New Insights into the Pro-Inflammatory and Osteoclastogenic Profile of Circulating Monocytes in Osteoarthritis Patients
Abstract
1. Introduction
2. Results
2.1. Characteristics of OA Patients
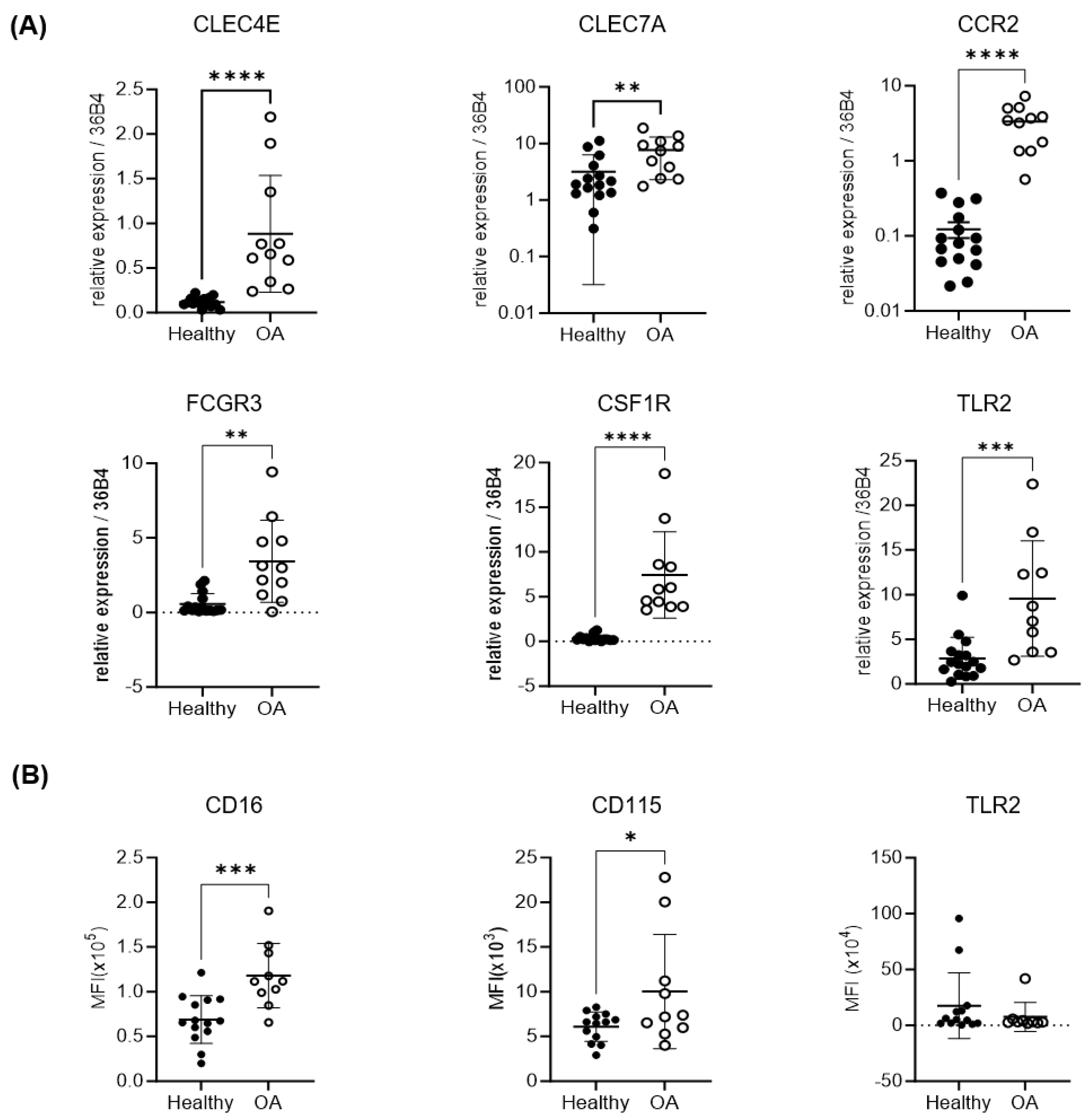
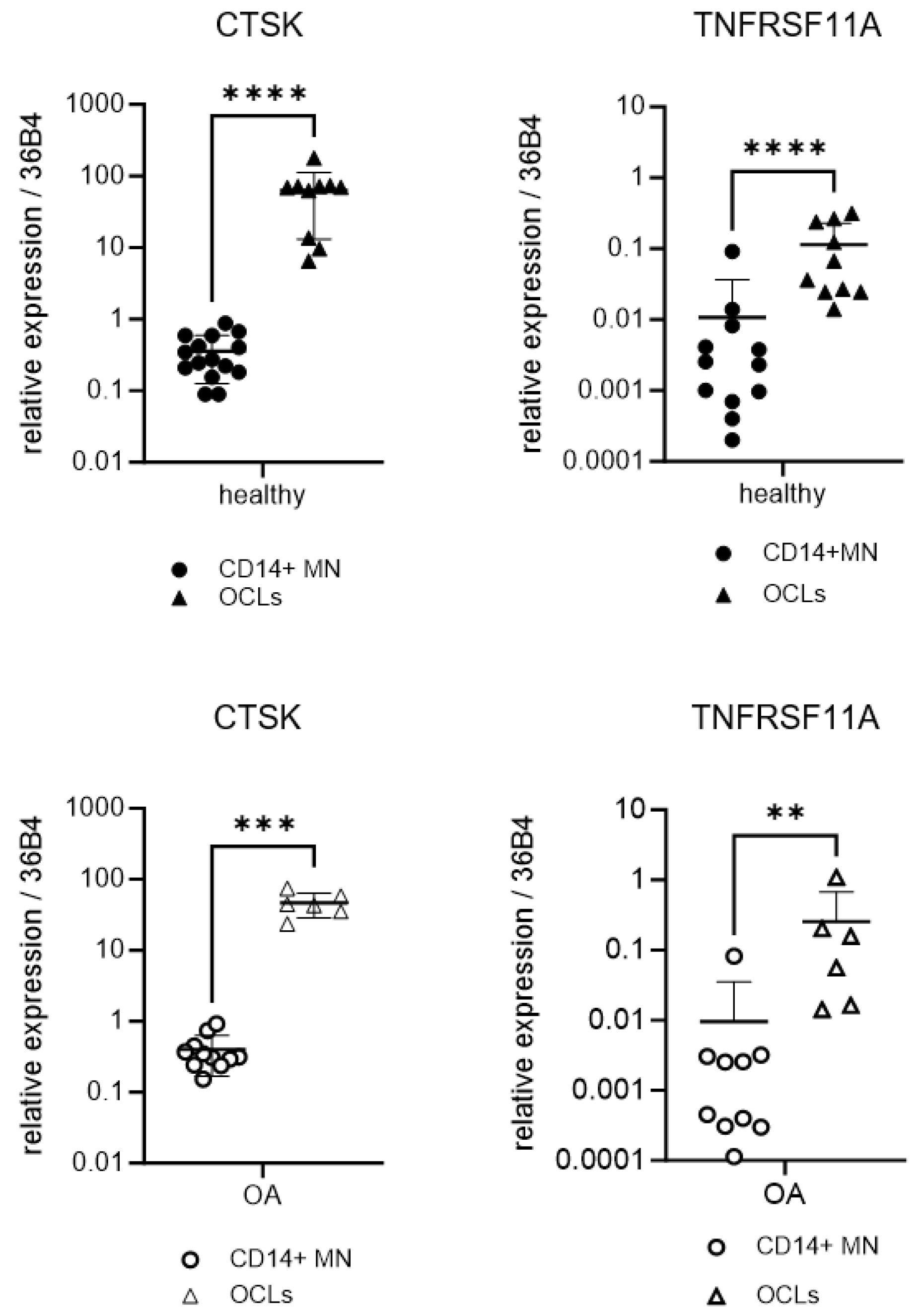
2.2. Expression of Osteoclastogenic and Inflammatory Markers of OA Monocytes
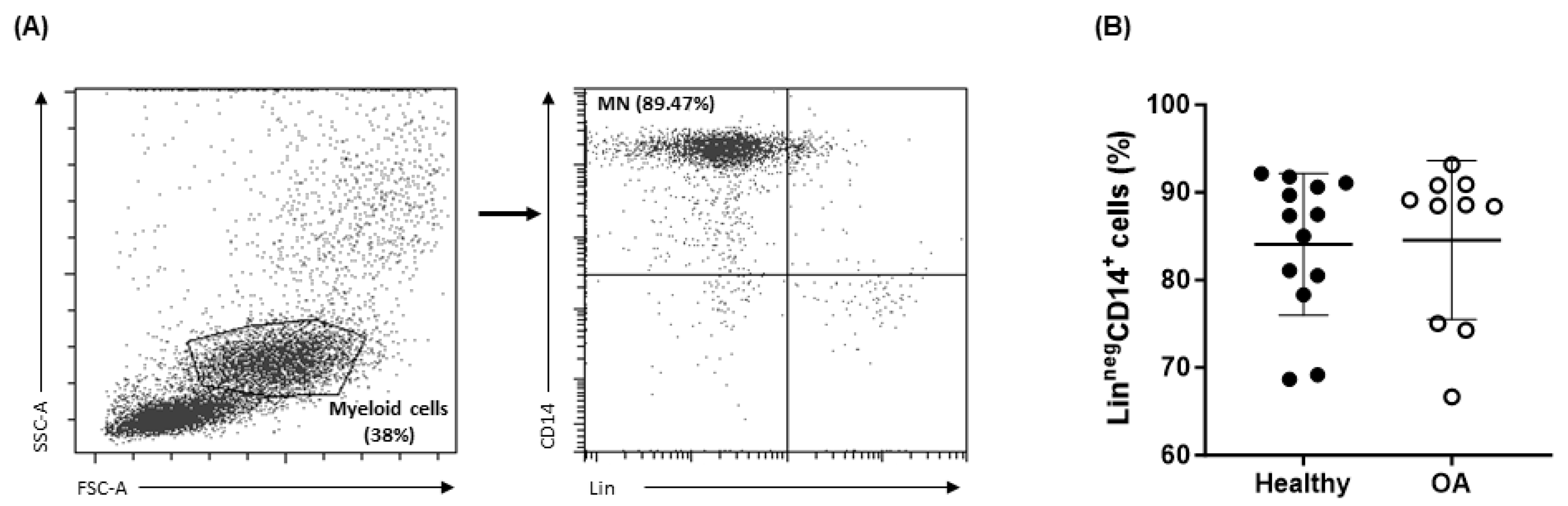
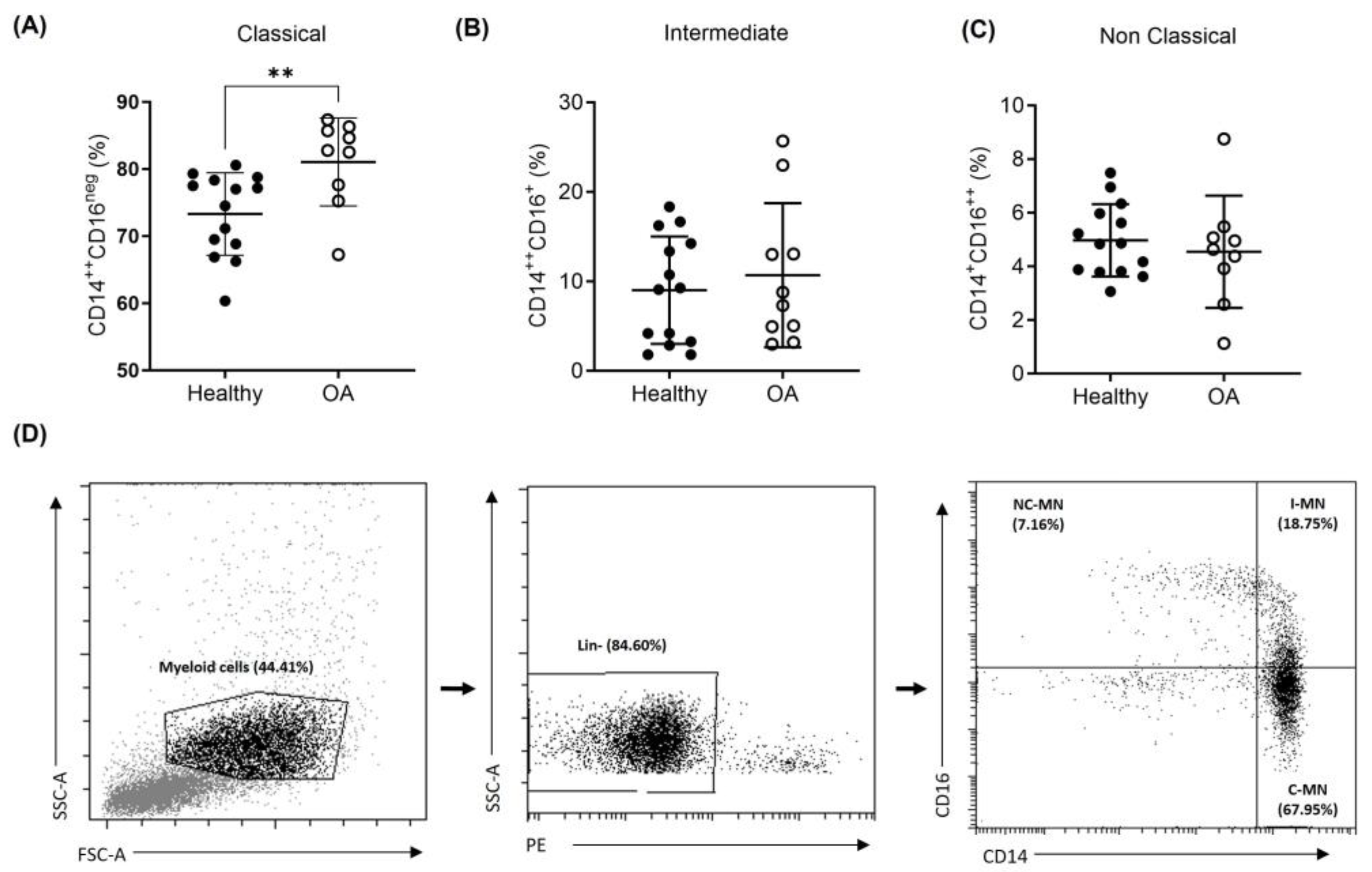
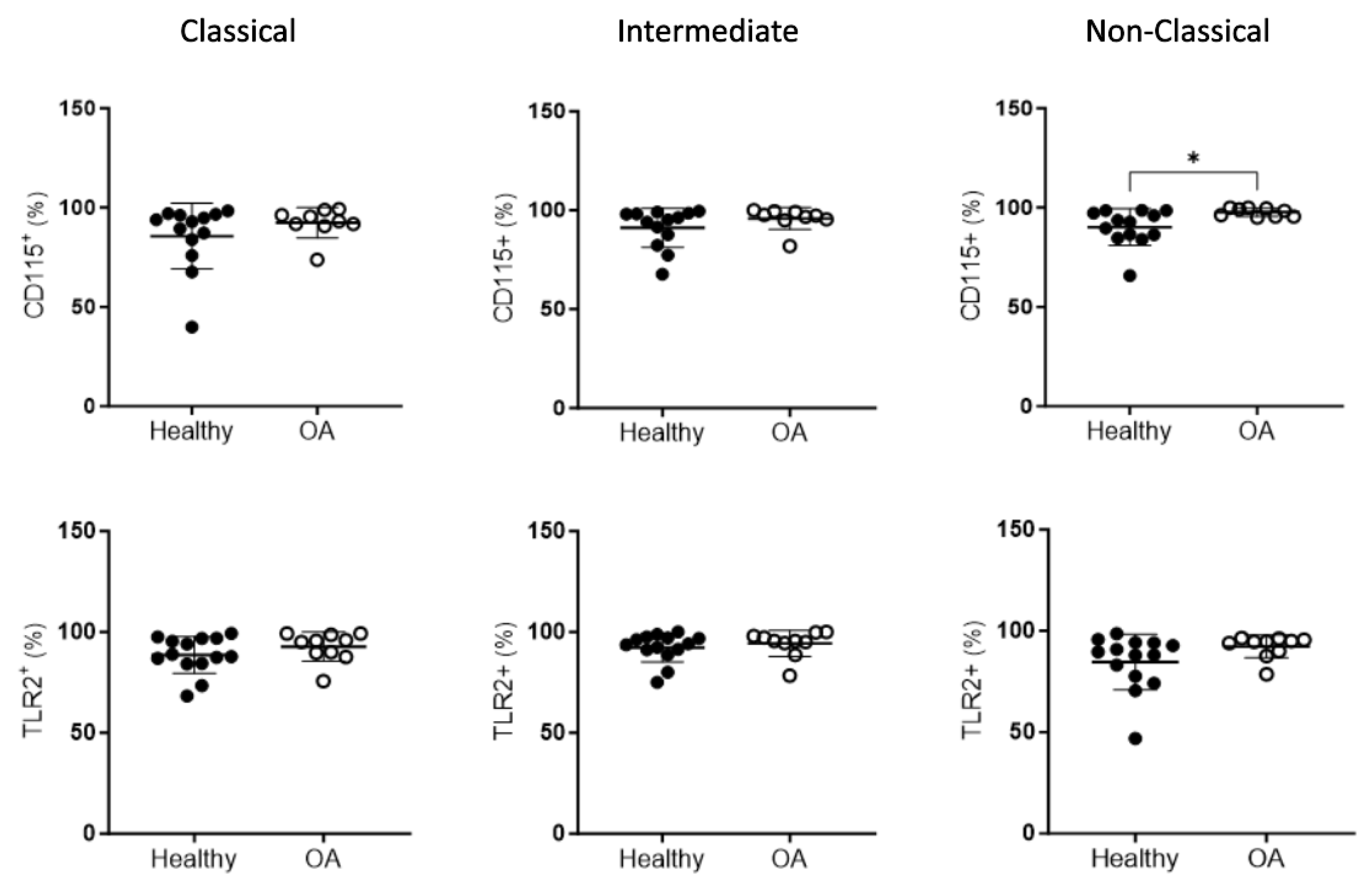
2.3. Peripheral Blood Monocyte Subsets in OA Patients
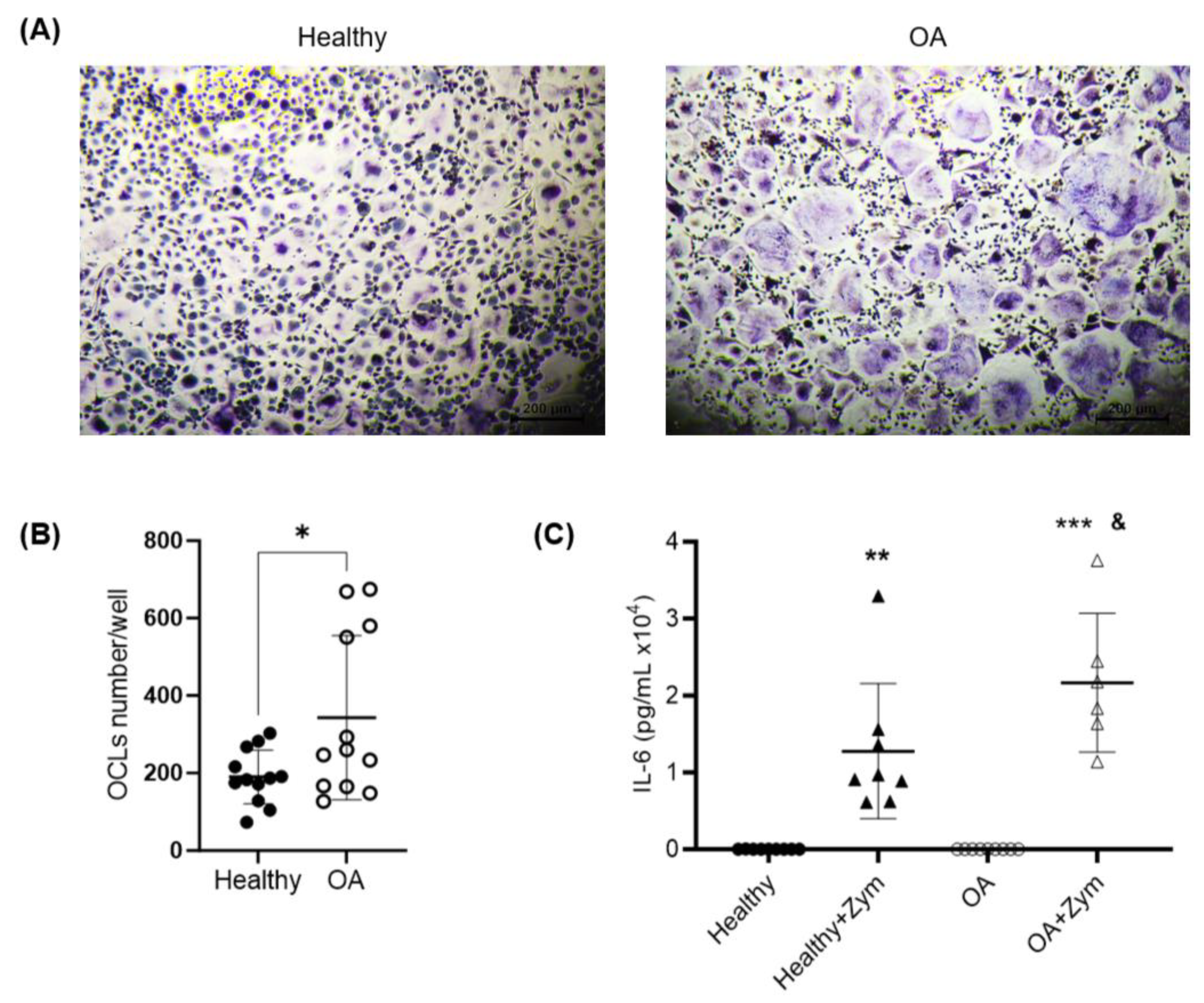
2.4. Osteoclastogenesis Potential of Monocytes from OA Patients
2.5. Inflammatory Potential of Monocytes from OA Patients
3. Discussion
4. Materials and Methods
4.1. Human Sample Collection
4.2. Isolation of Human PBMCs and CD14+ OCPs
4.3. ARN Extraction and qPCR
4.4. Flow Cytometry Analysis
4.5. CD14+ Cell Culture and Stimulation
4.6. ELISA on the IL-6 Protein Production
4.7. Osteoclast Differentiation and TRAP Staining
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, H.; Cao, J.; Chen, M.; Chen, M.; Chen, W.; Chen, X.; Chen, Y.; Chen, Y.; Chen, Y.; Chen, Z.; et al. Biomarkers of aging. Sci. China Life Sci. 2023, 66, 893–1066. [Google Scholar] [PubMed]
- Chung, P.L.; Zhou, S.; Eslami, B.; Shen, L.; LeBoff, M.S.; Glowacki, J. Effect of age on regulation of human osteoclast differentiation. J. Cell. Biochem. 2014, 115, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.; Loeser, R. Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Weiskopf, D.; Weinberger, B.; Grubeck-Loebenstein, B. The aging of the immune system. Transpl. Int. 2009, 22, 1041–1050. [Google Scholar] [CrossRef]
- Motta, F.; Barone, E.; Sica, A.; Selmi, C. Inflammaging and Osteoarthritis. Clin. Rev. Allergy Immunol. 2022, 64, 222–238. [Google Scholar] [CrossRef]
- Sharma, A.R.; Jagga, S.; Lee, S.S.; Nam, J.S. Interplay between cartilage and subchondral bone contributing to pathogenesis of osteoarthritis. Int. J. Mol. Sci. 2013, 14, 19805–19830. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Wang, S.; Jing, Y.; Su, J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021, 9, 20. [Google Scholar] [CrossRef]
- Pippenger, B.E.; Duhr, R.; Muraro, M.G.; Pagenstert, G.I.; Hügle, T.; Geurts, J. Multicolor flow cytometry-based cellular phenotyping identifies osteoprogenitors and inflammatory cells in the osteoarthritic subchondral bone marrow compartment. Osteoarthr. Cartil. 2015, 23, 1865–1869. [Google Scholar] [CrossRef]
- Madel, M.B.; Ibáñez, L.; Wakkach, A.; de Vries, T.J.; Teti, A.; Apparailly, F.; Blin-Wakkach, C. Immune Function and Diversity of Osteoclasts in Normal and Pathological Conditions. Front. Immunol. 2019, 10, 1408. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, L.; Guillem-Llobat, P.; Marín, M.; Guillén, M.I. Connection between Mesenchymal Stem Cells Therapy and Osteoclasts in Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 4693. [Google Scholar] [CrossRef] [PubMed]
- Elson, A.; Anuj, A.; Barnea-Zohar, M.; Reuven, N. The origins and formation of bone-resorbing osteoclasts. Bone 2022, 164, 116538. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef] [PubMed]
- Mori, G.; D’Amelio, P.; Faccio, R.; Brunetti, G. The Interplay between the bone and the immune system. Clin. Dev. Immunol. 2013, 2013, 720504. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, C. TNF-α and IL-6: The Link between Immune and Bone System. Curr. Drug Targets 2020, 21, 213–227. [Google Scholar] [PubMed]
- Rivollier, A.; Mazzorana, M.; Tebib, J.; Piperno, M.; Aitsiselmi, T.; Rabourdin-Combe, C.; Jurdic, P.; Servet-Delprat, C. Immature dendritic cell transdifferentiation into osteoclasts: A novel pathway sustained by the rheumatoid arthritis microenvironment. Blood 2004, 104, 4029–4037. [Google Scholar] [CrossRef]
- Kiesel, J.R.; Buchwald, Z.S.; Aurora, R. Cross-presentation by osteoclasts induces FoxP3 in CD8+ T cells. J. Immunol. 2009, 182, 5477–5487. [Google Scholar] [CrossRef]
- Ibáñez, L.; Abou-Ezzi, G.; Ciucci, T.; Amiot, V.; Belaïd, N.; Obino, D.; Mansour, A.; Rouleau, M.; Wakkach, A.; Blin-Wakkach, C. Inflammatory Osteoclasts Prime TNFα-Producing CD4+ T Cells and Express CX3CR1. J. Bone Miner. Res. 2016, 31, 1899–1908. [Google Scholar] [CrossRef]
- Madel, M.B.; Ibáñez, L.; Ciucci, T.; Halper, J.; Rouleau, M.; Boutin, A.; Hue, C.; Duroux-Richard, I.; Apparailly, F.; Garchon, H.J.; et al. Dissecting the phenotypic and functional heterogeneity of mouse inflammatory osteoclasts by the expression of Cx3cr1. Elife 2020, 9, e54493. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Aristizábal, A.; Gandhi, R.; Mahomed, N.N.; Marshall, K.W.; Viswanathan, S. Synovial fluid monocyte/macrophage subsets and their correlation to patient-reported outcomes in osteoarthritic patients: A cohort study. Arthritis Res. Ther. 2019, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhu, J.; Zhen, G.; Hu, Y.; An, S.; Li, Y.; Zheng, Q.; Chen, Z.; Yang, Y.; Wan, M.; et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J. Clin. Invest. 2019, 129, 1076–1093. [Google Scholar] [CrossRef] [PubMed]
- Rezuș, E.; Cardoneanu, A.; Burlui, A.; Luca, A.; Codreanu, C.; Tamba, B.I.; Stanciu, G.D.; Dima, N.; Bădescu, C.; Rezuș, C. The Link Between Inflammaging and Degenerative Joint Diseases. Int. J. Mol. Sci. 2019, 20, 614. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.; Kaneko, K.; Suh, A.J.; Bockman, R.; Park-Min, K.H. Origin of Osteoclasts: Osteoclast Precursor Cells. J. Bone Metab. 2023, 30, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Yeap, W.H.; Tai, J.J.; Ong, S.M.; Dang, T.M.; Wong, S.C. The three human monocyte subsets: Implications for health and disease. Immunol. Res. 2012, 53, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, S.; Schoenmaker, T.; Cao, Y.; Everts, V.; de Vries, T.J. Different Blood-Borne Human Osteoclast Precursors Respond in Distinct Ways to IL-17A. J. Cell. Physiol. 2015, 231, 1249–1260. [Google Scholar] [CrossRef]
- Durand, M.; Komarova, S.V.; Bhargava, A.; Trebec-Reynolds, D.P.; Li, K.; Fiorino, C.; Maria, O.; Nabavi, N.; Manolson, M.F.; Harrison, R.E.; et al. Monocytes from patients with osteoarthritis display increased osteoclastogenesis and bone resorption: The In Vitro Osteoclast Differentiation in Arthritis study. Arthritis Rheum. 2012, 65, 148–158. [Google Scholar] [CrossRef]
- Loukov, D.; Karampatos, S.; Maly, M.R.; Bowdish, D.M.E. Monocyte activation is elevated in women with knee-osteoarthritis and associated with inflammation, BMI and pain. Osteoarthr. Cartil. 2018, 26, 255–263. [Google Scholar] [CrossRef]
- Hirose, S.; Lin, Q.; Ohtsuji, M.; Nishimura, H.; Verbeek, J.S. Monocyte subsets involved in the development of systemic lupus erythematosus and rheumatoid arthritis. Int. Immunol. 2019, 31, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Shen, X.; Gao, G. Gene Expression Profiles of Peripheral Blood Monocytes in Osteoarthritis and Analysis of Differentially Expressed Genes. BioMed Res. Int. 2019, 2019, 4291689. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef]
- Chiu, Y.G.; Shao, T.; Feng, C.; Mensah, K.A.; Thullen, M.; Schwarz, E.M.; Ritchlin, C.T. CD16 (FcRgammaIII) as a potential marker of osteoclast precursors in psoriatic arthritis. Arthritis Res. Ther. 2010, 12, R14. [Google Scholar] [CrossRef]
- Mata-Martínez, P.; Bergón-Gutiérrez, M.; Del Fresno, C. Dectin-1 Signaling Update: New Perspectives for Trained Immunity. Front. Immunol. 2022, 13, 812148. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Nagata, M.; Yamasaki, S. Mincle: 20 years of a versatile sensor of insults. Int Immunol. 2018, 30, 233–239. [Google Scholar] [CrossRef]
- Gantner, B.N.; Simmons, R.M.; Canavera, S.J.; Akira, S.; Underhill, D.M. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 2003, 197, 1107–1117. [Google Scholar] [CrossRef]
- Zimmermann, H.W.; Sterzer, V.; Sahin, H. CCR1 and CCR2 antagonists. Curr. Top. Med. Chem. 2014, 14, 1539–1552. [Google Scholar] [CrossRef]
- Ożańska, A.; Szymczak, D.; Rybka, J. Pattern of human monocyte subpopulations in health and disease. Scand. J. Immunol. 2020, 92, e12883. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L. Monocyte subsets in man and other species. Cell. Immunol. 2014, 289, 135–139. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L. Blood Monocytes and Their Subsets: Established Features and Open Questions. Front. Immunol. 2015, 6, 423. [Google Scholar] [CrossRef]
- Flegar, D.; Filipović, M.; Šućur, A.; Markotić, A.; Lukač, N.; Šisl, D.; Ikić Matijašević, M.; Jajić, Z.; Kelava, T.; Katavić, V.; et al. Preventive CCL2/CCR2 Axis Blockade Suppresses Osteoclast Activity in a Mouse Model of Rheumatoid Arthritis by Reducing Homing of CCR2(hi) Osteoclast Progenitors to the Affected Bone. Front. Immunol. 2021, 12, 767231. [Google Scholar] [CrossRef]
- van den Bosch, M.H.J. Inflammation in osteoarthritis: Is it time to dampen the alarm(in) in this debilitating disease? Clin. Exp. Immunol. 2019, 195, 153–166. [Google Scholar] [CrossRef]
- Dillon, S.; Agrawal, S.; Banerjee, K.; Letterio, J.; Denning, T.L.; Oswald-Richter, K.; Kasprowicz, D.J.; Kellar, K.; Pare, J.; van Dyke, T.; et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Invest. 2006, 116, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Sano, H.; Iwaki, D.; Kudo, K.; Konishi, M.; Takahashi, H.; Takahashi, T.; Imaizumi, H.; Asai, Y.; Kuroki, Y. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J. Immunol. 2003, 171, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A. Influences of the IL-6 cytokine family on bone structure and function. Cytokine 2021, 146, 155655. [Google Scholar] [CrossRef] [PubMed]
- Haubruck, P.; Pinto, M.M.; Moradi, B.; Little, C.B.; Gentek, R. Monocytes, Macrophages, and Their Potential Niches in Synovial Joints-Therapeutic Targets in Post-Traumatic Osteoarthritis? Front. Immunol. 2021, 12, 763702. [Google Scholar] [CrossRef] [PubMed]
- Andreev, D.; Liu, M.; Weidner, D.; Kachler, K.; Faas, M.; Grüneboom, A.; Schlötzer-Schrehardt, U.; Muñoz, L.E.; Steffen, U.; Grötsch, B.; et al. Osteocyte necrosis triggers osteoclast-mediated bone loss through macrophage-inducible C-type lectin. J. Clin. Invest. 2020, 130, 4811–4830. [Google Scholar] [CrossRef] [PubMed]
- Plantinga, T.S.; Fransen, J.; Takahashi, N.; Stienstra, R.; van Riel, P.L.; van den Berg, W.B.; Netea, M.G.; Joosten, L.A. Functional consequences of DECTIN-1 early stop codon polymorphism Y238X in rheumatoid arthritis. Arthritis Res. Ther. 2010, 12, R26. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R. Chemokines and inflammation in osteoarthritis: Insights from patients and animal models. J. Orthop. Res. 2016, 35, 735–739. [Google Scholar] [CrossRef]
- Elsori, D.H.; Yakubenko, V.P.; Roome, T.; Thiagarajan, P.S.; Bhattacharjee, A.; Yadav, S.P.; Cathcart, M.K. Protein kinase Cδ is a critical component of Dectin-1 signaling in primary human monocytes. J. Leukoc. Biol. 2011, 90, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.M.; Kirkland, M.A.; Nicholson, G.C. Multiple roles of M-CSF in human osteoclastogenesis. J. Cell. Biochem. 2007, 102, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Glantschnig, H.; Fisher, J.E.; Wesolowski, G.; Rodan, G.A.; Reszka, A.A. M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003, 10, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Toh, M.L.; Bonnefoy, J.Y.; Accart, N.; Cochin, S.; Pohle, S.; Haegel, H.; De Meyer, M.; Zemmour, C.; Preville, X.; Guillen, C.; et al. Bone-and Cartilage-Protective Effects of a Monoclonal Antibody Against Colony-Stimulating Factor 1 Receptor in Experimental Arthritis. Arthritis Rheumatol. 2014, 66, 2989–3000. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Hartkamp, L.M.; Malvar-Fernandez, B.; van Es, I.E.; Lin, H.; Wong, J.; Long, L.; Zanghi, J.A.; Rankin, A.L.; Masteller, E.L.; et al. Colony-stimulating factor (CSF) 1 receptor blockade reduces inflammation in human and murine models of rheumatoid arthritis. Arthritis Res. Ther. 2016, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Pers, Y.M.; Quentin, J.; Feirreira, R.; Espinoza, F.; Abdellaoui, N.; Erkilic, N.; Cren, M.; Dufourcq-Lopez, E.; Pullig, O.; Nöth, U.; et al. Injection of Adipose-Derived Stromal Cells in the Knee of Patients with Severe Osteoarthritis has a Systemic Effect and Promotes an Anti-Inflammatory Phenotype of Circulating Immune Cells. Theranostics 2018, 8, 5519–5528. [Google Scholar] [CrossRef] [PubMed]
- Hofer, T.P.; Zawada, A.M.; Frankenberger, M.; Skokann, K.; Satzl, A.A.; Gesierich, W.; Schuberth, M.; Levin, J.; Danek, A.; Rotter, B.; et al. slan-defined subsets of CD16-positive monocytes: Impact of granulomatous inflammation and M-CSF receptor mutation. Blood 2015, 126, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Dayyani, F.; Belge, K.U.; Frankenberger, M.; Mack, M.; Berki, T.; Ziegler-Heitbrock, L. Mechanism of glucocorticoid-induced depletion of human CD14+CD16+ monocytes. J. Leukoc. Biol. 2003, 74, 33–39. [Google Scholar] [CrossRef]
- Komano, Y.; Nanki, T.; Hayashida, K.; Taniguchi, K.; Miyasaka, N. Identification of a human peripheral blood monocyte subset that differentiates into osteoclasts. Arthritis Res. Ther. 2006, 8, R152. [Google Scholar] [CrossRef]
- Xue, J.; Xu, L.; Zhu, H.; Bai, M.; Li, X.; Zhao, Z.; Zhong, H.; Cheng, G.; Li, X.; Hu, F.; et al. CD14+CD16− monocytes are the main precursors of osteoclasts in rheumatoid arthritis via expressing Tyro3TK. Arthritis Res. Ther. 2020, 22, 221. [Google Scholar] [CrossRef]
- Rana, A.K.; Li, Y.; Dang, Q.; Yang, F. Monocytes in rheumatoid arthritis: Circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis. Int. Immunopharmacol. 2018, 65, 348–359. [Google Scholar] [CrossRef] [PubMed]
| PATIENT | SEX | AGE (Years) | KL GRADE | ANALGESIA | CRP (mg/L) |
|---|---|---|---|---|---|
| 1 | Female | 73 | IV | INITIAL.MINOR | 2.1 |
| 2 | Female | 80 | IV | NO | 4 |
| 3 | Female | 72 | IV | NO | 4.5 |
| 4 | Female | 82 | III | SECOND STEP | <1 |
| 5 | Female | 74 | II | NO | 2 |
| 6 | Female | 71 | IV | NO | 2.5 |
| 7 | Male | 69 | III | NO | <1 |
| 8 | Female | 76 | IV | NO | <5 |
| 9 | Female | 74 | IV | OCASSIONAL | 4 |
| 10 | Female | 73 | II | DAILY | <1 |
| 11 | Female | 78 | IV | NO | <1 |
| 12 | Female | 69 | IV | OCASSIONAL | <1 |
| 13 | Female | 83 | IV | OCASSIONAL | 4 |
| 14 | Female | 63 | IV | NO | <1 |
| 15 | Female | 66 | IV | NO | 5 |
| 16 | Female | 81 | III | DAILY | <5 |
| 17 | Female | 70 | IV | DAILY | <5 |
| 18 | Male | 56 | IV | OCASSIONAL | <5 |
| 19 | Male | 58 | III | NO | <5 |
| 20 | Female | 78 | IV | NO | <1 |
| 21 | Male | 83 | III | NO | <5 |
| 22 | Male | 70 | IV | NO | <1 |
| 23 | Female | 70 | IV | NO | <1 |
| 24 | Female | 74 | IV | INITIAL.MINOR | 5 |
| 25 | Female | 77 | IV | INITIAL.MINOR | <5 |
| 36B4: | Fwd-TGCATCAGTACCCCATTCTATCAT Rv-AGGCAGATGGATCAGCCAAGA |
| CLEC4E: | Fwd-CTGAAACACAATGCACAGAGAGA Rv-AAAGATGCGAAATGTCACAACAC |
| CLEC7A: | Fwd-GGAAGCAACACATTGGAGAATGG Rv-CTTTGGTAGGAGTCACACTGTC |
| CCR2: | Fwd-GATCTGCTTTTTCTTATTACTCTCCCA Rv-TCCGCCAAAATAACCGATGT |
| FCGR3: | Fwd-GTCACTGTCCCAAGTTGCTAAG Rv-TCCTTCCTGTGTGCTTGTGG |
| CSF1R: | Fwd-CCAGCAGCGTTGATGTTAACTTT Rv-CGGCATGTTGGAAATCTACTTG |
| TLR2: | Fwd-TTGTGACCGCAATGGTATCTG Rv-TGTTGGACAGGTCAAGGCTTT |
| CTSK: | Fwd-TGAGGCTTCTCTTGGTGTCCATAC Rv-AAAGGGTGTCATTACTGCGGG |
| TNFRSF11A: | Fwd-ACCTTGCCTTGCAGGCTACTT Rv-CAAACCGCATCGGATTTCTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillem-Llobat, P.; Marín, M.; Rouleau, M.; Silvestre, A.; Blin-Wakkach, C.; Ferrándiz, M.L.; Guillén, M.I.; Ibáñez, L. New Insights into the Pro-Inflammatory and Osteoclastogenic Profile of Circulating Monocytes in Osteoarthritis Patients. Int. J. Mol. Sci. 2024, 25, 1710. https://doi.org/10.3390/ijms25031710
Guillem-Llobat P, Marín M, Rouleau M, Silvestre A, Blin-Wakkach C, Ferrándiz ML, Guillén MI, Ibáñez L. New Insights into the Pro-Inflammatory and Osteoclastogenic Profile of Circulating Monocytes in Osteoarthritis Patients. International Journal of Molecular Sciences. 2024; 25(3):1710. https://doi.org/10.3390/ijms25031710
Chicago/Turabian StyleGuillem-Llobat, Paloma, Marta Marín, Matthieu Rouleau, Antonio Silvestre, Claudine Blin-Wakkach, María Luisa Ferrándiz, María Isabel Guillén, and Lidia Ibáñez. 2024. "New Insights into the Pro-Inflammatory and Osteoclastogenic Profile of Circulating Monocytes in Osteoarthritis Patients" International Journal of Molecular Sciences 25, no. 3: 1710. https://doi.org/10.3390/ijms25031710
APA StyleGuillem-Llobat, P., Marín, M., Rouleau, M., Silvestre, A., Blin-Wakkach, C., Ferrándiz, M. L., Guillén, M. I., & Ibáñez, L. (2024). New Insights into the Pro-Inflammatory and Osteoclastogenic Profile of Circulating Monocytes in Osteoarthritis Patients. International Journal of Molecular Sciences, 25(3), 1710. https://doi.org/10.3390/ijms25031710








