Reinforced Defenses: R-Genes, PTI, and ETI in Modern Wheat Breeding for Blast Resistance
Abstract
1. Introduction
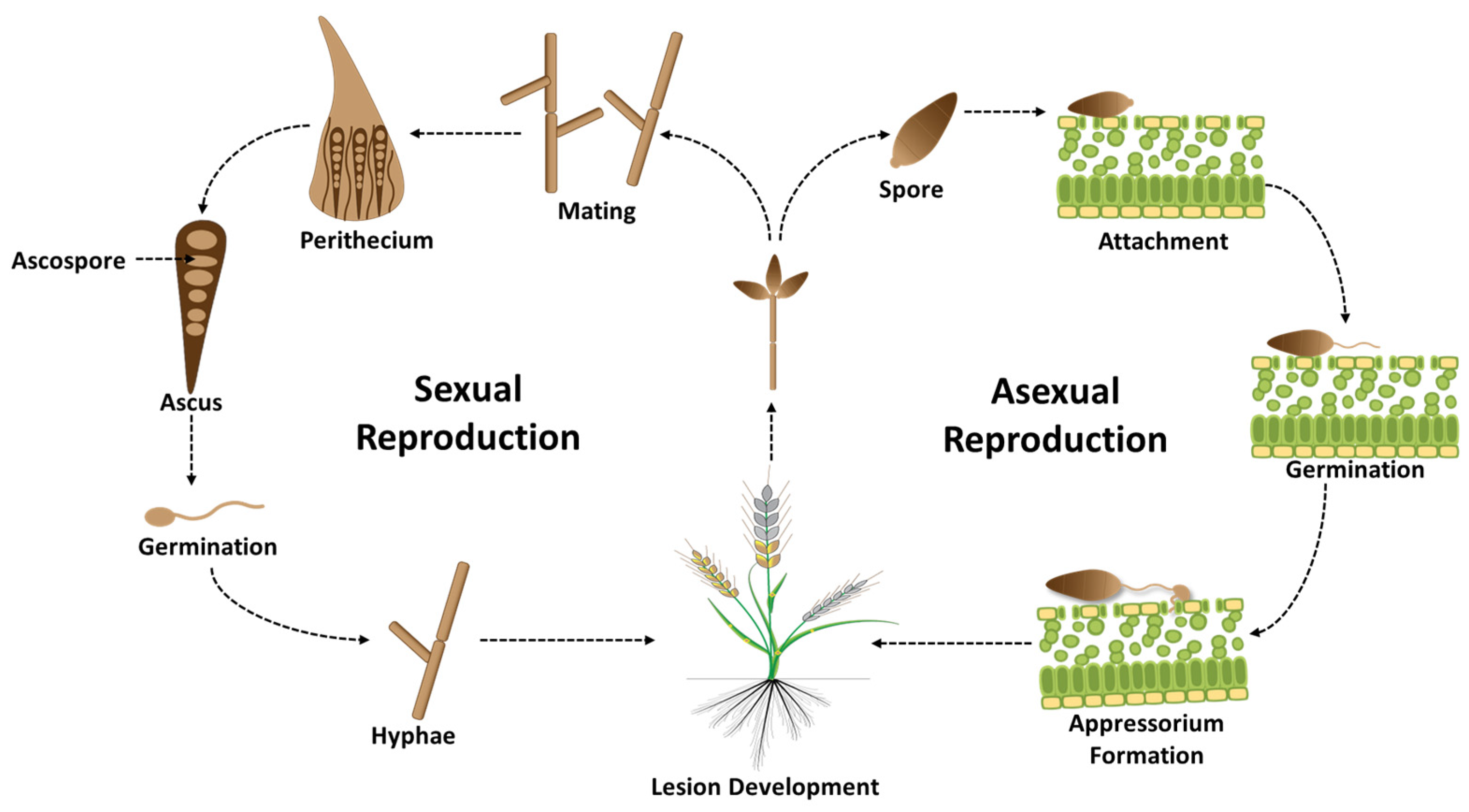
2. Biology of the Wheat Blast Pathogen
3. Wheat Blast: An Emerging Threat
| Country | Region | Year of Emergence | Severity of the Wheat Blast Outbreaks | Current Status of the Disease | References |
|---|---|---|---|---|---|
| Brazil | Paraná, Sao Paulo, Mato Grosso do Sul, Rio Grande do Sul, Minas Gerais, Goias, Brasília | 1985–1993 | Initial yield losses of 10–12%; in some areas, widespread outbreaks with yield losses up to 100% during epidemics | Widespread across all wheat-producing zones in Brazil, with recent epidemics in 2009 and 2012 | [24,39] |
| Bolivia | Santa Cruz | 1996 | During the first epidemic in 1996, up to 100% yield loss in early-sown fields | Widespread across all wheat-producing zones in Bolivia, with a recent epidemic in 2014 | [39,40] |
| Paraguay | Alto Parana, Itapua, Caaguazu, Caazapa, Canindeyu and Guaira | 2002 | Yield losses up to 80% in early-sown crops during the first epidemic | significant impact on major wheat production zones | [39,41] |
| Argentina | Chaco and Corrientes | 2007 | Limited impact initially | Presence in major wheat areas was noted, and concern grew after detection in the major wheat-producing province of Buenos Aires | [39,42] |
| USA | Kentucky | 2011 | Only a single spike was infected | Since then, the disease has not occurred. | [43] |
| Bangladesh | Kushtia, Meherpur, Chuadanga, Pabna, Jessore, Jhenaidah, Bhola, Barisal, Magura, Faridpur, and Rajshahi | 2016–2017 | First Asian outbreak affected ~15,000 ha with yield losses up to 51% in some districts | Spread to additional districts and is now present in all wheat-growing areas and affects wheat at various intensities—average yield loss of 15 to 24.5% | [4,5,29,44] |
| Zambia | Mpika district, Muchinga Province | 2017–2018 | Limited to the experimental field | Spread to the farmer’s field, but still with limited impact | [35] |
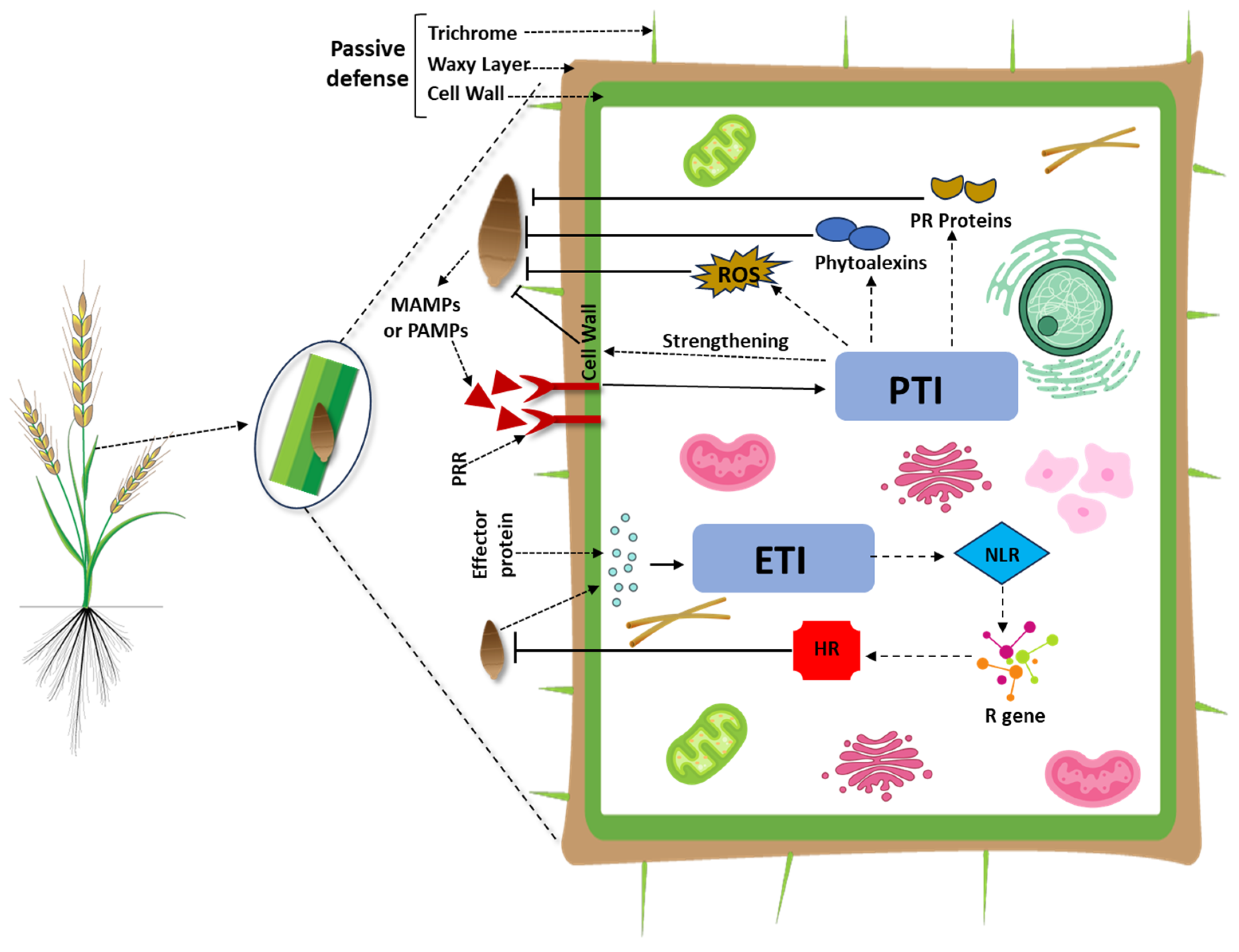
4. Plant Immune System: An Overview
5. Roles of Rmg Genes in Wheat Blast Resistance
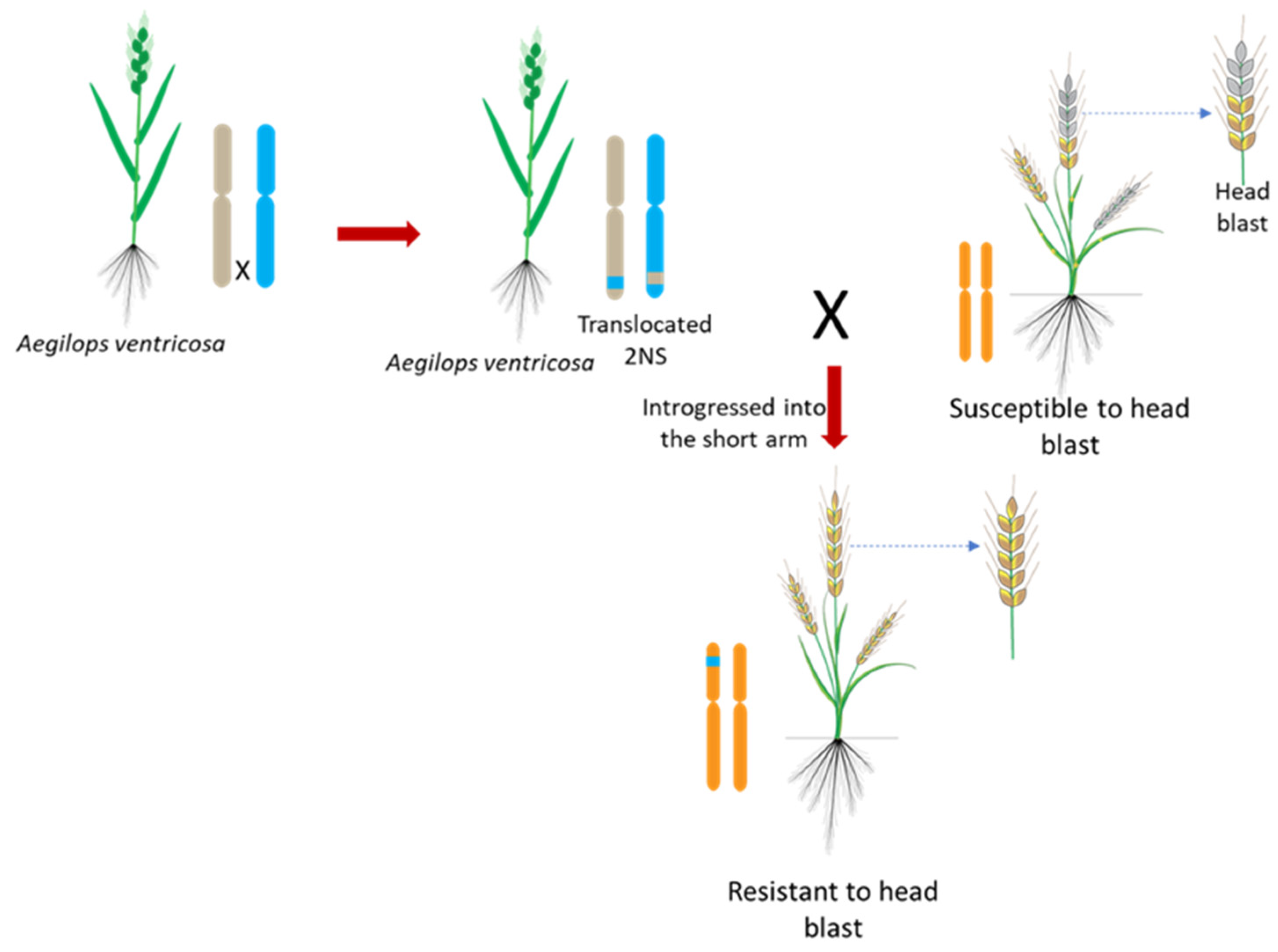
6. Role of 2NS Translocation and QTL Mapping for Wheat Blast Resistance
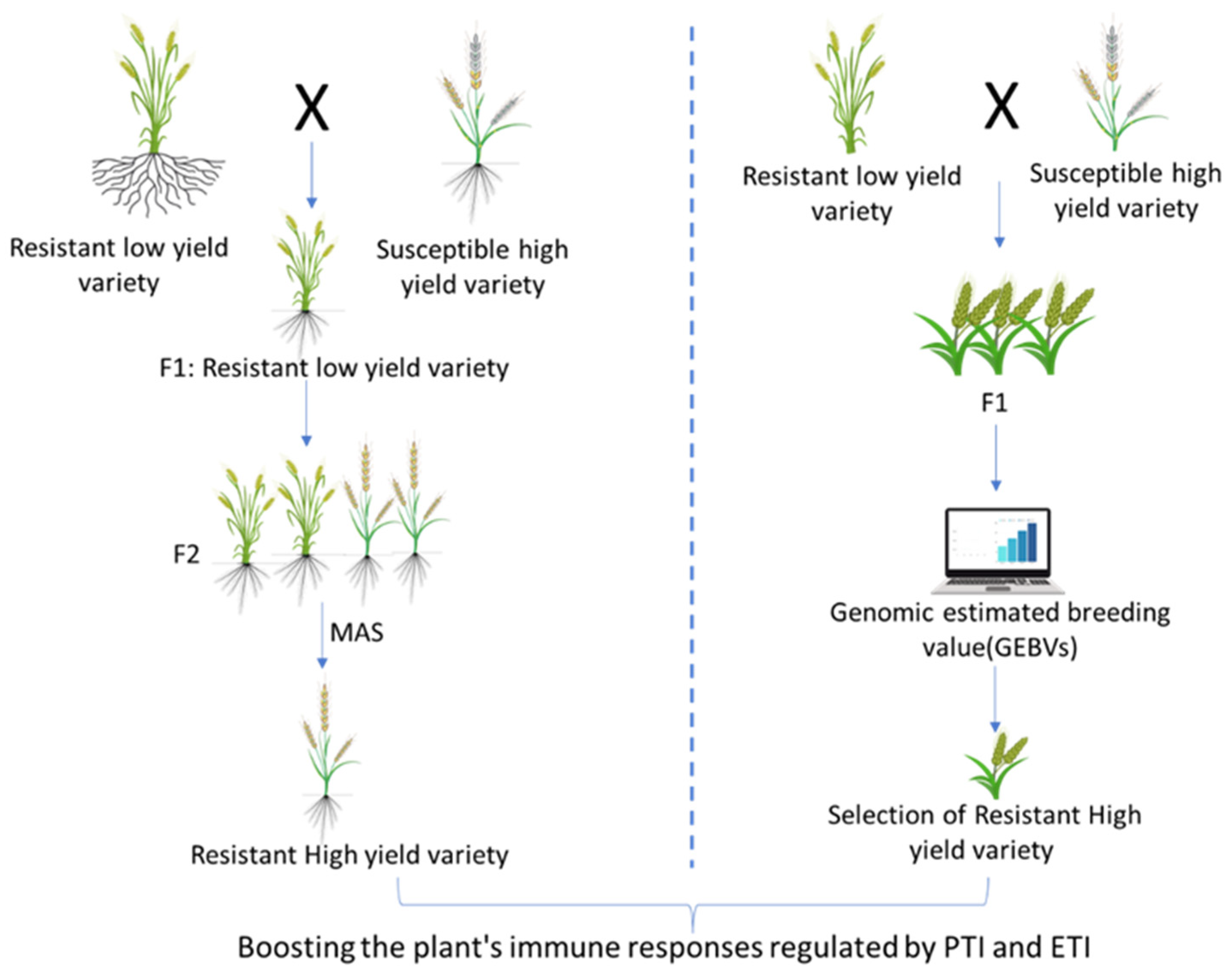
7. Advances in Molecular Breeding for Wheat Blast Resistance
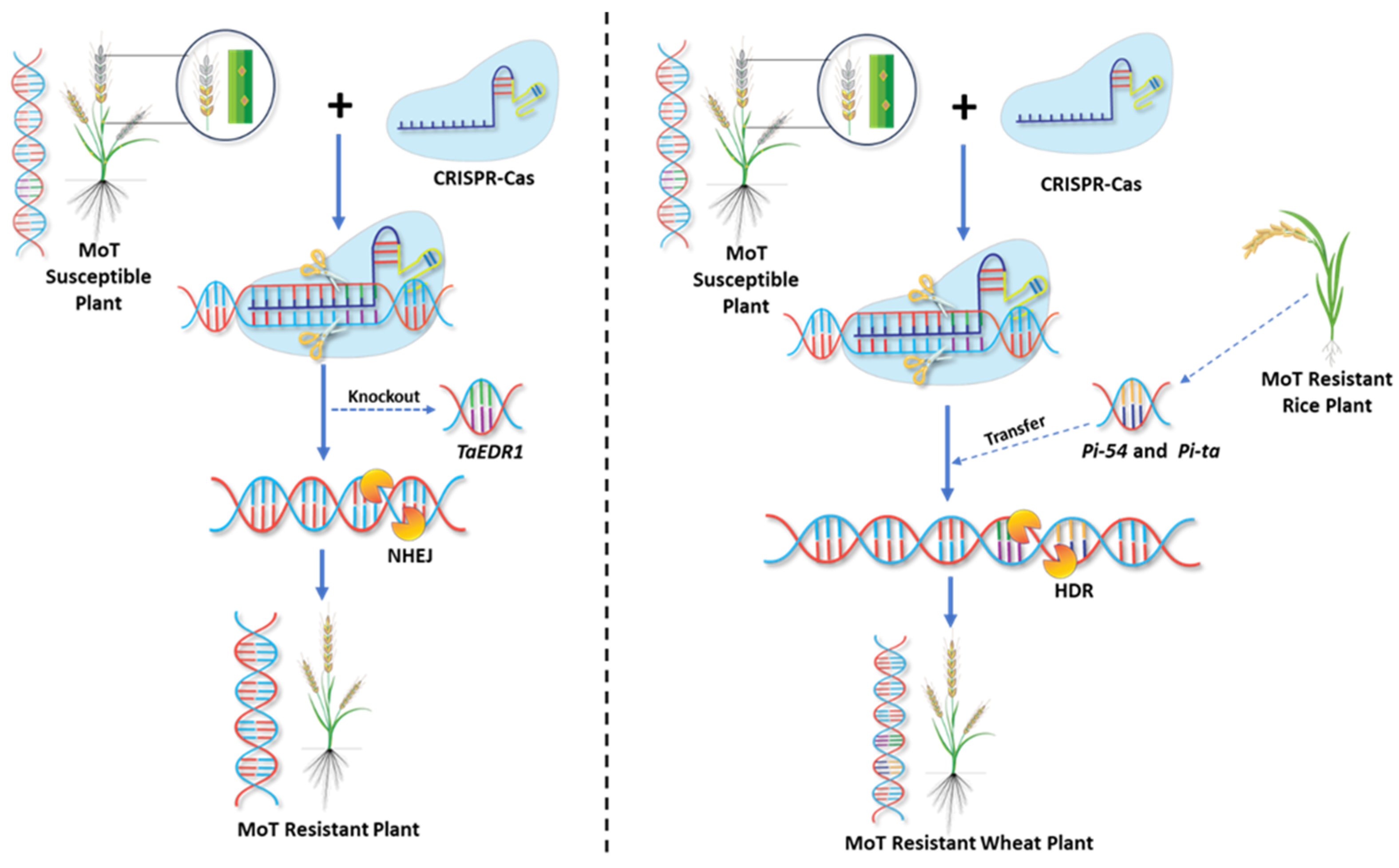
8. Genome Editing in Enhancing Wheat Blast Resistance
9. Development of Wheat Varieties for Blast Resistance
| Variety | Country | Resistance Level | Background | Reference |
|---|---|---|---|---|
| BARI Gom 33 | Bangladesh | High | 2NS | [122] |
| Borloug 100 | Bangladesh, Bolivia, Nepal | High | 2NS | [31] |
| BR 18-Terena | Brazil | High | Non 2NS | [83] |
| BR8 | Brazil | High | 2AS/2NS | [119] |
| BRS 229 | Brazil | High | Non 2NS | [125] |
| Caninde 1“S” | Paraguay | High | 2AS/2NS | [119] |
| Milan | South America | High | 2AS/2NS | [119] |
| Paragua CIAT | Bolivia | High | - | [9] |
| Parapeti CIAT | Bolivia | High | - | [9] |
| BRS 120 | Brazil | Moderate | 2NS | [126] |
| BRS 220 | Brazil | Moderate | 2NS | [126] |
| BRS 49 | Brazil | Moderate | 2NS | [126] |
| Caninde 1 | Paraguay | Moderate | 2NS | [9] |
| CD 116 | Brazil | Moderate | 2NS | [126] |
| IAPAR 53 | Brazil | Moderate | - | [126] |
| IPR 85 | Brazil | Moderate | - | [9] |
| Itapua 75 | Paraguay | Moderate | 2NS | [127] |
| Motacu CIAT | Bolivia | Moderate | Non 2NS | [127] |
| Patuju CIAT | Bolivia | Moderate | Non 2NS | [127] |
| Sausal CIAT | Bolivia | Moderate | 2AS/2NS | [127] |
10. Potential Challenges and Opportunities
11. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oerke, E.-C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Mostafa, M.; Ferdus, H.; Rahman, M.; Rana, J.A.; Islam, S.S.; Adhikary, S.; Sannal, A.; Hosen, M.A.E.; et al. Plant disease dynamics in a changing climate: Impacts, molecular mechanisms, and climate-informed strategies for sustainable management. Discov. Agric. 2024, 2, 132. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Mottaleb, K.A.; Sonder, K.; Donovan, J.; Braun, H.J. Global trends in wheat production, consumption and trade. In Wheat Improvement: Food Security in a Changing Climate; Springer International Publishing: Cham, Switzerland, 2022; pp. 47–66. [Google Scholar] [CrossRef]
- Islam, M.T.; Croll, D.; Gladieux, P.; Soanes, D.M.; Persoons, A.; Bhattacharjee, P.; Hossain, M.S.; Gupta, D.R.; Rahman, M.M.; Mahboob, M.G.; et al. Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biol. 2016, 14, 84. [Google Scholar] [CrossRef]
- Hossain, M.M. Wheat blast: A review from a genetic and genomic perspective. Front. Microbiol. 2022, 13, 983243. [Google Scholar] [CrossRef]
- Castroagudín, V.L.; Moreira, S.I.; Pereira, D.A.; Moreira, S.S.; Brunner, P.C.; Maciel, J.L.; Crous, P.W.; McDonald, B.A.; Alves, E.; Ceresini, P.C. Pyricularia graminis-tritici, a new Pyricularia species causing wheat blast. Persoonia 2016, 37, 199–216. [Google Scholar] [CrossRef]
- de Oliveira Nascimento, I.; Rodrigues, A.N.A.C.; Moraes, F.H.; Corsi, M.C.F. Isolation, identification and in vitro evaluation of Bacillus spp. in control of Magnaporthe oryzae comparing evaluation methods. Afr. J. Agric. Res. 2016, 11, 1743–1749. [Google Scholar] [CrossRef]
- Goulart, A.C.P.; Sousa, P.G.; Urashima, A.S. Danos em trigo causados pela infecção de Pyricularia grisea. Summa Phytopathol. 2007, 33, 358–363. [Google Scholar] [CrossRef]
- Kohli, M.M.; Mehta, Y.R.; Guzman, L.; Viedma, L.D.; Cubilla, L.E. Pyricularia blast—A threat to wheat cultivation. Czech J. Genet. Plant Breed. 2011, 47, S130–S134. [Google Scholar] [CrossRef]
- Castroagudín, V.L.; Ceresini, P.C.; de Oliveira, S.C.; Reges, J.T.A.; Maciel, J.L.N.; Bonato, A.L.V.; Dorigan, A.F.; McDonald, B.A. Resistance to QoI fungicides is widespread in Brazilian populations of the wheat blast pathogen Magnaporthe oryzae. Phytopathology 2015, 105, 284–294. [Google Scholar] [CrossRef]
- Dorigan, A.F.; de Carvalho, G.; Poloni, N.M.; Negrisoli, M.M.; Maciel, J.L.N.; Ceresini, P.C. Resistance to triazole fungicides in Pyricularia species is associated with invasive plants from wheat fields in Brazil. Acta Sci. Agron. 2019, 41, e39332. [Google Scholar] [CrossRef]
- Cruz, C.D.; Valent, B. Wheat blast disease: Danger on the move. Trop. Plant Pathol. 2017, 42, 210–222. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.M.; Iswanto, A.B.B.; Son, G.H.; Kim, S.H. Recent advances in effector-triggered immunity in plants: New pieces in the puzzle create a different paradigm. Int. J. Mol. Sci. 2021, 22, 4709. [Google Scholar] [CrossRef] [PubMed]
- Couch, B.C.; Kohn, L.M. A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae, from M. grisea. Mycologia 2002, 94, 683–693. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Z.; Zong, Y.; Wang, Y.; Liu, J.; Chen, K.; Qiu, J.L.; Gao, C. Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat. Commun. 2016, 7, 12617. [Google Scholar] [CrossRef]
- Chuma, I.; Shinogi, T.; Hosogi, N.; Ikeda, K.I.; Nakayashiki, H.; Park, P.; Tosa, Y. Cytological characteristics of microconidia of Magnaporthe oryzae. J. Gen. Plant Pathol. 2009, 75, 353–358. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Z.; Wang, C.; Li, Y.; Xu, J.R. Germination and infectivity of microconidia in the rice blast fungus Magnaporthe oryzae. Nat. Commun. 2014, 5, 4518. [Google Scholar] [CrossRef]
- Araujo, L.; Soares, J.M.; Filippi, M.C.C.D.; Rodrigues, F.Á. Cytological aspects of incompatible and compatible interactions between rice, wheat and the blast pathogen Pyricularia oryzae. Sci. Agric. 2016, 73, 177–183. [Google Scholar] [CrossRef]
- Saharan, M.S.; Bhardwaj, S.C.; Chatrath, R.; Sharma, P.; Choudhary, A.K.; Gupta, R.K. Wheat blast disease—An overview. J. Wheat Res. 2016, 8, 1–5. [Google Scholar]
- Castroagudín, V.L.; Danelli, A.L.; Moreira, S.I.; Reges, J.T.; de Carvalho, G.; Maciel, J.L.; Ceresini, P.C. The wheat blast pathogen Pyricularia graminis-tritici has complex origins and a disease cycle spanning multiple grass hosts. bioRxiv 2017, 203455. [Google Scholar] [CrossRef]
- O’Leary 2019. What Is Wheat Blast? Available online: https://www.cimmyt.org/news/what-is-wheat-blast/ (accessed on 12 October 2025).
- Islam, M.T.; Gupta, D.R.; Hossain, A.; Roy, K.K.; He, X.; Kabir, M.R.; Singh, P.K.; Khan, M.A.R.; Rahman, M.; Wang, G.L. Wheat blast: A new threat to food security. Phytopathol. Res. 2020, 2, 28. [Google Scholar] [CrossRef]
- Igarashi, S.; Utiamada, C.M.; Igarashi, L.C.; Kazuma, A.H.; Lopes, R.S. Pyricularia in wheat. 1. Occurrence of Pyricularia sp. in Paraná State. Fitopatol. Bras. 1986, 11, 351–352. [Google Scholar]
- CONAB—Companhia Nacional de Abastecimento. Follow-Up of the Brazilian Grain Crop. 2017. Available online: http://www.conab.gov.br/conteudos.php?a=1252&t=2 (accessed on 7 March 2025).
- Mottaleb, K.A.; Singh, P.K.; Sonder, K.; Kruseman, G.; Tiwari, T.P.; Barma, N.C.; Malaker, P.K.; Braun, H.J.; Erenstein, O. Threat of wheat blast to South Asia’s food security: An ex-ante analysis. PLoS ONE 2018, 13, e0197555. [Google Scholar] [CrossRef]
- Duveiller, E.; He, X.; Singh, P.K. Wheat blast: An emerging disease in South America potentially threatening wheat production. In The World Wheat Book; Bonjean, A., van Ginkel, M., Eds.; Lavoisier: Paris, France, 2016; Volume 3, pp. 1107–1122. [Google Scholar]
- Cruz, C.D.; Peterson, G.L.; Bockus, W.W.; Kankanala, P.; Dubcovsky, J.; Jordan, K.W.; Akhunov, E.; Chumley, F.; Baldelomar, F.D.; Valent, B. The 2NS translocation from Aegilops ventricosa confers resistance to the Triticum pathotype of Magnaporthe oryzae. Crop Sci. 2016, 56, 990–1000. [Google Scholar] [CrossRef]
- Malaker, P.K.; Barma, N.C.; Tiwari, T.P.; Collis, W.J.; Duveiller, E.; Singh, P.K.; Joshi, A.K.; Singh, R.P.; Braun, H.J.; Peterson, G.L.; et al. First report of wheat blast caused by Magnaporthe oryzae pathotype triticum in Bangladesh. Plant Dis. 2016, 100, 2330. [Google Scholar] [CrossRef]
- Sadat, M.A.; Choi, J. Wheat blast: A new fungal inhabitant to Bangladesh threatening world wheat production. Plant Pathol. J. 2017, 33, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Gahtyari, N.C.; Roy, C.; Roy, K.K.; He, X.; Tembo, B.; Xu, K.; Juliana, P.; Sonder, K.; Kabir, M.R.; et al. Wheat blast: A disease spreading by intercontinental jumps and its management strategies. Front. Plant Sci. 2021, 12, 710707. [Google Scholar] [CrossRef]
- Islam, M.T.; Kim, K.H.; Choi, J. Wheat blast in Bangladesh: The current situation and future impacts. Plant Pathol. J. 2019, 35, 1. [Google Scholar] [CrossRef]
- Bhatt, R.; Singh, P.; Hossain, A.; Timsina, J. Rice–wheat system in the northwest Indo-Gangetic plains of South Asia: Issues and technological interventions for increasing productivity and sustainability. Paddy Water Environ. 2021, 19, 345–365. [Google Scholar] [CrossRef]
- Mottaleb, K.A.; Govindan, V.; Singh, P.K.; Sonder, K.; He, X.; Singh, R.P.; Joshi, A.K.; Barma, N.C.; Kruseman, G.; Erenstein, O. Economic benefits of blast-resistant biofortified wheat in Bangladesh: The case of BARI Gom 33. Crop Prot. 2019, 123, 45–58. [Google Scholar] [CrossRef]
- Tembo, B.; Mulenga, R.M.; Sichilima, S.; M’siska, K.K.; Mwale, M.; Chikoti, P.C.; Singh, P.K.; He, X.; Pedley, K.F.; Peterson, G.L.; et al. Detection and characterization of fungus (Magnaporthe oryzae pathotype Triticum) causing wheat blast disease on rain-fed grown wheat (Triticum aestivum L.) in Zambia. PLoS ONE 2020, 15, e0238724. [Google Scholar] [CrossRef] [PubMed]
- Pequeno, D.N.; Ferreira, T.B.; Fernandes, J.M.; Singh, P.K.; Pavan, W.; Sonder, K.; Robertson, R.; Krupnik, T.J.; Erenstein, O.; Asseng, S. Production vulnerability to wheat blast disease under climate change. Nat. Clim. Chang. 2024, 14, 178–183. [Google Scholar] [CrossRef]
- Chávez, A.R.; Tellez, L.C.; Cazal-Martinez, C.C.; Kohli, M.M.; Carmona, M.A. Further progress on wheat blast epidemiology: Identification of novel alternate hosts of Magnaporthe oryzae Triticum pathotype in Paraguay. Eur. J. Plant Pathol. 2022, 164, 365–373. [Google Scholar] [CrossRef]
- Maciel, J.L.N.; Ceresini, P.C.; Castroagudin, V.L.; Zala, M.; Kema, G.H.; McDonald, B.A. Population structure and pathotype diversity of the wheat blast pathogen Magnaporthe oryzae 25 years after its emergence in Brazil. Phytopathology 2014, 104, 95–107. [Google Scholar] [CrossRef]
- Ceresini, P.C.; Castroagudín, V.L.; Rodrigues, F.Á.; Rios, J.A.; Aucique-Pérez, C.E.; Moreira, S.I.; Alves, E.; Croll, D.; Maciel, J.L.N. Wheat blast: Past, present, and future. Annu. Rev. Phytopathol. 2018, 56, 427–456. [Google Scholar] [CrossRef]
- Barea, G.; Toledo, J. Identificación y Zonificación de Pyricularia o Brusone (Pyricularia oryzae) en el Cultivo de Trigo en el Departamento de Santa Cruz; Informe Tecnico. Proyecto de Investigacion Trigo; Centro de Investigación Agrícola Tropical: Santa Cruz de la Sierra, Bolivia, 1996; pp. 76–86. [Google Scholar]
- Viedma, L.Q.; Morel, W. Añublo o Piricularia del Trigo; Díptico, MAG/DIA/CRIA, Programa de Investigación de Trigo; CRIA: Capitán Miranda, Paraguay, 2002. (In Spanish) [Google Scholar]
- Cabrera, M.G.; Gutierres, S.A. Primer Registro de Pyricularia grisea en Cultivos de Trigo del NE de Argentina; IFSC Press: Buenos Aires, Argentina, 2007; Available online: https://herbariofitopatologia.agro.uba.ar/?page_id=1689 (accessed on 9 March 2025).
- Farman, M.; Peterson, G.; Chen, L.; Starnes, J.; Valent, B.; Bachi, P.; Murdock, L.; Hershman, D.; Pedley, K.; Fernandes, J.M.; et al. The Lolium pathotype of Magnaporthe oryzae recovered from a single blasted wheat plant in the United States. Plant Dis. 2017, 101, 684–692. [Google Scholar] [CrossRef]
- Yesmin, N.; Jenny, F.; Abdullah, H.M.; Hossain, M.M.; Kader, M.A.; Solomon, P.S.; Bhuiyan, M.A. A review on South Asian wheat blast: The present status and future perspective. Plant Pathol. 2020, 69, 1618–1629. [Google Scholar] [CrossRef]
- Bednarek, P.; Osbourn, A. Plant–microbe interactions: Chemical diversity in plant defense. Science 2009, 324, 746–748. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Møller, B.L. Cyanogenic glycosides: Synthesis, physiology, and phenotypic plasticity. Annu. Rev. Plant Biol. 2014, 65, 155–185. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Shiu, S.H.; Bleecker, A.B. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. USA 2001, 98, 10763–10768. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Klessig, D.F. DAMPs, MAMPs, and NAMPs in plant innate immunity. BMC Plant Biol. 2016, 16, 232. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Vance, R.E.; Dangl, J.L. Intracellular innate immune surveillance devices in plants and animals. Science 2016, 354, aaf6395. [Google Scholar] [CrossRef]
- Naito, K.; Ishiga, Y.; Toyoda, K.; Shiraishi, T.; Ichinose, Y. N-terminal domain including conserved flg22 is required for flagellin-induced hypersensitive cell death in Arabidopsis thaliana. J. Gen. Plant Pathol. 2007, 73, 281–285. [Google Scholar] [CrossRef]
- Bettgenhaeuser, J.; Gardiner, M.; Spanner, R.; Green, P.; Hernández-Pinzón, I.; Hubbard, A.; Ayliffe, M.; Moscou, M.J. The genetic architecture of colonization resistance in Brachypodium distachyon to non-adapted stripe rust (Puccinia striiformis) isolates. PLoS Genet. 2018, 14, e1007637. [Google Scholar] [CrossRef]
- Thomma, B.P.; Nürnberger, T.; Joosten, M.H. Of PAMPs and effectors: The blurred PTI-ETI dichotomy. Plant Cell 2011, 23, 4–15. [Google Scholar] [CrossRef]
- Gilbert, B.; Bettgenhaeuser, J.; Upadhyaya, N.; Soliveres, M.; Singh, D.; Park, R.F.; Moscou, M.J.; Ayliffe, M. Components of Brachypodium distachyon resistance to nonadapted wheat stripe rust pathogens are simply inherited. PLoS Genet. 2018, 14, e1007636. [Google Scholar] [CrossRef]
- Cevik, V.; Boutrot, F.; Apel, W.; Robert-Seilaniantz, A.; Furzer, O.J.; Redkar, A.; Castel, B.; Kover, P.X.; Prince, D.C.; Holub, E.B.; et al. Transgressive segregation reveals mechanisms of Arabidopsis immunity to Brassica-infecting races of white rust (Albugo candida). Proc. Natl. Acad. Sci. USA 2019, 116, 2767–2773. [Google Scholar] [CrossRef]
- Bourras, S.; Kunz, L.; Xue, M.; Praz, C.R.; Müller, M.C.; Kälin, C.; Schläfli, M.; Ackermann, P.; Flückiger, S.; Parlange, F.; et al. The AvrPm3–Pm3 effector–NLR interactions control both race-specific resistance and host-specificity of cereal mildews on wheat. Nat. Commun. 2019, 10, 2292. [Google Scholar] [CrossRef]
- Cumagun, C.J.R.; Anh, V.L.; Vy, T.T.P.; Inoue, Y.; Asano, H.; Hyon, G.S.; Chuma, I.; Tosa, Y. Identification of a hidden resistance gene in tetraploid wheat using laboratory strains of Pyricularia oryzae produced by backcrossing. Phytopathology 2014, 6, 634–640. [Google Scholar] [CrossRef]
- Zhan, S.W.; Mayama, S.; Tosa, Y. Identification of two genes for resistance to Triticum isolates of Magnaporthe oryzae in wheat. Genome 2008, 51, 216–221. [Google Scholar] [CrossRef]
- Tagle, A.G.; Chuma, I.; Tosa, Y. Rmg7, a new gene for resistance to Triticum isolates of Pyricularia oryzae identified in tetraploid wheat. Phytopathology 2015, 105, 495–499. [Google Scholar] [CrossRef]
- Takabayashi, N.; Tosa, Y.; Oh, H.S.; Mayama, S. A gene-for-gene relationship underlying the species-specific parasitism of Avena/Triticum isolates of Magnaporthe grisea on wheat cultivars. Phytopathology 2002, 92, 1182–1188. [Google Scholar] [CrossRef]
- Nga, N.; Hau, V.T.; Tosa, Y. Identification of genes for resistance to a Digitaria isolate of Magnaporthe grisea in common wheat cultivars. Genome 2009, 52, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Vy, T.T.P.; Hyon, G.S.; Nga, N.T.T.; Inoue, Y.; Chuma, I.; Tosa, Y. Genetic analysis of host–pathogen incompatibility between Lolium isolates of Pyricularia oryzae and wheat. J. Gen. Plant Pathol. 2014, 80, 59–65. [Google Scholar] [CrossRef]
- Anh, V.L.; Anh, N.T.; Tagle, A.G.; Vy, T.T.P.; Inoue, Y.; Takumi, S.; Chuma, I.; Tosa, Y. Rmg8, a new gene for resistance to Triticum isolates of Pyricularia oryzae in hexaploid wheat. Phytopathology 2015, 105, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Anh, V.L.; Inoue, Y.; Asuke, S.; Vy, T.T.P.; Anh, N.T.; Wang, S.; Chuma, I.; Tosa, Y. Rmg8 and Rmg7, wheat genes for resistance to the wheat blast fungus, recognize the same avirulence gene AVR-Rmg8. Mol. Plant Pathol. 2018, 19, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Asuke, S.; Vy, T.T.P.; Inoue, Y.; Chuma, I.; Win, J.; Kato, K.; Tosa, Y. A new resistance gene in combination with Rmg8 confers strong resistance against Triticum isolates of Pyricularia oryzae in a common wheat landrace. Phytopathology 2018, 108, 1299–1306. [Google Scholar] [CrossRef]
- Tosa, Y.; Tamba, H.; Tanaka, K.; Mayama, S. Genetic analysis of host species specificity of Magnaporthe oryzae isolates from rice and wheat. Phytopathology 2006, 96, 480–484. [Google Scholar] [CrossRef]
- Chuma, I.; Zhan, S.W.; Asano, S.; Nga, N.T.T.; Vy, T.T.P.; Shirai, M.; Ibaragi, K.; Tosa, Y. PWT1, an avirulence gene of Magnaporthe oryzae tightly linked to the rDNA locus, is recognized by two staple crops, common wheat and barley. Phytopathology 2010, 100, 436–443. [Google Scholar] [CrossRef]
- Asuke, S.; Morita, K.; Shimizu, M.; Abe, F.; Terauchi, R.; Nago, C.; Takahashi, Y.; Shibata, M.; Yoshioka, M.; Iwakawa, M.; et al. Evolution of wheat blast resistance gene Rmg8 accompanied by differentiation of variants recognizing the powdery mildew fungus. Nat. Plants 2024, 10, 971–983. [Google Scholar] [CrossRef]
- He, X.; Kabir, M.R.; Roy, K.K.; Anwar, M.B.; Xu, K.; Marza, F.; Odilbekov, F.; Chawade, A.; Duveiller, E.; Huttner, E.; et al. QTL mapping for field resistance to wheat blast in the Caninde#1/Alondra population. Theor. Appl. Genet. 2020, 133, 2673–2683. [Google Scholar] [CrossRef]
- Horo, J.T.; Asuke, S.; Vy, T.T.P.; Tosa, Y. Effectiveness of the wheat blast resistance gene Rmg8 in Bangladesh suggested by distribution of an AVR-Rmg8 allele in the Pyricularia oryzae population. Phytopathology 2020, 110, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Asuke, S.; Umehara, Y.; Inoue, Y.; Vy, T.T.P.; Iwakawa, M.; Matsuoka, Y.; Kato, K.; Tosa, Y. Origin and dynamics of Rwt6, a wheat gene for resistance to nonadapted pathotypes of Pyricularia oryzae. Phytopathology 2021, 111, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Steed, A.; Goddard, R.; Gaurav, K.; O’Hara, T.; Schoen, A.; Rawat, N.; Elkot, A.F.; Korolev, A.V.; Chinoy, C.; et al. A wheat kinase and immune receptor form host-specificity barriers against the blast fungus. Nat. Plants 2023, 9, 385–392. [Google Scholar] [CrossRef]
- Inoue, Y.; Vy, T.T.P.; Tani, D.; Tosa, Y. Suppression of wheat blast resistance by an effector of Pyricularia oryzae is counteracted by a host specificity resistance gene in wheat. New Phytol. 2021, 229, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Nizolli, V.O.; Viana, V.E.; Pegoraro, C.; Maia, L.C.D.; Oliveira, A.C. Wheat blast: The last enemy of hunger fighters. Genet. Mol. Biol. 2023, 46 (Suppl. 1), e20220002. [Google Scholar] [CrossRef]
- He, X.; Kabir, M.R.; Roy, K.K.; Marza, F.; Chawade, A.; Duveiller, E.; Pierre, C.S.; Singh, P.K. Genetic dissection for head blast resistance in wheat using two mapping populations. Heredity 2021, 128, 402–410. [Google Scholar] [CrossRef]
- Roy, K.K.; Rahman, M.M.E.; Mustarin, K.; Reza, M.M.A.; Barma, N.C.D.; He, X.; Singh, P.K. First report of durum wheat (Triticum turgidum var. durum) blast caused by the fungus Magnaporthe oryzae pathotype Triticum in Bangladesh. Phytopathol. Mediterr. 2021, 60, 107–113. [Google Scholar] [CrossRef]
- Juliana, P.; He, X.; Kabir, M.R.; Roy, K.K.; Anwar, M.B.; Marza, F.; Poland, J.; Shrestha, S.; Singh, R.P.; Singh, P.K. Genome-wide association mapping for wheat blast resistance in CIMMYT’s international screening nurseries evaluated in Bolivia and Bangladesh. Sci. Rep. 2020, 10, 15972. [Google Scholar] [CrossRef]
- Juliana, P.; Poland, J.; Huerta-Espino, J.; Shrestha, S.; Crossa, J.; Crespo-Herrera, L.; Toledo, F.H.; Govindan, V.; Mondal, S.; Kumar, U.; et al. Improving grain yield, stress resilience and quality of bread wheat using large-scale genomics. Nat. Genet. 2019, 51, 1530–1539. [Google Scholar] [CrossRef]
- Wu, L.; He, X.; Kabir, M.R.; Roy, K.K.; Anwar, M.B.; Marza, F.; He, Y.; Jiang, P.; Zhang, X.; Singh, P.K. Genetic sources and loci for wheat head blast resistance identified by genome-wide association analysis. Crop J. 2022, 10, 793–801. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Torres, G.A.M.; Consoli, L.; Camilotti, G.A.; Scagliusi, S.M.M.; Nhani, A.; Turchetto, C.; Deuner, C.C.; Goddard, R.; Nicholson, P. Quantitative trait loci conferring blast resistance in hexaploid wheat at adult plant stage. Plant Pathol. 2021, 70, 100–109. [Google Scholar] [CrossRef]
- Cruppe, G.; Cruz, C.D.; Peterson, G.; Pedley, K.; Asif, M.; Fritz, A.; Calderon, L.; Lemes da Silva, C.; Todd, T.; Kuhnem, P.; et al. Novel sources of wheat head blast resistance in modern breeding lines and wheat wild relatives. Plant Dis. 2020, 104, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Goddard, R.; Steed, A.; Chinoy, C.; Ferreira, J.R.; Scheeren, P.L.; Maciel, J.L.N.; Caierão, E.; Torres, G.A.M.; Consoli, L.; Santana, F.M.; et al. Dissecting the genetic basis of wheat blast resistance in the Brazilian wheat cultivar BR 18-Terena. BMC Plant Biol. 2020, 20, 398. [Google Scholar] [CrossRef]
- Cruppe, G.; Silva, P.; Lemes da Silva, C.; Peterson, G.; Pedley, K.F.; Cruz, C.D.; Asif, M.; Lollato, R.P.; Fritz, A.K.; Valent, B. Genome-wide association reveals limited benefits of pyramiding the 1B and 1D loci with the 2NvS translocation for wheat blast control. Crop Sci. 2021, 61, 1089–1103. [Google Scholar] [CrossRef]
- Phuke, R.M.; He, X.; Juliana, P.; Kabir, M.R.; Roy, K.K.; Marza, F.; Roy, C.; Singh, G.P.; Chawade, A.; Joshi, A.K.; et al. Identification of genomic regions and sources for wheat blast resistance through GWAS in Indian wheat genotypes. Genes 2022, 13, 596. [Google Scholar] [CrossRef] [PubMed]
- Heffner, E.L.; Sorrells, M.E.; Jannink, J.L. Genomic selection for crop improvement. Crop Sci. 2009, 49, 1–12. [Google Scholar] [CrossRef]
- Ornella, L.; Singh, S.; Perez, P.; Burgueño, J.; Singh, R.; Tapia, E.; Bhavani, S.; Dreisigacker, S.; Braun, H.J.; Mathews, K.; et al. Genomic prediction of genetic values for resistance to wheat rusts. Plant Genome 2012, 5, 135–148. [Google Scholar] [CrossRef]
- Rutkoski, J.E.; Poland, J.A.; Singh, R.P.; Huerta-Espino, J.; Bhavani, S.; Barbier, H.; Rouse, M.N.; Jannink, J.L.; Sorrells, M.E. Genomic selection for quantitative adult plant stem rust resistance in wheat. Plant Genome 2014, 7, 1–10. [Google Scholar] [CrossRef]
- Heffner, E.L.; Lorenz, A.J.; Jannink, J.L.; Sorrells, M.E. Plant breeding with genomic selection: Gain per unit time and cost. Crop Sci. 2010, 50, 1681–1690. [Google Scholar] [CrossRef]
- Voss-Fels, K.P.; Cooper, M.; Hayes, B.J. Accelerating crop genetic gains with genomic selection. Theor. Appl. Genet. 2019, 132, 669–686. [Google Scholar] [CrossRef]
- Juliana, P.; He, X.; Marza, F.; Islam, R.; Anwar, B.; Poland, J.; Shrestha, S.; Singh, G.P.; Chawade, A.; Joshi, A.K.; et al. Genomic selection for wheat blast in a diversity panel, breeding panel and full-sibs panel. Front. Plant Sci. 2022, 12, 745379. [Google Scholar] [CrossRef]
- He, X.; Li, C.; Kishii, M.; Asuke, S.; Kabir, M.R.; Roy, K.K.; Butron, R.; Chawade, A.; Tosa, Y.; Singh, P.K. A novel quantitative trait locus on chromosome 7D derived from Aegilops tauschii confers moderate field resistance to wheat blast. Phytopathology 2025, 115, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, K.; Jia, Z.; Gong, Q.; Lin, Z.; Du, L.; Pei, X.; Ye, X. Efficient induction of haploid plants in wheat by editing of TaMTL using an optimized Agrobacterium-mediated CRISPR system. J. Exp. Bot. 2020, 71, 1337–1349. [Google Scholar] [CrossRef]
- Li, S.; Xia, L. Precise gene replacement in plants through CRISPR/Cas genome editing technology: Current status and future perspectives. Abiotech 2020, 1, 58–73. [Google Scholar] [CrossRef]
- Zhan, X.; Lu, Y.; Zhu, J.K.; Botella, J.R. Genome editing for plant research and crop improvement. J. Integr. Plant Biol. 2021, 63, 3–33. [Google Scholar] [CrossRef]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; Van Der Oost, J.; Regev, A.; et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Danner, E.; Bashir, S.; Yumlu, S.; Wurst, W.; Wefers, B.; Kühn, R. Control of gene editing by manipulation of DNA repair mechanisms. Mamm. Genome 2017, 28, 262–274. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Rubayet, M.T.; Khan, S.; Mostafa, M.; Mishu, N.J.; Sabbir, M.A.A.; Akter, N.; Kabir, A.; Mostofa, M.G. White mold: A global threat to crops and key strategies for its sustainable management. Microorganisms 2024, 13, 4. [Google Scholar] [CrossRef]
- Puchta, H.; Fauser, F. Synthetic nucleases for genome engineering in plants: Prospects for a bright future. Plant J. 2014, 78, 727–741. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; He, Y.; Xu, M.; Zhang, J.; Du, W.; Zhao, Y.; Xia, L. Precise gene replacement in rice by RNA transcript-templated homologous recombination. Nat. Biotechnol. 2019, 37, 445–450. [Google Scholar] [CrossRef]
- Lu, Y.; Tian, Y.; Shen, R.; Yao, Q.; Wang, M.; Chen, M.; Dong, J.; Zhang, T.; Li, F.; Lei, M.; et al. Targeted, efficient sequence insertion and replacement in rice. Nat. Biotechnol. 2020, 38, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Coaker, G.; Zhou, J.M.; Dong, X. Plant immune mechanisms: From reductionistic to holistic points of view. Mol. Plant 2020, 13, 1358–1378. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Jiao, C.; Hou, J.; Li, T.; Liu, H.; Wang, Y.; Zheng, J.; Liu, H.; Bi, Z.; Xu, F.; et al. Resequencing of 145 landmark cultivars reveals asymmetric sub-genome selection and strong founder genotype effects on wheat breeding in China. Mol. Plant 2020, 13, 1733–1751. [Google Scholar] [CrossRef]
- Li, S.; Zhang, C.; Li, J.; Yan, L.; Wang, N.; Xia, L. Present and future prospects for wheat improvement through genome editing and advanced technologies. Plant Commun. 2021, 2, 100211. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Wang, Y.; Li, J.; Gao, C. Genome editing in rice and wheat using the CRISPR/Cas system. Nat. Protoc. 2014, 9, 2395–2410. [Google Scholar] [CrossRef]
- Ouyang, X.; Hong, X.; Zhao, X.; Zhang, W.; He, X.; Ma, W.; Teng, W.; Tong, Y. Knock out of the PHOSPHATE 2 gene TaPHO2-A1 improves phosphorus uptake and grain yield under low phosphorus conditions in common wheat. Sci. Rep. 2016, 6, 29850. [Google Scholar] [CrossRef]
- Wang, W.; Simmonds, J.; Pan, Q.; Davidson, D.; He, F.; Battal, A.; Akhunova, A.; Trick, H.N.; Uauy, C.; Akhunov, E. Gene editing and mutagenesis reveal inter-cultivar differences and additivity in the contribution of TaGW2 homoeologues to grain size and weight in wheat. Theor. Appl. Genet. 2018, 131, 2463–2475. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Zhang, D.; Zhao, X.; Cao, X.; Dong, L.; Liu, J.; Chen, K.; Zhang, H.; Gao, C.; et al. Analysis of the functions of TaGW2 homoeologs in wheat grain weight and protein content traits. Plant J. 2018, 94, 857–866. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, Y.; Wu, G.; Zou, S.; Chen, Y.; Gao, C.; Tang, D. Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. Plant J. 2017, 91, 714–724. [Google Scholar] [CrossRef]
- Su, Z.; Bernardo, A.; Tian, B.; Chen, H.; Wang, S.; Ma, H.; Cai, S.; Liu, D.; Zhang, D.; Li, T.; et al. A deletion mutation in TaHRC confers Fhb1 resistance to Fusarium head blight in wheat. Nat. Genet. 2019, 51, 1099–1105. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, J.; Qin, L.; Shi, W.; Xia, G.; Liu, S. TaCYP81D5, one member in a wheat cytochrome P450 gene cluster, confers salinity tolerance via reactive oxygen species scavenging. Plant Biotechnol. J. 2020, 18, 791–804. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Torres, G.A.M.; Consoli, L.; Binneck, E.; Camilotti, G.A.; Scagliusi, S.M.M.; Deuner, C.C.; de Campos Dianese, A.; Goulart, A.C.P.; Seixas, C.D.S.; et al. Genetic and molecular basis of wheat–Magnaporthe oryzae Triticum interaction. In Wheat Blast; Kumar, S., Kashyap, P.L., Singh, G.P., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 69–104. [Google Scholar] [CrossRef]
- Ratna Madhavi, K.; Rambabu, R.; Abhilash Kumar, V.; Vijay Kumar, S.; Aruna, J.; Ramesh, S.; Sundaram, R.M.; Laha, G.S.; Sheshu Madhav, M.; Ravindra Babu, V.; et al. Marker assisted introgression of blast (Pi-2 and Pi-54) genes into the genetic background of elite, bacterial blight resistant indica rice variety, Improved Samba Mahsuri. Euphytica 2016, 212, 331–342. [Google Scholar] [CrossRef]
- Chen, X.; Jia, Y.; Jia, M.H.; Pinson, S.R.; Wang, X.; Wu, B.M. Functional interactions between major rice blast resistance genes, Pi-ta and Pi-b, and minor blast resistance quantitative trait loci. Phytopathology 2018, 108, 1095–1103. [Google Scholar] [CrossRef]
- Barman, D.; Yadav, P.; Priya, J.; Divya, B.R.; Khan, F.N.; Nagar, S.; Yadava, P.; Watts, A.; Ray, S.; Chinnusamy, V. Combating plant diseases through CRISPR-based genome-editing approaches. In Advances in Plant Disease Management; CRC Press: Boca Raton, FL, USA, 2023; pp. 147–174. [Google Scholar]
- Maciel, J.L.N. Magnaporthe oryzae, the blast pathogen: Current status and options for its control. CABI Rev. 2012, 2011, 1–8. [Google Scholar] [CrossRef]
- Marangoni, M.S.; Nunes, M.P.; Fonseca, N., Jr.; Mehta, Y.R. Pyricularia blast on white oats: A new threat to wheat cultivation. Trop. Plant Pathol. 2013, 38, 198–202. [Google Scholar] [CrossRef]
- Ha, X.; Koopmann, B.; von Tiedemann, A. Wheat blast and Fusarium head blight display contrasting interaction patterns on ears of wheat genotypes differing in resistance. Phytopathology 2016, 106, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Campos, M.; Góngora-Canul, C.; Das, S.; Kabir, M.; Valent, B.; Cruz, C. Epidemiological criteria to support breeding tactics against the emerging, high-consequence wheat blast disease. Plant Dis. 2020, 104, 2252–2261. [Google Scholar] [CrossRef]
- CGIAR. WHEAT Launches 2019 Annual Report. 2019. Available online: https://archive.wheat.org/wheat-launches-2019-annual-report/ (accessed on 5 March 2025).
- Hossain, A.; Mottaleb, K.A.; Fafhad, M.; Barma, N.D. Mitigating the twin problems of malnutrition and wheat blast by one wheat variety, “BARI Gom 33”, in Bangladesh. Acta Agrobot. 2019, 72, 2. [Google Scholar] [CrossRef]
- Harun-Or-Rashid, M.; Meah, M.B.; Uddin, M.I.; Ahmed, S.; Kashem, M.A. Gamma radiated wheat for combating devastating blast disease (Magnaporthe oryzae Triticum) in Bangladesh. Agric. Sci. 2019, 1, 1. [Google Scholar] [CrossRef]
- IAEA. NEW CRP: Disease Resistance in Rice and Wheat for Better Adaptation to Climate Change (D23032). 2018. Available online: https://www.iaea.org/newscenter/news/new-crp-disease-resistance-in-rice-and-wheat-for-better-adaptation-to-climate-change-d23032 (accessed on 3 March 2025).
- Brunetta, D.; Bassoi, M.C.; Dotto, S.R.; Scheeren, P.L.; Miranda, M.Z.D.; Tavares, L.C.V.; Miranda, L.C. Characteristics and agronomic performance of wheat cultivar BRS 229 in Paraná State, Brazil. Pesqui. Agropecuária Bras. 2006, 41, 889–892. [Google Scholar] [CrossRef]
- Prestes, A.M.; Arendt, P.F.; Fernandes, J.M.C.; Scheeren, P.L. Resistance to Magnaporthe grisea among Brazilian wheat genotypes. In Wheat Production in Stressed Environments; Buck, H.T., Nisi, J.E., Salomón, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 119–123. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Mohler, V.; Kohli, M. Advances in control of wheat diseases: Fusarium head blight, wheat blast and powdery mildew. In Achieving Sustainable Cultivation of Wheat; Langridge, P., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; pp. 345–370. [Google Scholar] [CrossRef]
- Savadi, S.; Prasad, P.; Kashyap, P.L.; Bhardwaj, S.C. Molecular breeding technologies and strategies for rust resistance in wheat (Triticum aestivum) for sustained food security. Plant Pathol. 2018, 67, 771–791. [Google Scholar] [CrossRef]
- Inoue, Y.; Vy, T.T.; Yoshida, K.; Asano, H.; Mitsuoka, C.; Asuke, S.; Anh, V.L.; Cumagun, C.J.; Chuma, I.; Terauchi, R.; et al. Evolution of the wheat blast fungus through functional losses in a host specificity determinant. Science 2017, 357, 80–83. [Google Scholar] [CrossRef]
- Schoen, A.; Wallace, S.; Holbert, M.F.; Brown-Guidera, G.; Harrison, S.; Murphy, P.; Sanantonio, N.; Van Sanford, D.; Boyles, R.; Mergoum, M.; et al. Reducing the generation time in winter wheat cultivars using speed breeding. Crop Sci. 2023, 63, 2079–2090. [Google Scholar] [CrossRef]
- Krattinger, S.G.; Keller, B. Molecular genetics and evolution of disease resistance in cereals. New Phytol. 2016, 212, 320–332. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Ahn, H.K.; Ding, P.; Jones, J.D. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 2021, 592, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shi, H.; Li, N.; Wei, N.; Tian, Y.; Peng, J.; Chen, X.; Zhang, L.; Zhang, M.; Dong, H. Aquaporin OsPIP2;2 links the H2O2 signal and a membrane-anchored transcription factor to promote plant defense. Plant Physiol. 2022, 188, 2325–2341. [Google Scholar] [CrossRef]
- Kourelis, J.; Van Der Hoorn, R.A. Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. Plant Cell 2018, 30, 285–299. [Google Scholar] [CrossRef] [PubMed]


| Gene | Wheat Species | Cultivars | Chromosome | Pathotype | Isolate | Efficacy | References |
|---|---|---|---|---|---|---|---|
| RmgTd(t) | Triticum dicoccoides | Ku109 (Tat4) | - | Avena, Triticum | A mutant progeny | Confer moderate resistance | [58] |
| Rmg1(Rwt4) | T. aestivum | Norin 4 | 1D | Avena | Isolate Br58 | Confers resistance in seedlings and heads, but is temperature sensitive | [61] |
| Rmg2 | T. aestivum | Thatcher | 7A | Triticum | Isolate Br48 | Confer resistance during the seedling stage; it is temperature sensitive | [59] |
| Rmg3 | T. aestivum | Thatcher | 6B | Digitaria | Isolate Br49 | Provide high resistance even at elevated temperatures (26 °C) | [59] |
| Rmg4 | T. aestivum | Norin 4 | 4A | Digitaria | Unkniwn isolate | Provide high resistance even at elevated temperatures (26 °C) | [62] |
| Rmg5 | T. aestivum | 6D | Digitaria | Unknown isolate | Confer resistance in seedlings and heads, but temperature-sensitive | [62] | |
| Rmg6(Rwt3) | T. aestivum | Red Egyptian | 1D | Lolium, Eleusine, Avenae | Ryegrass isolate TP2 | Confer resistance at the heading stage, but ineffective at 26 °C | [63] |
| Rmg7 | T. dicoccum | Norin 4 | 2A | Triticum | Br48 | Confer resistance at the heading stage, but ineffective at 26 °C | [60] |
| Rmg8 | T. aestivum | KU120 (St24), KU112 (ST17), KU122 (ST25) | 2B | Triticum | Br49 | Confer resistance at the heading stage and even at 26 °C | [64,65] |
| RmgGR119 | Albanian Wheat | S-615 | - | Triticum | Br50 | Confer high resistance to all Triticum isolates tested | [66] |
| Rwt1, Rwt2, Rwt5 | T. aestivum (implied) | Not specified | - | Setaria, Oryzae | - | Host-specificity barriers; recognize effectors (PWT3, PWT4) | [67,68] |
| QTL Number | DNA Markers a | Mapping Population | Reference |
|---|---|---|---|
| QWbr.emt-2 a | KASP and SSRs | Backcross population | [81] |
| QPag.emt-2 a | |||
| QWbr.emt-5B | |||
| QWbr.emt-7B | |||
| Loco 2AS | DArTSeq and STS | Backcross population | [76] |
| Loco 2DL | |||
| Loco 7AL | |||
| Loco 7DS | |||
| Loco 2AS | SNP | Designed panel | [78] |
| Loco 3BL | |||
| Loco 4AL | |||
| Loco 7BL | |||
| Loco 1AS | SNP | Designed panel | [70] |
| Loco 2BL | |||
| Loco 3AL | |||
| Loco 4BS | |||
| Loco 4DL | |||
| Loco 7BS | |||
| Loco 2A | SNP | Designed panel | [84] |
| Loco 1BS | SNP and STS | Designed panel | [76] |
| Loco 2AS | |||
| Loco 6BS | |||
| Loco 7BL | |||
| Loco 1A | SNP | Designed panel | [83] |
| Loco 2B | |||
| Loco 4A | |||
| Loco 5A |
| Gene/QTL | Chromosome | Marker Type | Flanking/Associated Markers | Validation & Utility | Reference |
|---|---|---|---|---|---|
| Rmg8 | 2BL | SSR | Xwmc317–Xbarc159 | Flanking SSRs identified via bulked segregant analysis; suitable for MAS in segregating and breeding populations | [64] |
| Rmg7 | 2AL | SSR | Xcfd50–Xhbg327 | Flanking SSRs validated in segregating lines; suitable for tracking in variety screening | [64] |
| Non-2NS SNPs (GWAS) | 2BS, 5AL, 7AL | SNP (DArTseq) | 2B_180938790; 5A_618682953; 7A_752501634 | Repeatedly detected across 12 multi-location trials; genotypes carrying all three alleles showed <30% wheat blast index | [85] |
| Qwb.cim-7D | 7DL | KASP | K3222157–K1061589 | Major QTL explaining up to 50.6% variation; KASP markers developed and validated for high-throughput MAS | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.M.; Sultana, F.; Mostafa, M.; Khan, I.; Tran, L.-S.P.; Mostofa, M.G. Reinforced Defenses: R-Genes, PTI, and ETI in Modern Wheat Breeding for Blast Resistance. Int. J. Mol. Sci. 2025, 26, 10078. https://doi.org/10.3390/ijms262010078
Hossain MM, Sultana F, Mostafa M, Khan I, Tran L-SP, Mostofa MG. Reinforced Defenses: R-Genes, PTI, and ETI in Modern Wheat Breeding for Blast Resistance. International Journal of Molecular Sciences. 2025; 26(20):10078. https://doi.org/10.3390/ijms262010078
Chicago/Turabian StyleHossain, Md. Motaher, Farjana Sultana, Mahabuba Mostafa, Imran Khan, Lam-Son Phan Tran, and Mohammad Golam Mostofa. 2025. "Reinforced Defenses: R-Genes, PTI, and ETI in Modern Wheat Breeding for Blast Resistance" International Journal of Molecular Sciences 26, no. 20: 10078. https://doi.org/10.3390/ijms262010078
APA StyleHossain, M. M., Sultana, F., Mostafa, M., Khan, I., Tran, L.-S. P., & Mostofa, M. G. (2025). Reinforced Defenses: R-Genes, PTI, and ETI in Modern Wheat Breeding for Blast Resistance. International Journal of Molecular Sciences, 26(20), 10078. https://doi.org/10.3390/ijms262010078







