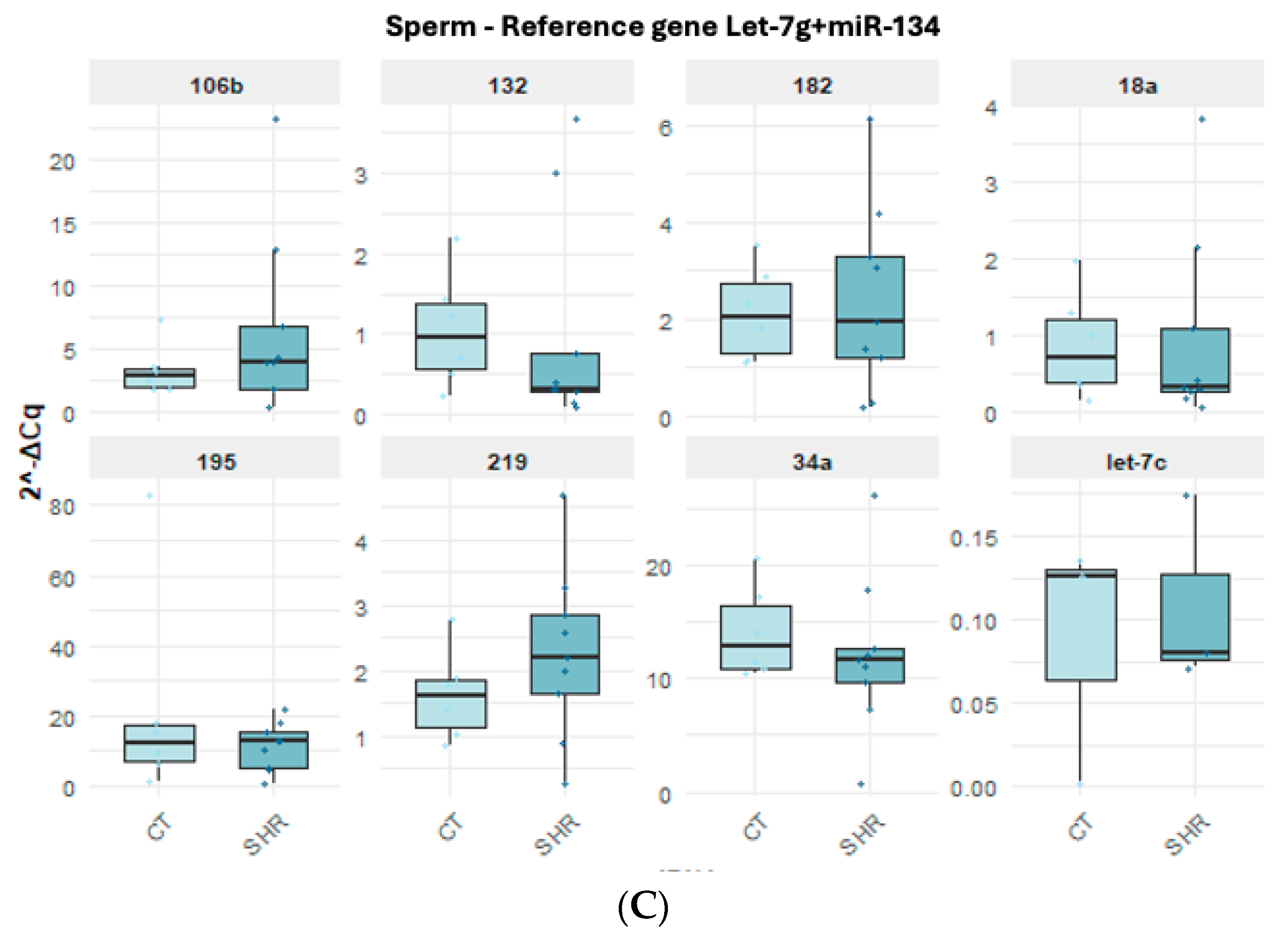
| GenEx (V. 7) | RefFinder (V. 1) | Bestkeeper (V. 1) | NormFinder (V. 0.953) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NormFinder | Genorm | RefFinder | ΔCt Method | Bestkeeper | NormFinder | Genorm | |||
| All tissues | Let7g | miR-34a | miR-Let7g | miR-Let7g | miR-195 | miR-Let7g | miR-34a | miR-34a | miR-Let7g |
| miR-34a | miR-Let7g | miR-34a | miR-34a | miR-34a | miR-34a | miR-Let7g | miR-Let7g | miR-34a | |
| Sperm | Let7g | miR-34a | miR-Let7g | miR-Let7g | miR-219 | miR-Let7g | miR-34a | miR-219 | miR-Let7g |
| miR-219 | miR-132 | miR-34a | miR-34a | miR-Let7g | miR-219 | miR-132 | miR-132 | miR-219 | |
| PFC | miR-34a | miR-132 | miR-195 | miR-219 | miR-132 | miR-34a | miR-195 | miR-195 | miR-34a |
| Let7g | miR-195 | miR-219 | miR-Let7g | miR-195 | miR-Let7g | miR-219 | miR-132 | miR-132 | |
| HIPPO | miR-34a | miR-34a | miR-195 | miR-Let7g | miR-195 | miR-34a | miR-195 | miR-34a | miR-34a |
| miR-132 | miR-Let7g | miR-Let7g | miR-195 | miR-34a | miR-132 | miR-Let7g | miR-Let7g | miR-132 | |
| miRNA | Score | |
|---|---|---|
| All samples combined | Let7g | ★★★★★ * |
| miR-34a | ★★★★★ | |
| Sperm | Let7g | ★★★★ |
| miR-219 | ★★★ | |
| miR-34a | ★★ | |
| miR-132 | ★★ | |
| PFC | miR-195 | ★★★ |
| Let7g | ★★ | |
| miR-34a | ★★ | |
| miR-219 | ★★ | |
| miR-132 | ★★ | |
| HIPPO | miR-34a | ★★★★ |
| Let-7g | ★★★ | |
| miR-132 | ★★ | |
| miR-195 | ★★ |
| Gene | Assay Name | Assay ID | miRNA Sequence |
|---|---|---|---|
| miR-132-5p | rno-miR-132-5p | rno481320_mir | ACCGUGGCUUUCGAUUGUUACU |
| miR-195-5p | rno-miR-195-5p | rno480882_mir | UAGCAGCACAGAAAUAUUGGC |
| miR-34a-5p | rno-miR-34a-5p | rno481304_mir | UGGCAGUGUCUUAGCUGGUUGU |
| miR-219a-5p | rno-miR-219a-5p | rno481348_mir | UGAUUGUCCAAACGCAAUUCU |
| miR-134-5p | rno-miR-134-5p | rno480922_mir | UGUGACUGGUUGACCAGAGGGG |
| miR-18a-5p | rno-miR-18a-5p | rno480968_mir | UAAGGUGCAUCUAGUGCAGAUAG |
| miR-106b-5p | rno-miR-106b-5p | rno478412_mir | UAAAGUGCUGACAGUGCAGAU |
| miR-182 | rno-miR-182 | rno480960_mir | UUUGGCAAUGGUAGAACUCACACCG |
| miR-let-7g-5p | hsa-let-7g-5p | 478580_mir | UGAGGUAGUAGUUUGUACAGUU |
| miR-484 | hsa-miR-484 | 478308_mir | UCAGGCUCAGUCCCCUCCCGAU |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantão, I.H.; Campagnoli, G.M.; Stumpp, T. Analysis of Normalizers and Neurodevelopment-Related microRNAs in the Prefrontal Cortex and in the Sperm of SHR Rats. Int. J. Mol. Sci. 2025, 26, 10589. https://doi.org/10.3390/ijms262110589
Cantão IH, Campagnoli GM, Stumpp T. Analysis of Normalizers and Neurodevelopment-Related microRNAs in the Prefrontal Cortex and in the Sperm of SHR Rats. International Journal of Molecular Sciences. 2025; 26(21):10589. https://doi.org/10.3390/ijms262110589
Chicago/Turabian StyleCantão, Isabelle Hernandez, Gabriella Mesas Campagnoli, and Taiza Stumpp. 2025. "Analysis of Normalizers and Neurodevelopment-Related microRNAs in the Prefrontal Cortex and in the Sperm of SHR Rats" International Journal of Molecular Sciences 26, no. 21: 10589. https://doi.org/10.3390/ijms262110589
APA StyleCantão, I. H., Campagnoli, G. M., & Stumpp, T. (2025). Analysis of Normalizers and Neurodevelopment-Related microRNAs in the Prefrontal Cortex and in the Sperm of SHR Rats. International Journal of Molecular Sciences, 26(21), 10589. https://doi.org/10.3390/ijms262110589






