Age-Associated Proteomic Changes in Human Spermatozoa
Abstract
1. Introduction
2. Results
2.1. Semen Analysis
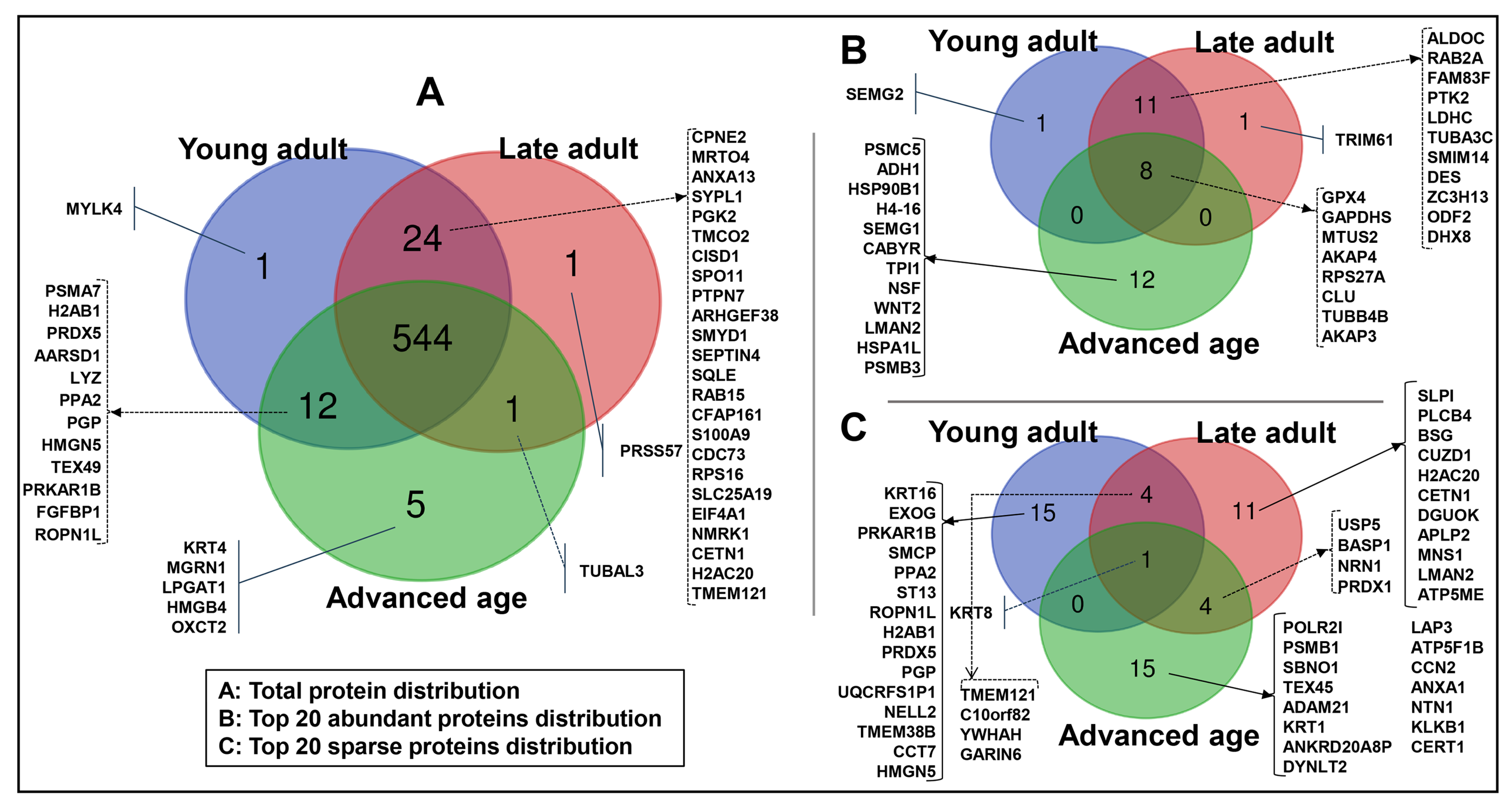
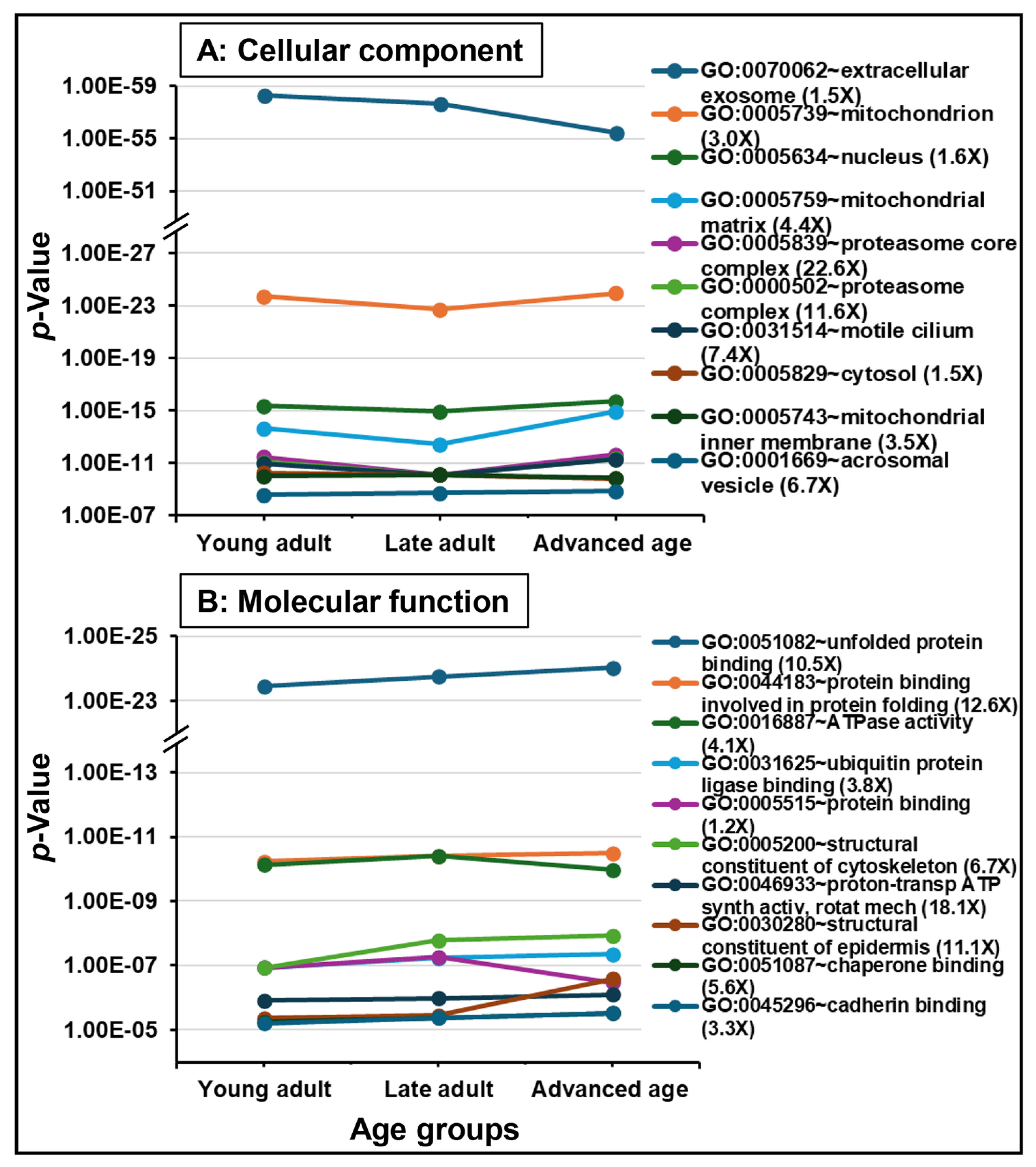
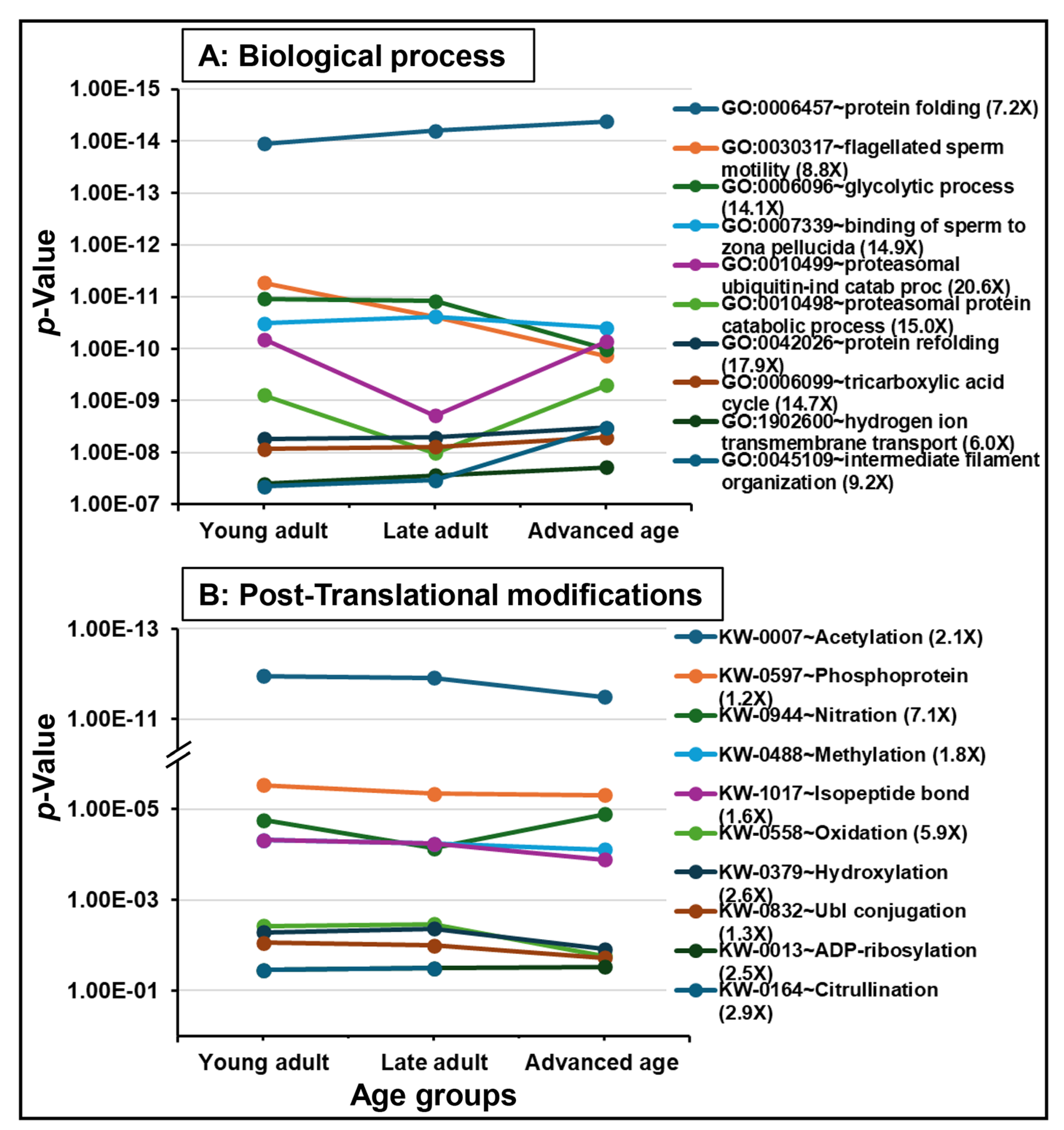
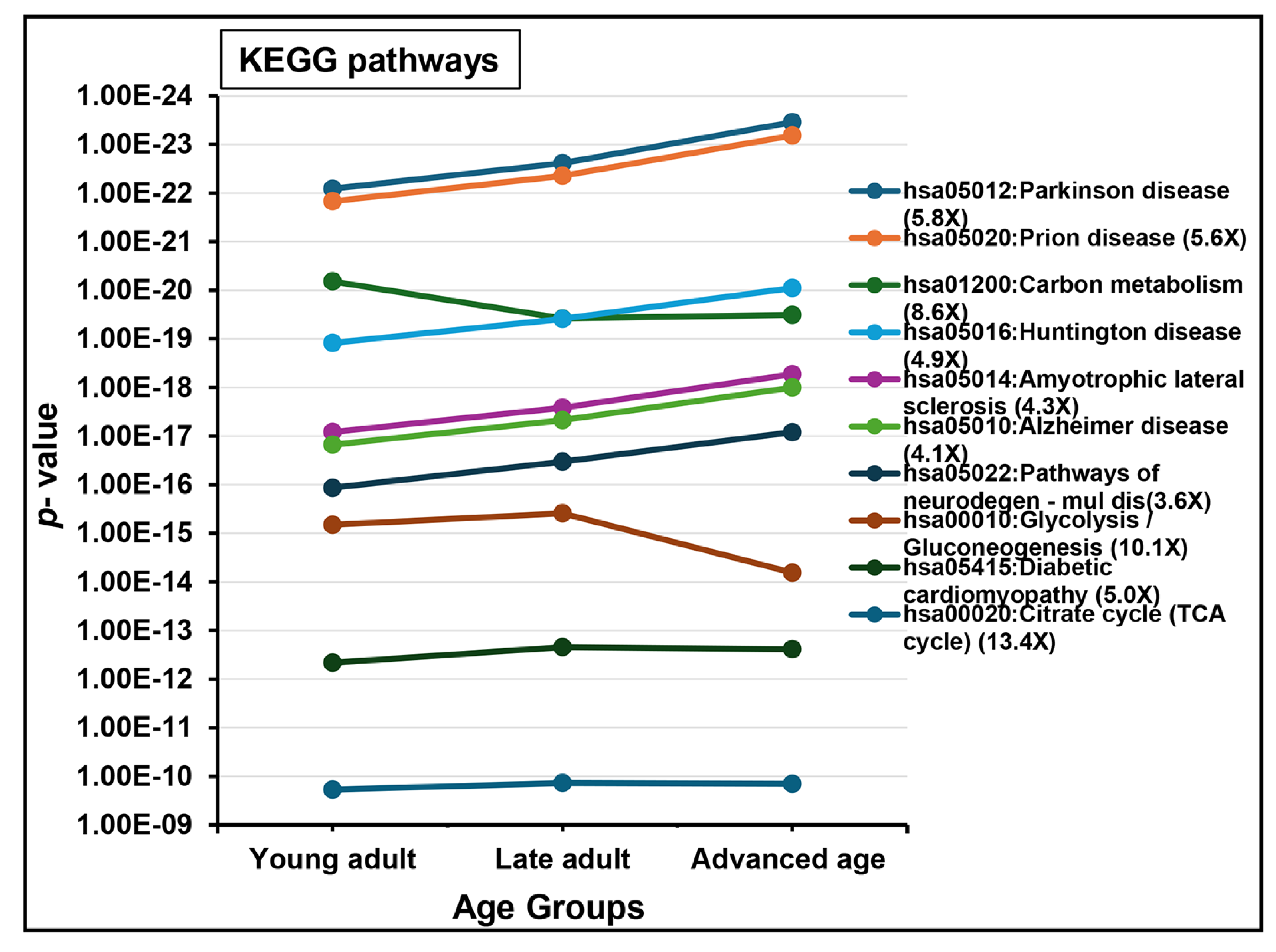
2.2. Protein Detection and Functional Analysis
3. Discussion
3.1. Semen Characteristics
3.2. Spermatozoal Proteome
3.3. Proteins Specific to Age Groups
3.4. Functional Enrichment and Key Aging-Related Proteins
4. Materials and Methods
4.1. Study Design and Sample Collection
4.2. Semen Analysis, Processing, and Sperm Purification
4.3. Proteome Analysis
4.3.1. Sperm Cell Preparation and Protein Extraction
4.3.2. In-Solution Protein Digestion
4.3.3. Protein Identification Using Mass Spectrometry-LC-MS/MS
4.4. Statistical Analysis, LC-MS/MS Data Analysis, and Bioinformatics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, R.; Agarwal, A.; Rohra, V.K.; Assidi, M.; Abu-Elmagd, M.; Turki, R.F. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod. Biol. Endocrinol. 2015, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, M.; Kunisaki, J.; Ghaed, M.; Yu, V.; Flores, H.A.; Hotaling, J.M. Fertility in the aging male: A systematic review. Fertil. Steril. 2022, 118, 1022–1034. [Google Scholar] [CrossRef]
- Martins da Silva, S.; Anderson, R.A. Reproductive axis ageing and fertility in men. Rev. Endocr. Metab. Disord. 2022, 23, 1109–1121. [Google Scholar] [CrossRef]
- Lahimer, M.; Montjean, D.; Cabry, R.; Capelle, S.; Lefranc, E.; Bach, V.; Ajina, M.; Ben Ali, H.; Khorsi-Cauet, H.; Benkhalifa, M. Paternal age matters: Association with sperm criteria, spermatozoa DNA integrity and methylation profile. J. Clin. Med. 2023, 12, 4928. [Google Scholar] [CrossRef]
- Aitken, R.J. Paternal age, de novo mutations, and offspring health? New directions for an ageing problem. Hum. Reprod. 2024, 39, 2645–2654. [Google Scholar] [CrossRef]
- Valizade, K.; Bayram, H.; Donmez Cakil, Y.; Selam, B.; Cincik, M. Age-related semen parameters and ICSI pregnancy outcomes of 8046 men in Turkey over a 9-year period. Aging Male 2024, 27, 2374724. [Google Scholar] [CrossRef]
- Verón, G.L.; Tissera, A.D.; Bello, R.; Beltramone, F.; Estofan, G.; Molina, R.I.; Vazquez-Levin, M.H. Impact of age, clinical conditions, and lifestyle on routine semen parameters and sperm kinematics. Fertil. Steril. 2018, 110, 68–75. [Google Scholar] [CrossRef]
- Kleshchev, M.; Osadchuk, L.; Osadchuk, A. Age-related changes in sperm morphology and analysis of multiple sperm defects. Front. Biosci. (Schol. Ed.) 2023, 15, 12. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, L.; Qiao, J. Effect of advanced parental age on pregnancy outcome and offspring health. J. Assist. Reprod. Genet. 2022, 39, 1969–1986. [Google Scholar] [CrossRef]
- Ketchem, J.M.; Bowman, E.J.; Isales, C.M. Male sex hormones, aging, and inflammation. Biogerontology 2023, 24, 1–25. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Barbagallo, F.; Alamo, A.; Mongioì, L.M.; Cannarella, R.; Aversa, A.; Calogero, A.E. Bio-functional sperm parameters: Does age matter? Front. Endocrinol. 2020, 11, 558374. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Wang, Y.F.; Liu, J.X.; Pan, Y.Y.; Liu, Y.F.; He, Z.C.; Mo, F.F.; Li, J.; Kang, L.H.; Gu, Y.J.; et al. Comparative analysis of proteomes between diabetic and normal human sperm: Insights into the effects of diabetes on male reproduction based on the regulation of mitochondria-related proteins. Mol. Reprod. Dev. 2018, 85, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Ameratunga, D.; Gebeh, A.; Amoako, A. Obesity and male infertility. Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 90, 102393. [Google Scholar] [CrossRef] [PubMed]
- Rabijewski, M. Male-specific consequences of obesity—Functional hypogonadism and fertility disorders. Endokrynol. Pol. 2023, 74, 480–489. [Google Scholar] [CrossRef]
- Schmid, T.E.; Grant, P.G.; Marchetti, F.; Weldon, R.H.; Eskenazi, B.; Wyrobek, A.J. Elemental composition of human semen is associated with motility and genomic sperm defects among older men. Hum. Reprod. 2013, 28, 274–282. [Google Scholar] [CrossRef]
- Wu, S.; Wu, F.; Ding, Y.; Hou, J.; Bi, J.; Zhang, Z. Advanced parental age and autism risk in children: A systematic review and meta-analysis. Acta Psychiatr. Scand. 2017, 135, 29–41. [Google Scholar] [CrossRef]
- Smith, K.R. Paternal age bioethics. J. Med. Ethics 2015, 41, 775–779. [Google Scholar] [CrossRef]
- Pennings, G.; Couture, V.; Ombelet, W. Social sperm freezing. Hum. Reprod. 2021, 36, 833–839. [Google Scholar] [CrossRef]
- Khandwala, Y.S.; Zhang, C.A.; Lu, Y.; Eisenberg, M.L. The age of fathers in the USA is rising: An analysis of 168,867,480 births from 1972 to 2015. Hum. Reprod. 2017, 32, 2110–2116. [Google Scholar] [CrossRef]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.; Driscoll, A.K.; Mathews, T.J. Births: Final data for 2015. Natl. Vital Stat. Rep. 2017, 66, 1. [Google Scholar]
- WHO. Ageing and Health, World Health Organization. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 21 February 2025).
- Abusaaq, H.I.; Population Aging in Saudi Arabia. SAMA Working Paper, Economic Research Department, Saudi Arabian Monetary Agency, Kingdom of Saudi Arabia 2015. Available online: https://www.sama.gov.sa/en-US/EconomicResearch/WorkingPapers/population%20aging%20in%20saudi%20arabia.pdf (accessed on 21 February 2025).
- Karlin, N.J.; Weil, J.; Felmban, W. Aging in Saudi Arabia: An exploratory study of contemporary older persons’ views about daily life, health, and the experience of aging. Gerontol. Geriatr. Med. 2016, 2, 2333721415623911. [Google Scholar] [CrossRef] [PubMed]
- Al-Khraif, R.; Salam, A.A.; Rashid, M.F.A. Family demographic transition in Saudi Arabia: Emerging issues and concerns. SAGE Open 2020, 10, 2158244020914556. [Google Scholar] [CrossRef]
- Salam, A.A. Ageing in Saudi Arabia: New dimensions and intervention strategies. Sci. Rep. 2023, 13, 4035. [Google Scholar] [CrossRef] [PubMed]
- KSA 2025. Saudi Arabia (KSA) Population Statistics 2025 [Infographics]. Available online: https://www.globalmediainsight.com/blog/saudi-arabia-population-statistics/ (accessed on 20 June 2025).
- O’Neill, A.; Saudi Arabia—Total Population from 2019 to 2029. Statista 2025. Available online: https://www.statista.com/statistics/262467/total-population-of-saudi-arabia/ (accessed on 21 February 2025).
- Brandt, J.S.; Cruz Ithier, M.A.; Rosen, T.; Ashkinadze, E. Advanced paternal age, infertility, and reproductive risks: A review of the literature. Prenat. Diagn. 2019, 39, 81–87. [Google Scholar] [CrossRef]
- Chan, P.T.K.; Robaire, B. Advanced paternal age and future generations. Front. Endocrinol. 2022, 13, 897101. [Google Scholar] [CrossRef]
- Greenberg, D.R.; Khandwala, Y.S.; Lu, Y.; Stevenson, D.K.; Shaw, G.M.; Eisenberg, M.L. Disease burden in offspring is associated with changing paternal demographics in the United States. Andrology 2020, 8, 342–347. [Google Scholar] [CrossRef]
- Sartorius, G.A.; Nieschlag, E. Paternal age and reproduction. Hum. Reprod. Update 2010, 16, 65–79. [Google Scholar] [CrossRef]
- Gunes, S.; Hekim, G.N.; Arslan, M.A.; Asci, R. Effects of aging on the male reproductive system. J. Assist. Reprod. Genet. 2016, 33, 441–454. [Google Scholar] [CrossRef]
- O’Leary, M.P.; Rhodes, T.; Girman, C.J.; Jacobson, D.J.; Roberts, R.O.; Lieber, M.M.; Jacobsen, S.J. Distribution of the brief male sexual inventory in community men. Int. J. Impot. Res. 2003, 15, 185–191. [Google Scholar] [CrossRef]
- Kidd, S.A.; Eskenazi, B.; Wyrobek, A.J. Effects of male age on semen quality and fertility: A review of the literature. Fertil. Steril. 2001, 75, 237–248. [Google Scholar] [CrossRef]
- Johnson, S.L.; Dunleavy, J.; Gemmell, N.J.; Nakagawa, S. Consistent age-dependent declines in human semen quality: A systematic review and meta-analysis. Ageing Res. Rev. 2015, 19, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Colasante, A.; Minasi, M.G.; Scarselli, F.; Casciani, V.; Zazzaro, V.; Ruberti, A.; Greco, P.; Varricchio, M.T.; Greco, E. The aging male: Relationship between male age, sperm quality and sperm DNA damage in an unselected population of 3124 men attending the fertility centre for the first time. Arch. Ital. Urol. Androl. 2019, 90, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Pino, V.; Sanz, A.; Valdés, N.; Crosby, J.; Mackenna, A. The effects of aging on semen parameters and sperm DNA fragmentation. JBRA Assist. Reprod. 2020, 24, 82–86. [Google Scholar] [CrossRef]
- Nago, M.; Arichi, A.; Omura, N.; Iwashita, Y.; Kawamura, T.; Yumura, Y. Aging increases oxidative stress in semen. Investig. Clin. Urol. 2021, 62, 233–238. [Google Scholar] [CrossRef]
- Setti, A.S.; Braga, D.P.A.F.; Guilherme, P.; Vingris, L.; Iaconelli, A., Jr.; Borges, E., Jr. Paternal ageing impacts blastulation and the outcomes of pregnancy at different levels of maternal age: A clustering analysis of 21,960 oocytes and 3837 ICSI cycles. Andrologia 2022, 54, e14485. [Google Scholar] [CrossRef]
- Jodar, M.; Soler-Ventura, A.; Oliva, R.; Molecular Biology of Reproduction and Development Research Group. Semen proteomics and male infertility. J. Proteomics 2017, 162, 125–134. [Google Scholar] [CrossRef]
- Agarwal, A.; Panner Selvam, M.K.; Baskaran, S. Proteomic analyses of human sperm cells: Understanding the role of proteins and molecular pathways affecting male reproductive health. Int. J. Mol. Sci. 2020, 21, 1621. [Google Scholar] [CrossRef]
- Castillo, J.; de la Iglesia, A.; Leiva, M.; Jodar, M.; Oliva, R. Proteomics of human spermatozoa. Hum. Reprod. 2023, 38, 2312–2320. [Google Scholar] [CrossRef]
- Parkes, R.; Garcia, T.X. Bringing proteomics to bear on male fertility: Key lessons. Expert Rev. Proteomics 2024, 21, 181–203. [Google Scholar] [CrossRef]
- Martínez-Heredia, J.; de Mateo, S.; Vidal-Taboada, J.M.; Ballescà, J.L.; Oliva, R. Identification of proteomic differences in asthenozoospermic sperm samples. Hum. Reprod. 2008, 23, 783–791. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Lepczynski, A.; Ozgo, M.; Kamieniczna, M.; Fraczek, M.; Stanski, L.; Olszewska, M.; Malcher, A.; Skrzypczak, W.; Kurpisz, M.K. Sperm mitochondrial dysfunction and oxidative stress as possible reasons for isolated asthenozoospermia. J. Physiol. Pharmacol. 2018, 69, 403–417. [Google Scholar]
- Schon, S.B.; Moritz, L.; Rabbani, M.; Meguid, J.; Juliano, B.R.; Ruotolo, B.T.; Aston, K.; Hammoud, S.S. Proteomic analysis of human sperm reveals changes in protamine 1 phosphorylation in men with infertility. F S Sci. 2024, 5, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Agarwal, A.; Mohanty, G.; Hamada, A.J.; Gopalan, B.; Willard, B.; Yadav, S.; du Plessis, S. Proteomic analysis of human spermatozoa proteins with oxidative stress. Reprod. Biol. Endocrinol. 2013, 11, 48. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Agarwal, A.; Pushparaj, P.N.; Baskaran, S.; Bendou, H. Sperm proteome analysis and identification of fertility-associated biomarkers in unexplained male infertility. Genes 2019, 10, 522. [Google Scholar] [CrossRef]
- Liu, X.; Teng, Z.; Wang, Z.; Zhu, P.; Song, Z.; Liu, F. Expressions of HSPA1L and HSPA9 are associated with poor sperm quality of low-motility spermatozoa in fertile men. Andrologia 2022, 54, e14321. [Google Scholar] [CrossRef]
- Greither, T.; Dejung, M.; Behre, H.M.; Butter, F.; Herlyn, H. The human sperm proteome-Toward a panel for male fertility testing. Andrology 2023, 11, 1418–1436. [Google Scholar] [CrossRef]
- Jodar, M.; Attardo-Parrinello, C.; Soler-Ventura, A.; Barrachina, F.; Delgado-Dueñas, D.; Cívico, S.; Calafell, J.M.; Ballescà, J.L.; Oliva, R. Sperm proteomic changes associated with early embryo quality after ICSI. Reprod. Biomed. Online 2020, 40, 700–710. [Google Scholar] [CrossRef]
- Liu, F.J.; Liu, X.; Han, J.L.; Wang, Y.W.; Jin, S.H.; Liu, X.X.; Liu, J.; Wang, W.T.; Wang, W.J. Aged men share the sperm protein PATE1 defect with young asthenozoospermia patients. Hum. Reprod. 2015, 30, 861–869. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Hao, F.; Yang, Y.; Yang, H.; Chang, Q.; Kong, P.; Liu, W.; Jiao, X.; Teng, X. A new perspective on semen quality of aged male: The characteristics of metabolomics and proteomics. Front. Endocrinol. 2023, 13, 1058250. [Google Scholar] [CrossRef]
- Salmon-Divon, M.; Shrem, G.; Balayla, J.; Nehushtan, T.; Volodarsky-Perel, A.; Steiner, N.; Son, W.Y.; Dahan, M.H. An age-based sperm nomogram: The McGill reference guide. Hum. Reprod. 2020, 35, 2213–2225. [Google Scholar] [CrossRef]
- Chen, G.X.; Li, H.Y.; Lin, Y.H.; Huang, Z.Q.; Huang, P.Y.; Da, L.C.; Shi, H.; Yang, L.; Feng, Y.B.; Zheng, B.H. The effect of age and abstinence time on semen quality: A retrospective study. Asian J. Androl. 2022, 24, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.D.; Ribeiro, J.C.; Ferreira, R.; Alves, M.G.; Oliveira, P.F. Understanding the age-related alterations in the testis-specific proteome. Expert Rev. Proteomics 2023, 20, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Siva, A.B.; Kameshwari, D.B.; Singh, V.; Pavani, K.; Sundaram, C.S.; Rangaraj, N.; Deenadayal, M.; Shivaji, S. Proteomics-based study on asthenozoospermia: Differential expression of proteasome alpha complex. Mol. Hum. Reprod. 2010, 16, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, M.; Joenväärä, S.; Jain, T.; Tomar, A.K.; Sinha, A.; Singh, S.; Yadav, S.; Renkonen, R. Human spermatozoa quantitative proteomic signature classifies normo- and asthenozoospermia. Mol. Cell Proteomics 2017, 16, 57–72. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, R.; Samanta, L.; Durairajanayagam, D.; Sabanegh, E. Proteomic signatures of infertile men with clinical varicocele and their validation studies reveal mitochondrial dysfunction leading to infertility. Asian J. Androl. 2016, 18, 282–291. [Google Scholar] [CrossRef]
- Sakakibara, I.; Yanagihara, Y.; Himori, K.; Yamada, T.; Sakai, H.; Sawada, Y.; Takahashi, H.; Saeki, N.; Hirakawa, H.; Yokoyama, A.; et al. Myofiber androgen receptor increases muscle strength mediated by a skeletal muscle splicing variant of Mylk4. iScience 2021, 24, 102303. [Google Scholar] [CrossRef]
- Zhang, W.; Zou, M.; Xiong, X.; Wei, Y.; Ke, C.; Li, H.; Xie, J.; Wei, Q.; Huang, J. Transcriptome analysis reveals the regulatory mechanism of myofiber development in male and female black Muscovy duck at different ages. Front. Vet. Sci. 2024, 11, 1484102. [Google Scholar] [CrossRef]
- Altab, G.; Merry, B.J.; Beckett, C.W.; Raina, P.; Lopes, I.; Goljanek-Whysall, K.; de Magalhães, J.P. Unravelling the transcriptomic symphony of muscle ageing: Key pathways and hub genes altered by ageing and caloric restriction in rat muscle revealed by RNA sequencing. BMC Genomics 2025, 26, 29. [Google Scholar] [CrossRef]
- Perera, N.C.; Schilling, O.; Kittel, H.; Back, W.; Kremmer, E.; Jenne, D.E. NSP4, an Elastase-Related Protease in Human Neutrophils with Arginine Specificity. Proc. Natl. Acad. Sci. USA 2012, 109, 6229–6234. [Google Scholar] [CrossRef]
- Kasperkiewicz, P.; Poreba, M.; Snipas, S.J.; Lin, S.J.; Kirchhofer, D.; Salvesen, G.S.; Drag, M. Design of a selective substrate and activity-based probe for human neutrophil serine protease 4. PLoS ONE 2015, 10, e0132818. [Google Scholar] [CrossRef]
- AhYoung, A.P.; Eckard, S.C.; Gogineni, A.; Xi, H.; Lin, S.J.; Gerhardy, S.; Cox, C.; Phung, Q.T.; Hackney, J.A.; Katakam, A.K.; et al. Neutrophil serine protease 4 is required for mast cell-dependent vascular leakage. Commun. Biol. 2020, 3, 687. [Google Scholar] [CrossRef]
- Barresi, V.; Di Bella, V.; Nigro, L.L.; Privitera, A.P.; Bonaccorso, P.; Scuderi, C.; Condorelli, D.F. Temporary Serine Protease Inhibition and the Role of SPINK2 in Human Bone Marrow. iScience 2023, 26, 106791. [Google Scholar] [CrossRef] [PubMed]
- Freeland, J.; Crowell, P.D.; Giafaglione, J.M.; Boutros, P.C.; Goldstein, A.S. Aging of the progenitor cells that initiate prostate cancer. Cancer Lett. 2021, 515, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Zmudzinska, A.; Wisniewski, J.; Mlynarz, P.; Olejnik, B.; Mogielnicka-Brzozowska, M. Age-dependent variations in functional quality and proteomic characteristics of Canine (Canis lupus familiaris) epididymal spermatozoa. Int. J. Mol. Sci. 2022, 23, 9143. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Xiong, C.; Li, J.; Sui, C.; Wang, S.; Li, H.; Jiang, X. The effect of mahogunin gene mutant on reproduction in male mice: A new sight for infertility? Andrologia 2014, 46, 98–105. [Google Scholar] [CrossRef]
- Gunn, T.M.; Silvius, D.; Lester, A.; Gibbs, B. Chronic and age-dependent effects of the spongiform neurodegeneration-associated MGRN1 E3 ubiquitin ligase on mitochondrial homeostasis. Mamm. Genome 2019, 30, 151–165. [Google Scholar] [CrossRef]
- Kawana, H.; Ozawa, M.; Shibata, T.; Onishi, H.; Sato, Y.; Kano, K.; Shindou, H.; Shimizu, T.; Kono, N.; Aoki, J. Identification and characterization of LPLAT7 as an sn-1-specific lysophospholipid acyltransferase. J. Lipid Res. 2022, 63, 100271. [Google Scholar] [CrossRef]
- Tabuchi, A.; Kashiwaya, N.; Sato, T.; Miura, S.; Hoshino, D.; Kano, Y. Intracellular calcium ion regulation in skeletal muscle of mice overexpressing acyltransferase LPGAT1/LPLAT7. J. Phys. Fitness Sports Med. 2024. Abstract 39. Available online: https://www.jstage.jst.go.jp/article/jpfsm/13/6/13_170/_pdf (accessed on 21 February 2025).
- Fang, T.; Shen, N.; Shi, Z.; Luo, W.; Di, Y.; Liu, X.; Ma, S.; Wang, J.; Hou, S. Biological mechanism and functional verification of key genes related to major depressive disorder and type 2 diabetes mellitus. Mamm. Genome 2025, 36, 66–82. [Google Scholar] [CrossRef]
- Shibata, T.; Kawana, H.; Nishino, Y.; Ito, Y.; Sato, H.; Onishi, H.; Kano, K.; Inoue, A.; Taketomi, Y.; Murakami, M.; et al. Abnormal male reproduction and embryonic development induced by downregulation of a phospholipid fatty acid-introducing enzyme Lpgat1 in zebrafish. Sci. Rep. 2022, 12, 7312. [Google Scholar] [CrossRef]
- Yang, L.; Li, S.; Mo, C.; Zhou, B.; Fan, S.; Shi, F.; Wei, X.; Zhao, Q.; Yang, G.; Li, S.; et al. Transcriptome analysis and identification of age-associated fertility decreased genes in hen uterovaginal junction. Poultry Sci. 2021, 100, 100892. [Google Scholar] [CrossRef]
- Catena, R.; Escoffier, E.; Caron, C.; Khochbin, S.; Martianov, I.; Davidson, I. HMGB4, a novel member of the HMGB family, is preferentially expressed in the mouse testis and localizes to the basal pole of elongating spermatids. Biol. Reprod. 2009, 80, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Junghare, V.; Hazra, S.; Singh, U.; Sengar, G.S.; Raja, T.V.; Kumar, S.; Tyagi, S.; Das, A.K.; Kumar, A.; et al. Database on spermatozoa transcriptogram of categorized Frieswal crossbred (Holstein Friesian × Sahiwal) bulls. Theriogenology 2019, 129, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Miyagawa, Y.; Tsujimura, A.; Nishimune, Y. Male infertility and single-nucleotide polymorphisms in the testis-specific succinyl CoA: 3-oxoacid CoA transferase (SCOT-T/OXCT2) gene in a Japanese cohort. Int. J. Reprod. Fertil. Sex Health 2018, S1, 1–6. [Google Scholar]
- Cheng, L.; Sun, P.; Xie, X.; Sun, D.; Zhou, Q.; Yang, S.; Xie, Q.; Zhou, X. Hepatitis B virus surface protein induces oxidative stress by increasing peroxides and inhibiting antioxidant defences in human spermatozoa. Reprod. Fertil. Dev. 2020, 32, 1180–1189. [Google Scholar] [CrossRef]
- Card, C.J.; Krieger, K.E.; Kaproth, M.; Sartini, B.L. Oligo-dT selected spermatozoal transcript profiles differ among higher and lower fertility dairy sires. Anim. Reprod. Sci. 2017, 177, 105–123. [Google Scholar] [CrossRef]
- Lu, Y.; Oura, S.; Matsumura, T.; Oji, A.; Sakurai, N.; Fujihara, Y.; Shimada, K.; Miyata, H.; Tobita, T.; Noda, T.; et al. CRISPR/Cas9-mediated genome editing reveals 30 testis-enriched genes dispensable for male fertility in mice. Biol. Reprod. 2019, 101, 501–511. [Google Scholar] [CrossRef]
- Huang, D.; Li, J.; He, L.Q. Influence of Tripterygium wilfordii on the expression of spermiogenesis related genes Herc4, Ipo11 and Mrto4 in mice. Yi Chuan 2009, 31, 941–946. [Google Scholar] [CrossRef]
- Antony, M.; Scranton, V.; Srivastava, P.; Verma, R. Micro RNA 181c-5p: A promising target for post-stroke recovery in socially isolated mice. Neurosci. Lett. 2020, 715, 134610. [Google Scholar] [CrossRef]
- Maltseva, D.V.; Raigorodskaya, M.P.; Zgoda, V.G.; Tonevitsky, E.A.; Knyazev, E.N. Intracellular transport of ribosome-inactivating proteins depends on Annexin 13. Dokl. Biochem. Biophys. 2020, 494, 219–221. [Google Scholar] [CrossRef]
- Seabright, A.P.; Fine, N.H.F.; Barlow, J.P.; Lord, S.O.; Musa, I.; Gray, A.; Bryant, J.A.; Banzhaf, M.; Lavery, G.G.; Hardie, D.G.; et al. AMPK activation induces mitophagy and promotes mitochondrial fission while activating TBK1 in a PINK1-Parkin independent manner. FASEB J. 2020, 34, 6284–6301. [Google Scholar] [CrossRef]
- Liu, X.X.; Zhang, H.; Shen, X.F.; Liu, F.J.; Liu, J.; Wang, W.J. Characteristics of testis-specific phosphoglycerate kinase 2 and its association with human sperm quality. Hum. Reprod. 2016, 31, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, W.; Qu, F.; Jing, J.; Hu, Y.; Liu, Y.; Ding, Z. Proteomic alterations underlie an association with teratozoospermia in obese mice sperm. Reprod. Biol. Endocrinol. 2019, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Beckers, A.; Fuhl, F.; Ott, T.; Boldt, K.; Brislinger, M.M.; Walentek, P.; Schuster-Gossler, K.; Hegermann, J.; Alten, L.; Kremmer, E.; et al. The highly conserved FOXJ1 target CFAP161 is dispensable for motile ciliary function in mouse and Xenopus. Sci. Rep. 2021, 11, 13333. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, J.; Liao, C.; Ni, Z.; Zheng, J.; Yu, F. System analysis of teratozoospermia mRNA profile based on integrated bioinformatics tools. Mol. Med. Rep. 2018, 18, 1297–1304. [Google Scholar] [CrossRef]
- Padyana, A.K.; Gross, S.; Jin, L.; Cianchetta, G.; Narayanaswamy, R.; Wang, F.; Wang, R.; Fang, C.; Lv, X.; Biller, S.A.; et al. Structure and inhibition mechanism of the catalytic domain of human squalene epoxidase. Nat. Commun. 2019, 10, 97. [Google Scholar] [CrossRef]
- Agarwal, A.; Durairajanayagam, D.; Halabi, J.; Peng, J.; Vazquez-Levin, M. Proteomics, oxidative stress and male infertility. Reprod. Biomed. Online 2014, 29, 32–58. [Google Scholar] [CrossRef]
- Ning, L.; Huo, Q.; Xie, N. Comprehensive analysis of the expression and prognosis for tripartite motif-containing genes in breast cancer. Front. Genet. 2022, 13, 876325. [Google Scholar] [CrossRef]
- Xue, R.; Lin, W.; Sun, J.; Watanabe, M.; Xu, A.; Araki, M.; Nasu, Y.; Tang, Z.; Huang, P. The role of Wnt signaling in male reproductive physiology and pathology. Mol. Hum. Reprod. 2021, 27, gaaa085. [Google Scholar] [CrossRef]
- Martins, A.D.; Panner Selvam, M.K.; Agarwal, A.; Alves, M.G.; Baskaran, S. Alterations in seminal plasma proteomic profile in men with primary and secondary infertility. Sci. Rep. 2020, 10, 7539. [Google Scholar] [CrossRef]
- Shuvalov, O.; Kizenko, A.; Petukhov, A.; Fedorova, O.; Daks, A.; Bottrill, A.; Snezhkina, A.V.; Kudryavtseva, A.V.; Barlev, N. SEMG1/2 augment energy metabolism of tumor cells. Cell Death Dis. 2020, 11, 1047. [Google Scholar] [CrossRef]
- Toste Rêgo, A.; da Fonseca, P.C.A. Characterization of fully recombinant human 20S and 20S-PA200 proteasome complexes. Mol. Cell 2019, 76, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.K.; Altuwaijri, S.; Lin, W.J.; Kan, P.Y.; Collins, L.L.; Chang, C. Proteasome activity is required for androgen receptor transcriptional activity via regulation of androgen receptor nuclear translocation and interaction with coregulators in prostate cancer cells. J. Biol. Chem. 2002, 277, 36570–36576. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Willis, W.D.; Brown, P.R.; Goulding, E.H.; Fulcher, K.D.; Eddy, E.M. Targeted Disruption of the Akap4 Gene Causes Defects in Sperm Flagellum and Motility. Dev. Biol. 2002, 248, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Cui, Y.; Zhang, X.; Lou, J.; Zhou, J.; Bei, H.; Wei, R. Proteomic profile of human spermatozoa in healthy and asthenozoospermic individuals. Reprod. Biol. Endocrinol. 2018, 16, 16. [Google Scholar] [CrossRef]
- Xie, Y.; Kang, R.; Klionsky, D.J.; Tang, D. GPX4 in cell death, autophagy, and disease. Autophagy 2023, 19, 2621–2638. [Google Scholar] [CrossRef]
- Flesch, F.M.; Gadella, B.M. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim. Biophys. Acta 2000, 1469, 197–235. [Google Scholar] [CrossRef]
- Rodríguez-Villamil, P.; Hoyos-Marulanda, V.; Martins, J.A.M.; Oliveira, A.N.; Aguiar, L.H.; Moreno, F.B.; Velho, A.L.; Monteiro-Moreira, A.C.; Moreira, R.A.; Vasconcelos, I.M.; et al. Purification of binder of sperm protein 1 (BSP1) and its effects on bovine in vitro embryo development after fertilization with ejaculated and epididymal sperm. Theriogenology 2016, 85, 540–554. [Google Scholar] [CrossRef]
- Mostek, A.; Westfalewicz, B.; Słowińska, M.; Dietrich, M.A.; Judycka, S.; Ciereszko, A. Differences in sperm protein abundance and carbonylation level in bull ejaculates of low and high quality. PLoS ONE 2018, 13, e0206150. [Google Scholar] [CrossRef]
- Samanta, L.; Sharma, R.; Cui, Z.; Agarwal, A. Proteomic analysis reveals dysregulated cell signaling in ejaculated spermatozoa from infertile men. Asian J. Androl. 2019, 21, 121–130. [Google Scholar]
- Samanta, L.; Swain, N.; Ayaz, A.; Venugopal, V.; Agarwal, A. Post-translational modifications in sperm proteome: The chemistry of proteome diversifications in the pathophysiology of male factor infertility. Biochim. Biophys. Acta 2016, 1860, 1450–1465. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, H.; Pan, T.; Hu, X.; Ding, J.; Chen, Y.; Li, J.; Chen, H.; Luo, T. Global proteomic analyses of lysine acetylation, malonylation, succinylation, and crotonylation in human sperm reveal their involvement in male fertility. J. Proteomics 2024, 303, 105213. [Google Scholar] [CrossRef] [PubMed]
- Mostek Majewska, A.; Majewska, A.; Janta, A.; Ciereszko, A. New insights into posttranslational modifications of proteins during bull sperm capacitation. Cell Commun. Signal. 2023, 21, 72. [Google Scholar] [CrossRef]
- Parte, P.P.; Rao, P.; Redij, S.; Lobo, V.; D’Souza, S.J.; Gajbhiye, R.; Kulkarni, V. Sperm phosphoproteome profiling by ultra-performance liquid chromatography followed by data-independent analysis (LC-MS(E)) reveals altered proteomic signatures in asthenozoospermia. J. Proteomics 2012, 75, 5861–5871. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 2017, 97, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Krejčí, J.; Stixová, L.; Pagáčová, E.; Legartová, S.; Kozubek, S.; Lochmanová, G.; Zdráhal, Z.; Sehnalová, P.; Dabravolski, S.; Hejátko, J.; et al. Post-translational modifications of histones in human sperm. J. Cell Biochem. 2015, 116, 2195–2209. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Samanta, L.; Samanta, A. Functional analysis of differentially expressed acetylated spermatozoal proteins in infertile men with unilateral and bilateral varicocele. Int. J. Mol. Sci. 2020, 21, 3155. [Google Scholar] [CrossRef]
- Singh, K.; Jaiswal, D. One-carbon metabolism, spermatogenesis, and male infertility. Reprod. Sci. 2013, 20, 622–630. [Google Scholar] [CrossRef]
- Hereng, T.H.; Elgstoen, K.B.; Cederkvist, F.H.; Eide, L.; Jahnsen, T.; Skalhegg, B.S.; Rosendal, K.R. Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa. Hum. Reprod. 2011, 26, 3249–3263. [Google Scholar] [CrossRef]
- du Plessis, S.S.; Agarwal, A.; Mohanty, G.; van der Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2015, 17, 230–235. [Google Scholar] [CrossRef]
- Shen, X.; Chen, C.; Wang, Y.; Zheng, W.; Zheng, J.; Jones, A.E.; Zhu, B.; Zhang, H.; Lyons, C.; Rijal, A.; et al. Role of histone variants H2BC1 and H2AZ.2 in H2AK119ub nucleosome organization and Polycomb gene silencing. bioRxiv 2024. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Y.; Xu, K.; Li, Y.; Riaz, F.; Lu, K.; Chen, Q.; Du, X.; Wu, L.; Cao, D.; et al. Cholesterol-induced leucine aminopeptidase 3 (LAP3) upregulation inhibits cell autophagy in pathogenesis of NAFLD. Aging 2022, 14, 3259–3275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, A.; Zhao, Z.; Chen, F.; Yan, X.; Han, Y.; Wu, D.; Wu, Y. Protein disulfide isomerase is essential for spermatogenesis in mice. JCI Insight 2024, 9, e177743. [Google Scholar] [CrossRef] [PubMed]
- Braschi, B.; Omran, H.; Witman, G.B.; Pazour, G.J.; Pfister, K.K.; Bruford, E.A.; King, S.M. Consensus nomenclature for dyneins and associated assembly factors. J. Cell Biol. 2022, 221, e202109014. [Google Scholar] [CrossRef]
- Paiano, J.; Wu, W.; Yamada, S.; Sciascia, N.; Callen, E.; Paola Cotrim, A.; Deshpande, R.A.; Maman, Y.; Day, A.; Paull, T.T.; et al. ATM and PRDM9 regulate SPO11-bound recombination intermediates during meiosis. Nat. Commun. 2020, 11, 857. [Google Scholar] [CrossRef]
- Ganetzky, R.D.; Markhard, A.L.; Yee, I.; Clever, S.; Cahill, A.; Shah, H.; Grabarek, Z.; To, T.L.; Mootha, V.K. Congenital hypermetabolism and uncoupled oxidative phosphorylation. N. Engl. J. Med. 2022, 387, 1395–1403. [Google Scholar] [CrossRef]
- Leung, M.R.; Zeng, J.; Wang, X.; Roelofs, M.C.; Huang, W.; Zenezini Chiozzi, R.; Hevler, J.F.; Heck, A.J.R.; Dutcher, S.K.; Brown, A.; et al. Structural specializations of the sperm tail. Cell 2023, 186, 2880–2896.e17. [Google Scholar] [CrossRef]
- Ghezzi, M.; Garolla, A.; Magagna, S.; Šabovich, I.; Berretta, M.; Foresta, C.; De Toni, L. Fertility outcomes and sperm-DNA parameters in metastatic melanoma survivors receiving vemurafenib or dabrafenib therapy: Case report. Front. Oncol. 2020, 10, 232. [Google Scholar] [CrossRef]
- Rahajeng, J.; Giridharan, S.S.; Cai, B.; Naslavsky, N.; Caplan, S. Important relationships between Rab and MICAL proteins in endocytic trafficking. World J. Biol. Chem. 2010, 1, 254. [Google Scholar] [CrossRef]
- Aslam, M.K.; Kumaresan, A.; Rajak, S.K.; Tajmul, M.; Datta, T.K.; Mohanty, T.K.; Srinivasan, A.; Yadav, S. Comparative proteomic analysis of Taurine, Indicine, and crossbred (Bos taurus × Bos indicus) bull spermatozoa for identification of proteins related to sperm malfunctions and subfertility in crossbred bulls. Theriogenology 2015, 84, 624–633. [Google Scholar] [CrossRef]
- Wang, H.Y.; Shen, Y.R.; Tsai, Y.C.; Wu, S.R.; Wang, C.Y.; Kuo, P.L. Proper phosphorylation of septin 12 regulates septin 4 and soluble adenylyl cyclase expression to induce sperm capacitation. J. Cell. Physiol. 2023, 238, 597–609. [Google Scholar] [CrossRef]
- Lin, Y.H.; Kuo, Y.C.; Chiang, H.S.; Kuo, P.L. The role of the septin family in spermiogenesis. Spermatogenesis 2011, 1, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Suryavathi, V.; Panneerdoss, S.; Wolkowicz, M.J.; Shetty, J.; Sherman, N.E.; Flickinger, C.J.; Herr, J.C. Dynamic changes in equatorial segment protein 1 (SPESP1) glycosylation during mouse spermiogenesis. Biol. Reprod. 2015, 92, 129. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, Y.; Murakami, M.; Inoue, N.; Satouh, Y.; Kaseda, K.; Ikawa, M.; Okabe, M. Sperm equatorial segment protein 1, SPESP1, is required for fully fertile sperm in mouse. J. Cell Sci. 2010, 123, 1531–1536. [Google Scholar] [CrossRef]
- Kosaka, A.; Yajima, Y.; Hatayama, M.; Ikuta, K.; Sasaki, T.; Hirai, N.; Yasuda, S.; Nagata, M.; Hayashi, R.; Harabuchi, S.; et al. A stealth antigen SPESP1, which is epigenetically silenced in tumors, is a suitable target for cancer immunotherapy. Cancer Sci. 2021, 112, 2705–2713. [Google Scholar] [CrossRef]
- Enoiu, S.I.; Nygaard, M.B.; Bungum, M.; Ziebe, S.; Petersen, M.R.; Almstrup, K. Expression of membrane fusion proteins in spermatozoa and total fertilisation failure during in vitro fertilisation. Andrology 2022, 10, 1317–1327. [Google Scholar] [CrossRef]
- Corda, P.O.; Moreira, J.; Howl, J.; Oliveira, P.F.; Fardilha, M.; Silva, J.V. Differential proteomic analysis of human sperm: A systematic review to identify candidate targets to monitor sperm quality. World J. Mens Health 2024, 42, 71–91. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhou, L.; Guo, Y.; Wang, F.; He, F.; Cheng, Y.; Meng, X.; Xie, H.; Zhang, Y.; Li, J. Downregulated SPESP1-driven fibroblast senescence decreases wound healing in aged mice. Clin. Transl. Med. 2024, 14, e1660. [Google Scholar] [CrossRef]
- Ning, F.; Xin, H.; Liu, J.; Lv, C.; Xu, X.; Wang, M.; Wang, Y.; Zhang, W.; Zhang, X. Structure and function of USP5: Insight into physiological and pathophysiological roles. Pharmacol. Res. 2020, 157, 104557. [Google Scholar] [CrossRef]
- Gao, S.T.; Xin, X.; Wang, Z.Y.; Hu, Y.Y.; Feng, Q. USP5: Comprehensive insights into structure, function, biological and disease-related implications, and emerging therapeutic opportunities. Mol. Cell Probes 2024, 73, 101944. [Google Scholar] [CrossRef]
- Munuce, M.J.; Marini, P.E.; Teijeiro, J.M. Expression profile and distribution of Annexin A1, A2 and A5 in human semen. Andrologia 2019, 51, e13224. [Google Scholar] [CrossRef]
- Ignotz, G.G.; Cho, M.Y.; Suarez, S.S. Annexins are candidate oviductal receptors for bovine sperm surface proteins and thus may serve to hold bovine sperm in the oviductal reservoir. Biol. Reprod. 2007, 77, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; San Gabriel, M.C.; Zini, A.; Chan, P.; O’Flaherty, C. Low amounts and high thiol oxidation of peroxiredoxins in spermatozoa from infertile men. J. Androl. 2012, 33, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.Y.; Kim, K.U.; Kwon, W.S.; Rahman, M.S.; Khatun, A.; Pang, M.G. Peroxiredoxin activity is a major landmark of male fertility. Sci. Rep. 2017, 7, 17174. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.Y.; Scarlata, E.; O’Flaherty, C. Long-term adverse effects of oxidative stress on rat epididymis and spermatozoa. Antioxidants 2020, 9, 170. [Google Scholar] [CrossRef]
- Shi, H.; Liu, J.; Zhu, P.; Wang, H.; Zhao, Z.; Sun, G.; Li, J. Expression of peroxiredoxins in the human testis, epididymis and spermatozoa and their role in preventing H₂O₂-induced damage to spermatozoa. Folia Histochem. Cytobiol. 2018, 56, 141–150. [Google Scholar] [CrossRef]
- Falahati, A.M.; Fallahi, S.; Allamehzadeh, Z.; Izadi Raieni, M.; Malekzadeh, K. Effects of date palm pollen supplementations on the expression of PRDX1 and PRDX6 genes in infertile men: A controlled clinical trial. Int. J. Fertil. Steril. 2023, 17, 201–207. [Google Scholar]
- Selvaratnam, J.S.; Robaire, B. Effects of aging and oxidative stress on spermatozoa of superoxide-dismutase 1- and catalase-null mice. Biol. Reprod. 2016, 95, 60. [Google Scholar] [CrossRef]
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Coviello, A.D.; Bremner, W.J.; McKinlay, J.B. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts male aging study. J. Clin. Endocrinol. Metab. 2002, 87, 589–598. [Google Scholar] [CrossRef]
- Ha, A.S.; Scott, M.; Zhang, C.A.; Li, S.; Langroudi, A.P.; Glover, F.; Basran, S.; Del Giudice, F.; Shaw, G.M.; Eisenberg, M.L. Sociodemographic Trends and Perinatal Outcomes in Fathers 50 Years and Older. JAMA Netw. Open 2024, 7, e2425269. [Google Scholar] [CrossRef]
- WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO Press, World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Alaiya, A.; Fox, J.; Bobis, S.; Matic, G.; Shinwari, Z.; Barhoush, E.; Márquez, M.; Nilsson, S.; Holmberg, A.R. Proteomic analysis of soft tissue tumor implants treated with a novel polybisphosphonate. Cancer Genom. Proteom. 2014, 11, 39–49. [Google Scholar]
- Alaiya, A.; Alshukairi, A.; Shinwari, Z.; Al-Fares, M.; Alotaibi, J.; AlOmaim, W.; Alsharif, I.; Bakheet, R.; Alharbi, L.; Allam, R.; et al. Alterations in the plasma proteome induced by SARS-CoV-2 and MERS-CoV reveal biomarkers for disease outcomes for COVID-19 patients. J. Inflamm. Res. 2021, 14, 4313–4328. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]


| Endpoints | Young Adult Group (n = 6) | Late Adult Group (n = 7) | Advanced Age Group (n = 5) | p-Value |
|---|---|---|---|---|
| Age (years) | 27.8 ± 2.6 a | 34.3 ± 2.9 b | 47.2 ± 4.2 c | 0.001 |
| BMI (kg/m2) | 28.68 ± 3.75 | 26.33 ± 2.88 | 25.90 ± 2.05 | 0.114 |
| Abstinence (days) | 4.2 ± 0.48 | 4.1 ± 2.0 | 3.6 ± 0.9 | 0.489 |
| Total Volume (mL) | 3.33 ± 1.84 | 2.47 ± 0.80 | 5.10 ± 2.79 | 0.141 |
| Sperm conc. (×106/mL) | 55.3 ± 25.54 a | 85.4 ± 22.06 b | 78.0 ± 38.24 ab | 0.022 |
| Total motility (%) | 63.17 ± 17.91 | 69.86 ± 9.06 | 67.40 ± 12.88 | 0.201 |
| Progression (%) | 39.83 ± 10.44 | 45.43 ± 13.79 | 48.40 ± 12.26 | 0.217 |
| Total sperm/Ejac. (×106) | 194.33 ±18.21 | 217 ± 11.36 | 337.90 ± 18.71 | 0.396 |
| Total motile sperm (×106) | 142.05 ± 19.21 | 153.44 ± 84.93 | 220.65 ± 115.68 | 0.443 |
| Normal morphology (%) | 4.5 ± 0.55 | 6.0 ± 3.61 | 5.4 ± 1.14 | 0.169 |
| (A) Key Enriched Biological Functions | Selected DEPs in Young Adult Group (YA) | |||
| Categories | FDR | Proteins | p-Values | YA/AA |
| kw-0007~Acetylation | 4.2 × 10−3 | Squalene monooxygenase (SQLE) | 9.5 × 10−10 | ND in AA |
| Kw-0597~Phosphoprotein | 1.3 × 10−2 | Histone H2B type 1 (H2BC1) | 8.4 × 10−6 | 982.5 |
| GO:0005739~mitochondrion | 2.1 × 10−3 | Cytosol aminopeptidase (LAP3) | 4.2 × 10−4 | 10397 |
| Protein d. isylfide-isomeraseA4 (PDIA4) | 1.4 × 10−4 | 12.6 | ||
| Lactotransferrin (LTF) | 3.5 × 10−4 | 75.9 | ||
| Dynein light chain Tctex-type protein 2 (DYNLT2) | 5.4 × 10−3 | 272.6 | ||
| (B) Key Enriched Biological Functions | Selected DEPs in Late Adult Group (LA) | |||
| Categories | FDR | Proteins | p-Values | LA/AA |
| GO:0070062~extracellular exosome | 9.2 × 10−6 | Meiotic recombination protein (SPO11) | 2.4 × 10−11 | ND in AA |
| KW-0007~Acetylation | 2.3 × 10−4 | ATP synthase subunit beta (ATP5F1B) | 1.5 × 10−6 | 12674.5 |
| GO:0005634~nucleus | 2.8 × 10−2 | Testis-expressed protein 45 (TEX45) | 4.1 × 10−4 | 5250.2 |
| hsa01200:Carbon metabolism | 2.8 × 10−2 | Calcium-binding protein 5 (CABP5) | 2.3 × 10−7 | 0.5 |
| KW-1017~Isopeptide bond | 3.3 × 10−2 | E3 ubiquitin-protein ligase MGRN1 (MGRN1) | 1.0 × 10−6 | ND in LA |
| Serine/threonine-protein kinase B-raf (BRAF) | 3.42 × 10−3 | 0.3 | ||
| (C) Key Enriched Biological Functions | Selected DEPs in Advanced Age Group (AA) | |||
| Categories | FDR | Proteins | p-Values | AA/LA |
| GO:0070062~extracellular exosome | 4.6 × 10−7 | Ras-related protein Rab-15 (RAB15) | 1.1 × 10−9 | ND in AA |
| KW-0007~Acetylation | 7.1 × 10−5 | Septin-4 (SEPTIN4) | 5.2 × 10−10 | ND in AA |
| GO:0005739~mitochondrion | 7.2 × 10−4 | Sperm equatorial segment protein 1 (SPESP1) | 2.4 × 10−6 | 0.003 |
| GO:0031514~motile cilium | 9.3 × 10−3 | Ubiquitin carboxyl-terminal hydrolase 5 (USP5) | 1.9 × 10−3 | 0.4 |
| KW-0597~Phosphoprotein | 2.2 × 10−3 | Annexin A1(ANXA1) | 2.3 × 10−3 | 0.03 |
| GO:0005856~cytoskeleton | 4.5 × 10−3 | Peroxiredoxin-1 (PRDX1) | 2.5 × 10−3 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beg, M.A.; Ismail, A.O.; Alaiya, A.; Khan, F.A.; Hamoda, T.A.-A.A.-M.; Sheikh, I.A.; Sharma, P.; Baothman, O.M.; Alkhzaim, A.H.; Shinwari, Z.; et al. Age-Associated Proteomic Changes in Human Spermatozoa. Int. J. Mol. Sci. 2025, 26, 6099. https://doi.org/10.3390/ijms26136099
Beg MA, Ismail AO, Alaiya A, Khan FA, Hamoda TA-AA-M, Sheikh IA, Sharma P, Baothman OM, Alkhzaim AH, Shinwari Z, et al. Age-Associated Proteomic Changes in Human Spermatozoa. International Journal of Molecular Sciences. 2025; 26(13):6099. https://doi.org/10.3390/ijms26136099
Chicago/Turabian StyleBeg, Mohd Amin, Abrar Osama Ismail, Ayodele Alaiya, Firdous Ahmad Khan, Taha Abo-Almagd Abdel-Meguid Hamoda, Ishfaq Ahmad Sheikh, Priyanka Sharma, Omar Mohammed Baothman, Ali Hasan Alkhzaim, Zakia Shinwari, and et al. 2025. "Age-Associated Proteomic Changes in Human Spermatozoa" International Journal of Molecular Sciences 26, no. 13: 6099. https://doi.org/10.3390/ijms26136099
APA StyleBeg, M. A., Ismail, A. O., Alaiya, A., Khan, F. A., Hamoda, T. A.-A. A.-M., Sheikh, I. A., Sharma, P., Baothman, O. M., Alkhzaim, A. H., Shinwari, Z., Abuzinadah, R. F., Mohammed, A., Assiri, A. M., Abuzenadah, A. M., Memili, E., & Feugang, J. M. (2025). Age-Associated Proteomic Changes in Human Spermatozoa. International Journal of Molecular Sciences, 26(13), 6099. https://doi.org/10.3390/ijms26136099








