β–Lactam TRPM8 Antagonist RGM8-51 Displays Antinociceptive Activity in Different Animal Models
Abstract
:1. Introduction
2. Results and Discussion
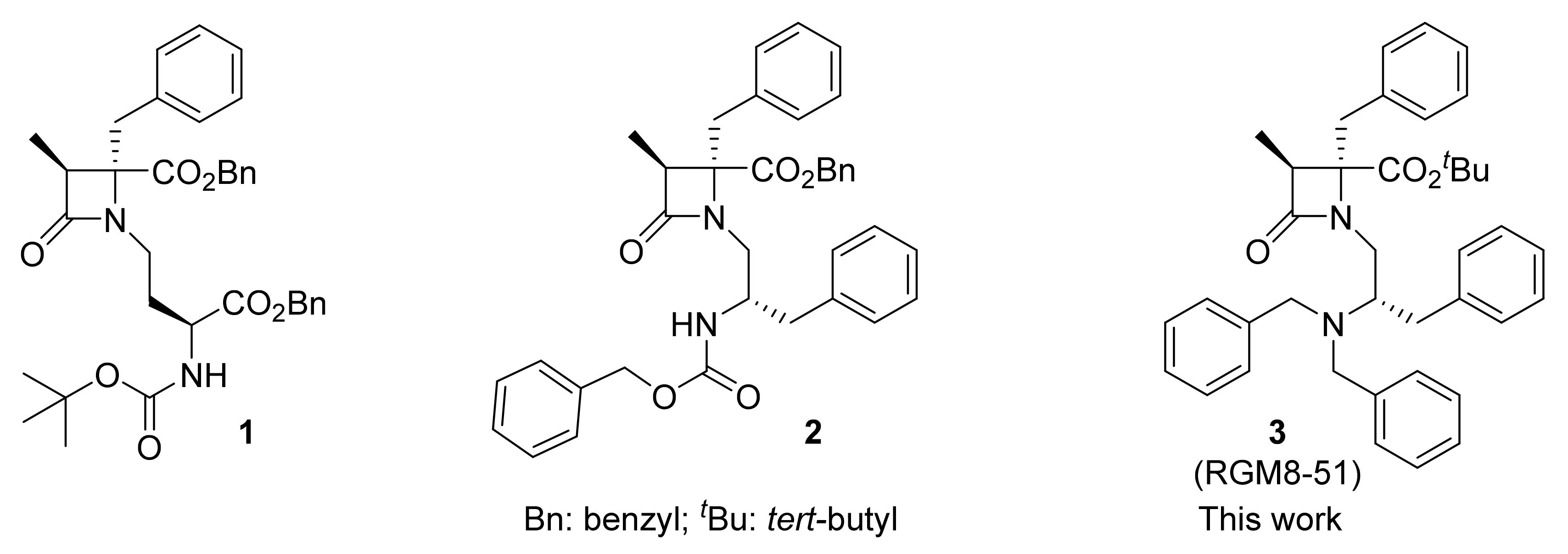
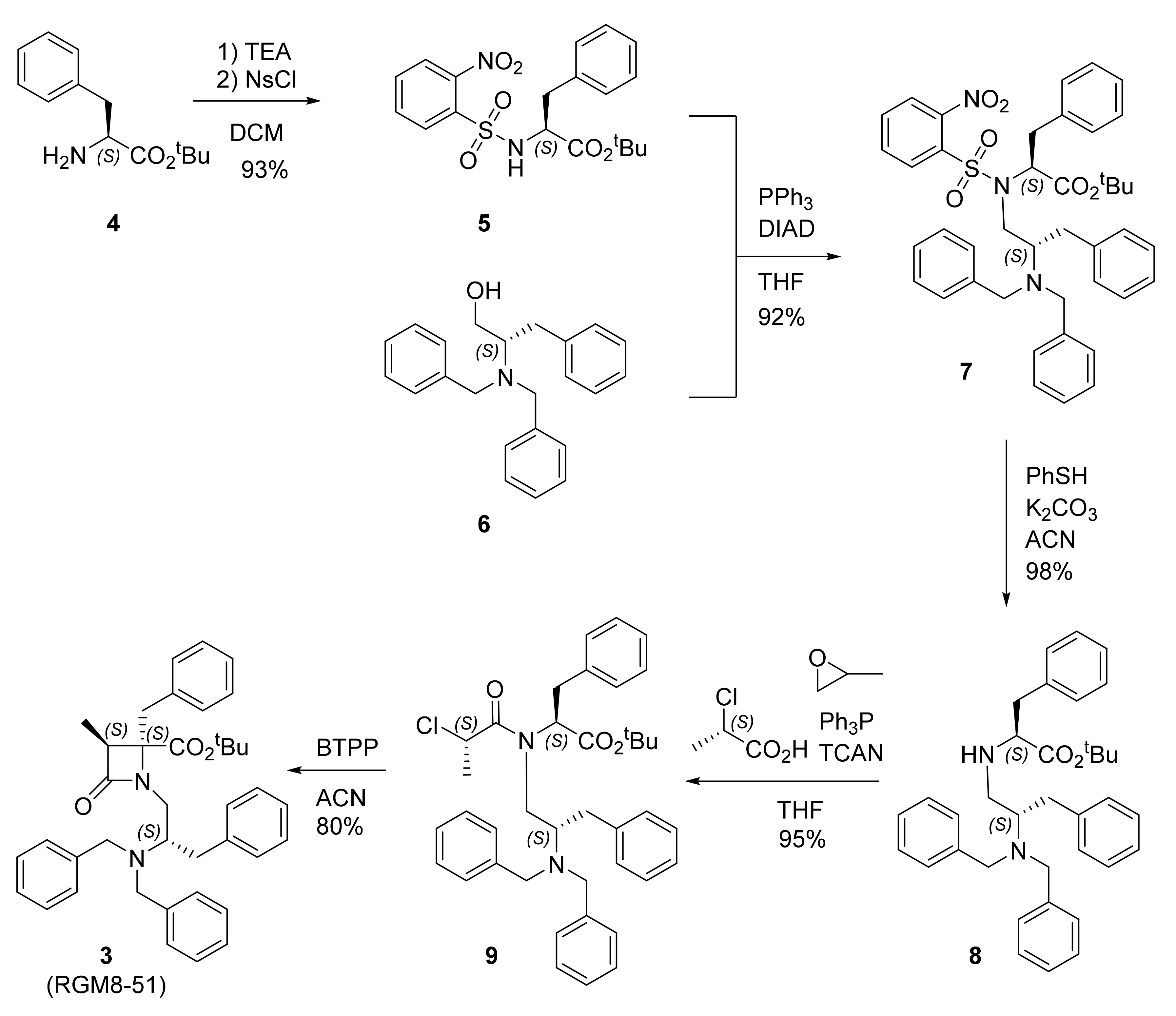
2.1. Synthesis
2.2. In Vitro TRPM8 Antagonist Activity
2.2.1. Experiments in Cell Lines
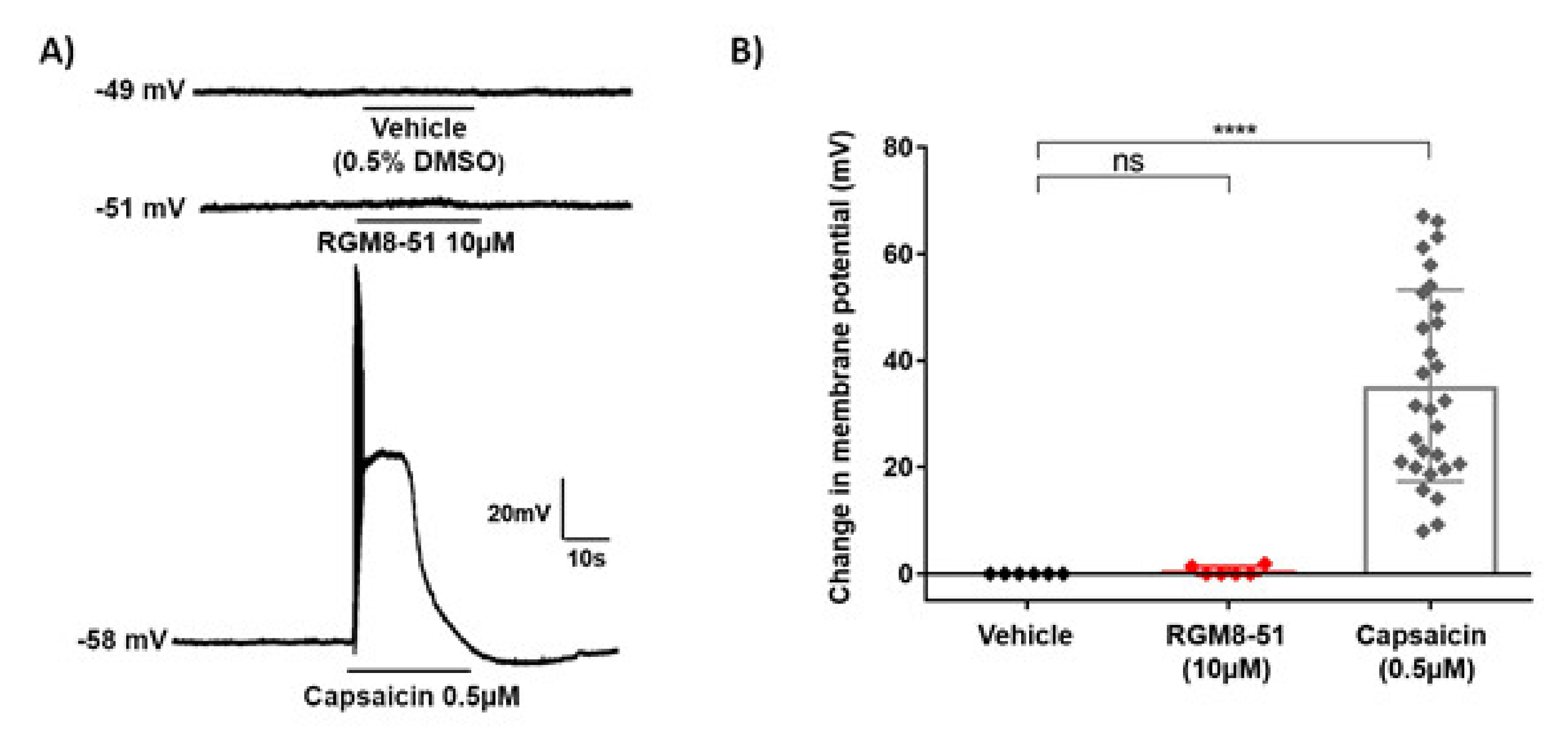
2.2.2. Experiments in DRG Neurons
2.2.3. In Vitro Preliminary Pharmacokinetic Studies
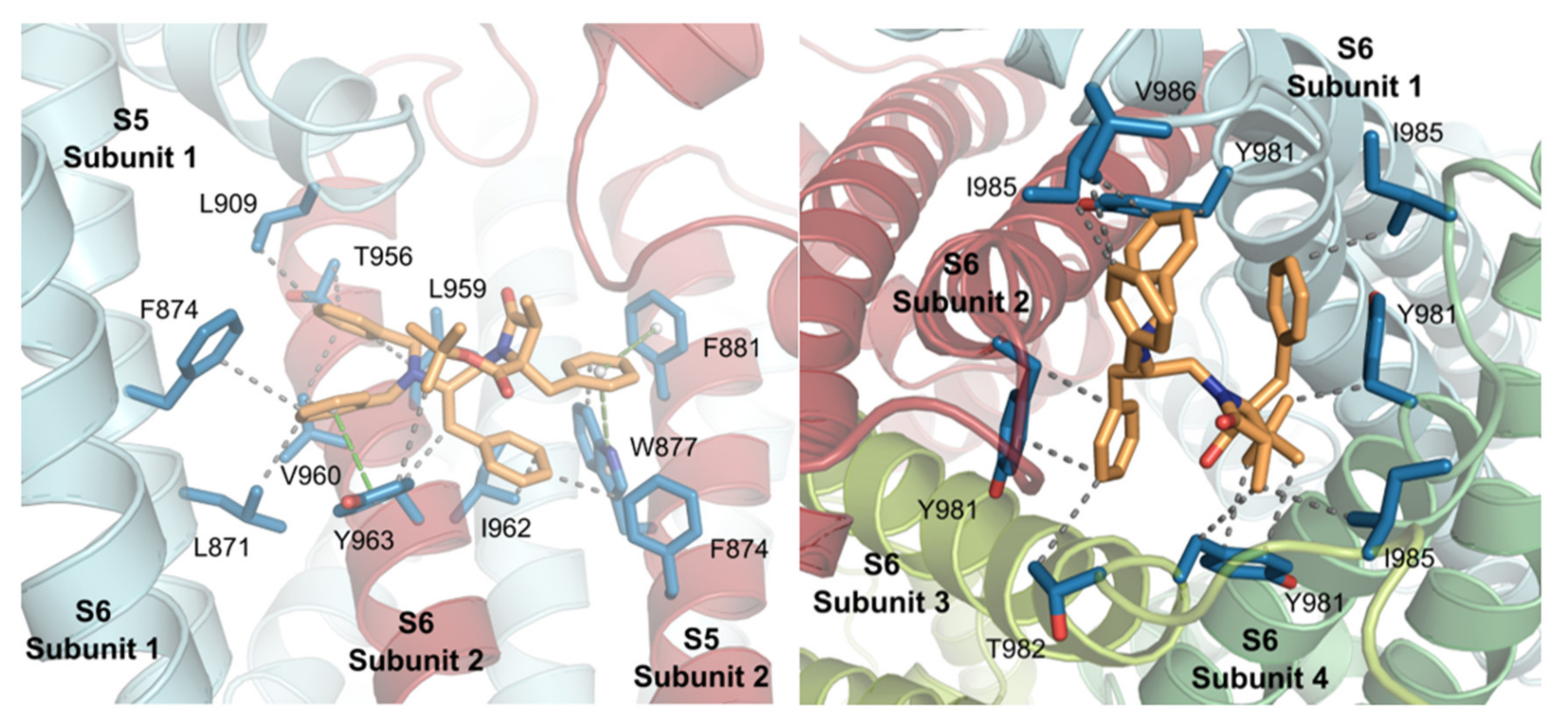
2.3. Molecular Modeling Studies
2.4. In Vivo Antinociceptive Activity
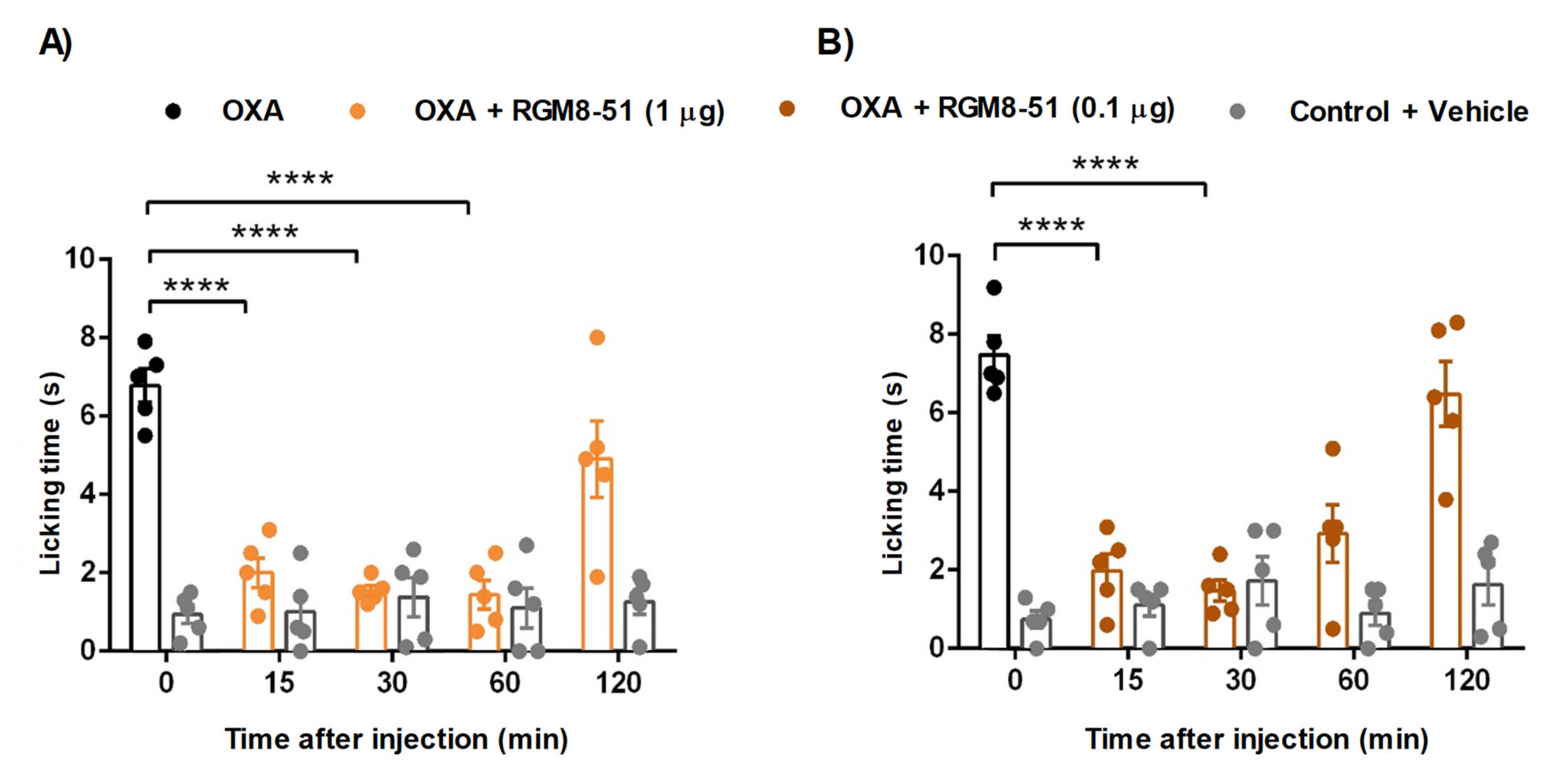
2.4.1. Mouse Chemotherapy-Induced Peripheral Neuropathy
2.4.2. Chronic Constriction Injury (CCI) of the Sciatic Nerve
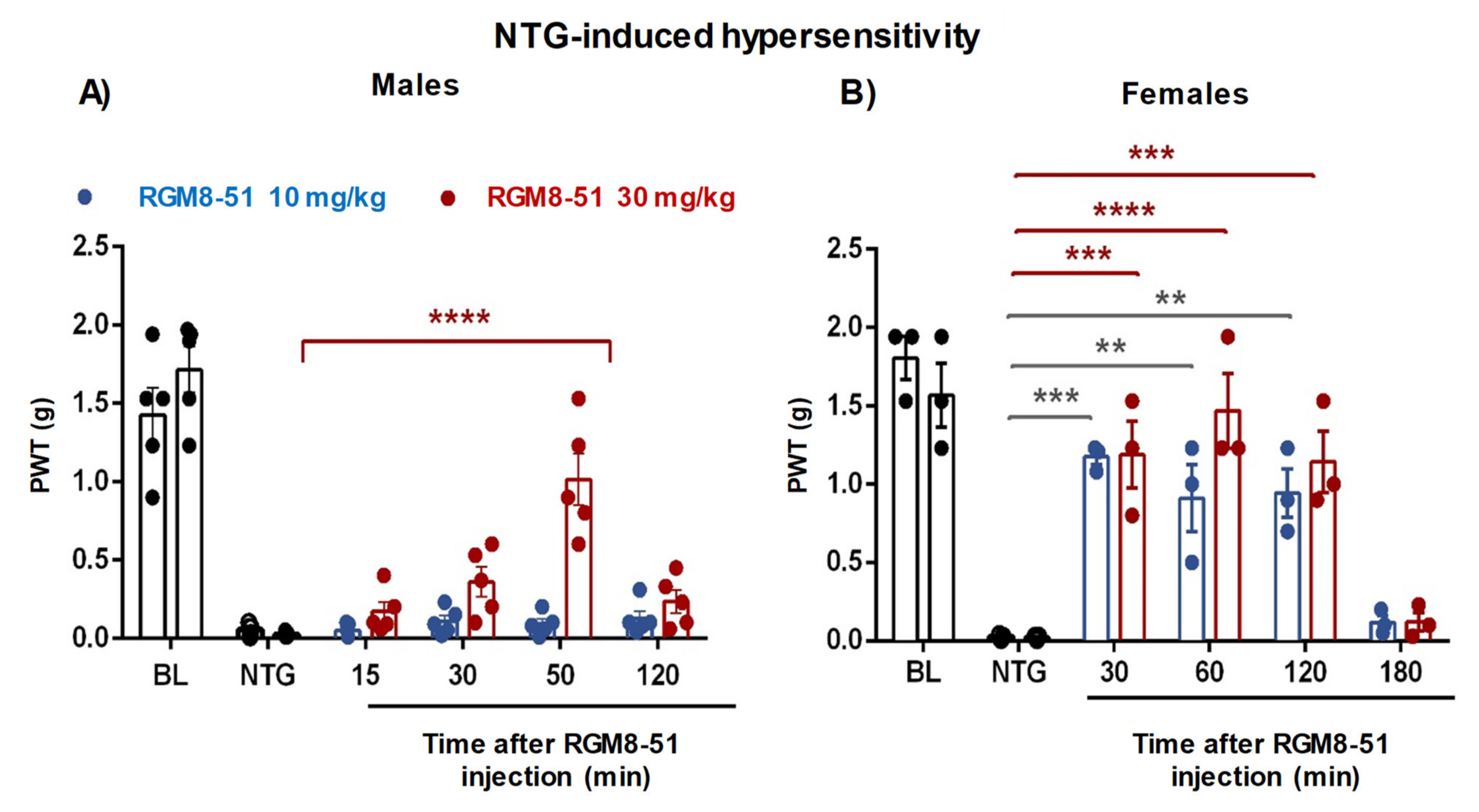
2.4.3. NTG-Induced Mechanical Hypersensitivity
3. Conclusions
4. Methods
4.1. Synthesis
4.1.1. Ns-L-Phe-OtBu (5)
4.1.2. N-[(2S-Dibenzylamino-3-phenyl)prop-1-yl]-Ns-L-Phe-OtBu (7)
4.1.3. N-[(2S-Dibenzylamino-3-phenyl)prop-1-yl]-L-Phe-OtBu (8)
4.1.4. N-[(2S-Dibenzylamino-3-phenyl)prop-1-yl]-N-(2′S-chloropropanoyl)-L-Phe-OtBu (9)
4.1.5. 4(S)-Benzyl-3S-methyl-4-(tert-butoxy)carbonyl-1-[(2′S-dibenzylamino-3′-phenyl)prop-1′-yl]-2-oxoazetidine (RGM8-51, 3)
4.2. Molecular Modeling Studies
4.3. Functional Assays by Calcium Microfluorimetry
4.4. Isolation of Rat DRG Neurons
4.5. Functional Assays by Patch-Clamp Electrophysiology
4.6. Selectivity and Preliminary PK Properties
4.7. Mouse Model of Oxaliplatin-Induced Peripheral Neuropathy
4.8. Rat Chronic Constriction Injury (CCI) Model
4.9. Mouse Model of NTG-Induced Mechanical Hypersensitivity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Mikrani, R.; He, Y.; Faran Ashraf Baig, M.M.; Abbas, M.; Naveed, M.; Tang, M.; Zhang, Q.; Li, C.; Zhou, X. TRPM8 channels: A review of distribution and clinical role. Eur. J. Pharmacol. 2020, 882, 173312. [Google Scholar] [CrossRef]
- Pérez De Vega, M.J.; Gómez-Monterrey, I.; Ferrer-Montiel, A.; González-Muñiz, R. Transient Receptor Potential Melastatin 8 Channel (TRPM8) Modulation: Cool Entryway for Treating Pain and Cancer. J. Med. Chem. 2016, 59, 10006–10029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Muñiz, R.; Bonache, M.A.; Martin-Escura, C.; Gomez-Monterrey, I. Recent Progress in TRPM8 Modulation: An Update. Int. J. Mol. Sci. 2019, 20, 2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lolignier, S.; Gkika, D.; Andersson, D.; Leipold, E.; Vetter, I.; Viana, F.; Noel, J.; Busserolles, J. New insight in cold pain: Role of ion channels, modulation, and clinical perspectives. J. Neurosci. 2016, 36, 11435–11439. [Google Scholar] [CrossRef] [Green Version]
- Bennett, G.J.; Xie, Y.-K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Caspani, O.; Zurborg, S.; Labuz, D.; Heppenstall, P.A. The contribution of TRPM8 and TRPA1 channels to cold allodynia and neuropathic pain. PLoS ONE 2009, 4, e7383. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Shu, R.; Song, C.; Yu, Y.; Wang, G.; Li, Y.; Liu, C. Downregulations of TRPM8 expression and membrane trafficking in dorsal root ganglion mediate the attenuation of cold hyperalgesia in CCI rats induced by GFRα3 knockdown. Brain Res. Bull. 2017, 135, 8–24. [Google Scholar] [CrossRef]
- Su, L.; Wang, C.; Yu, Y.-H.; Ren, Y.-Y.; Xie, K.-L.; Wang, G.-L. Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci. 2011, 12, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Caro, C.; Cristiano, C.; Avagliano, C.; Bertamino, A.; Ostacolo, C.; Campiglia, P.; Gomez-Monterrey, I.; La Rana, G.; Gualillo, O.; Calignano, A.; et al. Characterization of new TRPM8 modulators in pain perception. Int. J. Mol. Sci. 2019, 20, 5544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Hou, J.; Li, Z.; Cao, X.; Qin, B.; Cao, S.; Li, Q.; Liu, X. Intrathecal TRPM8 blocking attenuates cold hyperalgesia via PKC and NF-κB signaling in the dorsal root ganglion of rats with neuropathic pain. J. Pain Res. 2019, 12, 1287–1296. [Google Scholar] [CrossRef] [Green Version]
- Lehto, S.G.; Weyer, A.D.; Zhang, M.; Youngblood, B.D.; Wang, J.; Wang, W.; Kerstein, P.C.; Davis, C.; Wild, K.D.; Stucky, C.L.; et al. AMG2850, a potent and selective TRPM8 antagonist, is not effective in rat models of inflammatory mechanical hypersensitivity and neuropathic tactile allodynia. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 465–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertamino, A.; Ostacolo, C.; Medina, A.; Di Sarno, V.; Lauro, G.; Ciaglia, T.; Vestuto, V.; Pepe, G.; Basilicata, M.G.; Musella, S.; et al. Exploration of TRPM8 Binding Sites by β-Carboline-Based Antagonists and Their In Vitro Characterization and In Vivo Analgesic Activities. J. Med. Chem. 2020, 63, 9672–9694. [Google Scholar] [CrossRef]
- De Caro, C.; Russo, R.; Avagliano, C.; Cristiano, C.; Calignano, A.; Aramini, A.; Bianchini, G.; Allegretti, M.; Brandolini, L. Antinociceptive effect of two novel transient receptor potential melastatin 8 antagonists in acute and chronic pain models in rat. Br. J. Pharmacol. 2018, 175, 1691–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.B.; Goldstein, D.; Krishnan, A.V.; Lin, C.S.Y.; Friedlander, M.L.; Cassidy, J.; Koltzenburg, M.; Kiernan, M.C. Chemotherapy-Induced Peripheral Neurotoxicity: A Critical Analysis. CA Cancer J. Clin. 2013, 63, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Zanone, M.M.; Marinucci, C.; Ciancio, A.; Cocito, D.; Zardo, F.; Spagone, E.; Ferrero, B.; Cerruti, C.; Charrier, L.; Cavallo, F.; et al. Peripheral neuropathy after viral eradication with direct-acting antivirals in chronic HCV hepatitis: A prospective study. Liver Int. 2021, 41, 2611–2621. [Google Scholar] [CrossRef]
- Egan, K.E.; Caldwell, G.M.; Eckmann, M.S. HIV Neuropathy-a Review of Mechanisms, Diagnosis, and Treatment of Pain. Curr. Pain Headache Rep. 2021, 25, 55. [Google Scholar] [CrossRef] [PubMed]
- Nassini, R.; Benemei, S.; Fusi, C.; Trevisan, G.; Materazzi, S. Transient receptor potential channels in chemotherapy-induced neuropathy. Open Pain J. 2013, 6, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Rimola, V.; Osthues, T.; Koenigs, V.; Geisslinger, G.; Sisignano, M. Oxaliplatin causes transient changes in TRPM8 channel activity. Int. J. Mol. Sci. 2021, 22, 4962. [Google Scholar] [CrossRef]
- Mizoguchi, S.; Andoh, T.; Yakura, T.; Kuraishi, Y. Involvement of c-Myc-mediated transient receptor potential melastatin 8 expression in oxaliplatin-induced cold allodynia in mice. Pharmacol. Rep. 2016, 68, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chen, F.; Huang, S.; Cai, Z.; Lan, H.; Tong, Y.; Yu, X.; Zhao, G. TRPA1 and TRPM8 Receptors May Promote Local Vasodilation that Aggravates Oxaliplatin-Induced Peripheral Neuropathy Amenable to 17β-Estradiol Treatment. Curr. Neurovasc. Res. 2016, 13, 309–317. [Google Scholar] [CrossRef]
- Wu, B.; Su, X.; Zhang, W.; Zhang, Y.-H.; Feng, X.; Ji, Y.-H.; Tan, Z.-Y. Oxaliplatin depolarizes the IB4- dorsal root ganglion neurons to drive the development of neuropathic pain through TRPM8 in mice. Front. Mol. Neurosci. 2021, 14, 690858. [Google Scholar] [CrossRef]
- Kawashiri, T.; Egashira, N.; Kurobe, K.; Tsutsumi, K.; Yamashita, Y.; Ushio, S.; Yano, T.; Oishi, R. L type Ca2+ channel blockers prevent oxaliplatin-induced cold hyperalgesia and TRPM8 overexpression in rats. Mol. Pain 2012, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Hogan, D.; Baker, A.L.; Moron, J.A.; Carlton, S.M. Systemic morphine treatment induces changes in firing patterns and responses of nociceptive afferent fibers in mouse glabrous skin. Pain 2013, 154, 2297–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, K.; Jasmin, L. Sustained Morphine Administration Induces TRPM8-Dependent Cold Hyperalgesia. J. Pain 2017, 18, 212–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, D.; Zou, Z.; Zhou, C.; Mu, F. Role of transient receptor potential melastatin 8 channels in migraine mechanism in rats. Zhongguo Dangdai Erke Zazhi 2015, 17, 515–519. [Google Scholar] [PubMed]
- Ling, Y.H.; Chen, S.P.; Fann, C.S.J.; Wang, S.J.; Wang, Y.F. TRPM8 genetic variant is associated with chronic migraine and allodynia. J. Headache Pain 2019, 20, 115. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Ali, A.; Ahmad, U.; Pandey, A.K.; Singh, B. rs2651899 variant is associated with risk for migraine without aura from North Indian population. Mol. Biol. Rep. 2019, 46, 1247–1255. [Google Scholar] [CrossRef]
- Kozyreva, T.V.; Tkachenko, E.Y.; Potapova, T.A.; Romashchenko, A.G.; Voevoda, M.I. Single-nucleotide polymorphism rs11562975 of the thermosensitive ion channel TRPM8 gene and human sensitivity to cold and menthol. Hum. Physiol. 2011, 37, 188–192. [Google Scholar] [CrossRef]
- Horne, D.B.; Biswas, K.; Brown, J.; Bartberger, M.D.; Clarine, J.; Davis, C.D.; Gore, V.K.; Harried, S.; Horner, M.; Kaller, M.R.; et al. Discovery of TRPM8 Antagonist (S)-6-(((3-Fluoro-4-(trifluoromethoxy)phenyl)(3-fluoropyridin-2-yl)methyl)carbamoyl)nicotinic Acid (AMG 333), a Clinical Candidate for the Treatment of Migraine. J. Med. Chem. 2018, 61, 8186–8201. [Google Scholar] [CrossRef]
- De la Torre-Martinez, R.; Bonache, M.A.; Llabres-Campaner, P.J.; Balsera, B.; Fernandez-Carvajal, A.; Fernandez-Ballester, G.; Ferrer-Montiel, A.; Perez de Vega, M.J.; Gonzalez-Muniz, R. Synthesis, high-throughput screening and pharmacological characterization of β–lactam derivatives as TRPM8 antagonists. Sci. Rep. 2017, 7, 10766. [Google Scholar]
- Bonache, M.A.; Martin-Escura, C.; de la Torre Martinez, R.; Medina, A.; Gonzalez-Rodriguez, S.; Francesch, A.; Cuevas, C.; Roa, A.M.; Fernandez-Ballester, G.; Ferrer-Montiel, A.; et al. Highly functionalized β–lactams and 2-ketopiperazines as TRPM8 antagonists with antiallodynic activity. Sci. Rep. 2020, 10, 14154. [Google Scholar] [CrossRef]
- Perez-Faginas, P.; O’Reilly, F.; O’Byrne, A.; Garcia-Aparicio, C.; Martin-Martinez, M.; Perez de Vega, M.J.; Garcia-Lopez, M.T.; Gonzalez-Muniz, R. Exceptional Stereoselectivity in the Synthesis of 1,3,4-Trisubstituted 4-Carboxy β–Lactam Derivatives from Amino Acids. Org. Lett. 2007, 9, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Lashinger, E.S.R.; Steiginga, M.S.; Hieble, J.P.; Leon, L.A.; Gardner, S.D.; Nagilla, R.; Davenport, E.A.; Hoffman, B.E.; Laping, N.J.; Su, X. AMTB, a TRPM8 channel blocker: Evidence in rats for activity in overactive bladder and painful bladder syndrome. Am. J. Physiol. Ren. Physiol. 2008, 295, F803–F810. [Google Scholar] [CrossRef]
- Hung, C.-Y.; Tan, C.-H.; Tan, C.-H. TRP Channels in Nociception and Pathological Pain. Adv. Exp. Med. Biol. 2018, 1099, 13–27. [Google Scholar]
- Ferrer-Montiel, A.; Fernández-Carvajal, A.; Planells-Cases, R.; Fernández-Ballester, G.; González-Ros, J.M.; Messeguer, À.; González-Muñiz, R. Advances in modulating thermosensory TRP channels. Expert Opin. Ther. Pat. 2012, 22, 999–1017. [Google Scholar] [CrossRef]
- Li, W.-G.; Xu, T.-L. ASIC3 Channels in Multimodal Sensory Perception. ACS Chem. Neurosci. 2011, 2, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Schou, W.S.; Ashina, S.; Amin, F.M.; Goadsby, P.J.; Ashina, M. Calcitonin gene-related peptide and pain: A systematic review. J. Headache Pain 2017, 18, 34. [Google Scholar] [CrossRef] [Green Version]
- Benemei, S.; Nicoletti, P.; Capone, J.G.; Geppetti, P. CGRP receptors in the control of pain and inflammation. Curr. Opin. Pharmacol. 2009, 9, 9–14. [Google Scholar] [CrossRef]
- Xu, J.J.; Diaz, P.; Bie, B.; Astruc-Diaz, F.; Wu, J.; Yang, H.; Brown, D.L.; Naguib, M. Spinal gene expression profiling and pathways analysis of a CB2 agonist (MDA7)-targeted prevention of paclitaxel-induced neuropathy. Neuroscience 2014, 260, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Clapper, J.R.; Moreno-Sanz, G.; Russo, R.; Guijarro, A.; Vacondio, F.; Duranti, A.; Tontini, A.; Sanchini, S.; Sciolino, N.R.; Spradley, J.M.; et al. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat. Neurosci. 2010, 13, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Otto, W.R.; Sanchez-Herrera, D.; Facer, P.; Yiangou, Y.; Korchev, Y.; Birch, R.; Benham, C.; Bountra, C.; Chessell, I.P.; et al. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain 2008, 138, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, N.; Levey, A.I.; Augusti-Tocco, G. Rat dorsal root ganglia express m1-m4 muscarinic receptor proteins. J. Peripher. Nerv. Syst. 1999, 4, 222–232. [Google Scholar] [PubMed]
- Buchli, R.; Ndoye, A.; Arredondo, J.; Webber, R.J.; Grando, S.A. Identification and characterization of muscarinic acetylcholine receptor subtypes expressed in human skin melanocytes. Mol. Cell. Biochem. 2001, 228, 57–72. [Google Scholar] [CrossRef]
- Metzger, M.; Just, L.; Boss, A.; Drews, U. Identification and Functional Characterization of the Muscarinic Receptor M3 in the Human Keratinocyte Cell Line HaCaT. Cells Tissues Organs 2005, 180, 96–105. [Google Scholar] [CrossRef]
- Krieger, E.; Darden, T.; Nabuurs, S.B.; Finkelstein, A.; Vriend, G. Making optimal use of empirical energy functions: Force-field parameterization in crystal space. Proteins Struct. Funct. Bioinform. 2004, 57, 678–683. [Google Scholar] [CrossRef]
- Krieger, E.; Koraimann, G.; Vriend, G. Increasing the precision of comparative models with yasara NOVA-a self-parameterizing force field. Proteins Struct. Funct. Genet. 2002, 47, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wu, M.; Zubcevic, L.; Borschel, W.F.; Lander, G.C.; Lee, S.Y. Structure of the cold- and menthol-sensing ion channel TRPM8. Science 2018, 359, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diver, M.M.; Cheng, Y.; Julius, D. Structural insights into TRPM8 inhibition and desensitization. Science 2019, 365, 1434–1440. [Google Scholar] [CrossRef]
- Ewertz, M.; Qvortrup, C.; Eckhoff, L. Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol. 2015, 54, 587–591. [Google Scholar] [CrossRef]
- Journigan, V.B.; Feng, Z.; Rahman, S.; Wang, Y.; Amin, A.R.M.R.; Heffner, C.E.; Bachtel, N.; Wang, S.; Gonzalez-Rodriguez, S.; Fernandez-Carvajal, A.; et al. Structure-Based Design of Novel Biphenyl Amide Antagonists of Human Transient Receptor Potential Cation Channel Subfamily M Member 8 Channels with Potential Implications in the Treatment of Sensory Neuropathies. ACS Chem. Neurosci. 2020, 11, 268–290. [Google Scholar]
- Gavva, N.R.; Sandrock, R.; Arnold, G.E.; Davis, M.; Lamas, E.; Lindvay, C.; Li, C.-M.; Smith, B.; Gabriel, K.; Vargas, G.; et al. Reduced TRPM8 expression underpins reduced migraine risk and attenuated cold pain sensation in humans. Sci. Rep. 2019, 9, 19655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowlton, W.M.; Daniels, R.L.; Palkar, R.; McCoy, D.D.; McKemy, D.D. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS ONE 2011, 6, e25894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benemei, S.; Dussor, G. TRP Channels and Migraine: Recent Developments and New Therapeutic Opportunities. Pharmaceuticals 2019, 12, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghighi, A.B.; Motazedian, S.; Rezaii, R.; Mohammadi, F.; Salarian, L.; Pourmokhtari, M.; Khodaei, S.; Vossoughi, M.; Miri, R. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: A randomised, double-blind, placebo-controlled, crossed-over study. Int. J. Clin. Pract. 2010, 64, 451–456. [Google Scholar] [CrossRef]
- Moye, L.S.; Pradhan, A.A.A. Animal Model of Chronic Migraine-Associated Pain. Curr. Protoc. Neurosci. 2017, 80, 9.60.1–9.60.9. [Google Scholar] [CrossRef]
- Tipton, A.F.; Pradhan, A.A.; Tarash, I.; McGuire, B.; Charles, A. The effects of acute and preventive migraine therapies in a mouse model of chronic migraine. Cephalalgia 2016, 36, 1048–1056. [Google Scholar] [CrossRef] [Green Version]
- Wen, Q.; Zhang, D.; Qin, G.; Chen, L.; Wang, Y.; Pan, Q.; Tian, R.; Zhou, J.; Wang, Y. MicroRNA-155-5p promotes neuroinflammation and central sensitization via inhibiting SIRT1 in a nitroglycerin-induced chronic migraine mouse model. J. Neuroinflamm. 2021, 18, 287. [Google Scholar] [CrossRef]
- Asuthkar, S.; Elustondo, P.A.; Demirkhanyan, L.; Sun, X.; Baskaran, P.; Velpula, K.K.; Thyagarajan, B.; Pavlov, E.V.; Zakharian, E. The TRPM8 protein is a testosterone receptor: I. Biochemical evidence for direct TRPM8-testosterone interactions. J. Biol. Chem. 2015, 290, 2659–2669. [Google Scholar] [CrossRef] [Green Version]
- Asuthkar, S.; Demirkhanyan, L.; Sun, X.; Elustondo, P.A.; Krishnan, V.; Baskaran, P.; Velpula, K.K.; Thyagarajan, B.; Pavlov, E.V.; Zakharian, E. The TRPM8 Protein Is a Testosterone Receptor. II. Functional Evidence for an Ionotropic Effect of Testosterone on TRPM8. J. Biol. Chem. 2015, 290, 2670–2688. [Google Scholar] [CrossRef] [Green Version]
- Mohandass, A.; Krishnan, V.; Gribkova, E.D.; Asuthkar, S.; Baskaran, P.; Nersesyan, Y.; Hussain, Z.; Wise, L.M.; George, R.E.; Stokes, N.; et al. TRPM8 as the rapid testosterone signaling receptor: Implications in the regulation of dimorphic sexual and social behaviors. FASEB J. 2020, 34, 10887–10906. [Google Scholar] [CrossRef]
- Huo, C.; Wang, C.; Zhao, M.; Peng, S. Stereoselective synthesis of natural N-(1-deoxy-D-β-fructos-1-yl)-L- amino acids and their effect on lead decorporation. Chem. Res. Toxicol. 2004, 17, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Blazquez, M.; Olivos-Ore, L.A.; Barahona, M.V.; Sanchez de la Muela, M.; Solar, V.; Jimenez, E.; Gualix, J.; McIntosh, J.M.; Ferrer-Montiel, A.; Miras-Portugal, M.T.; et al. Overexpression of P2X3 and P2X7 receptors and TRPV1 channels in adrenomedullary chromaffin cells in a rat model of neuropathic pain. Int. J. Mol. Sci. 2019, 20, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaeva-Koleva, M.; Butron, L.; Gonzalez-Rodriguez, S.; Devesa, I.; Valente, P.; Serafini, M.; Genazzani, A.A.; Pirali, T.; Ballester, G.F.; Fernandez-Carvajal, A.; et al. A capsaicinoid-based soft drug, AG1529, for attenuating TRPV1-mediated histaminergic and inflammatory sensory neuron excitability. Sci. Rep. 2021, 11, 246. [Google Scholar] [CrossRef] [PubMed]



| Compd. | Ca2+ Microfluorography Assays | Patch-Clamp Assay | |||
|---|---|---|---|---|---|
| rTRPM 8 IC50 (μM) | Intervals | hTRPM8 IC50 (μM) | Intervals | rTRPM8 IC50 (μM) | |
| RGM8-51 (3) | 1.06 ± 1.21 | 0.72 to 1.55 | 1.74 ± 1.19 | 1.23 ± 2.45 | 0.97 ± 1.56 |
| 2 | 3.1 ± 1.1 | 2.57 to 3.99 | _ | _ | 0.9 ± 1.0 |
| AMTB | 7.3 ± 1.5 * | 6.23 ± 0.02 ** | _ | ||
| Compd. | hTRPV1 ago. (%) | hTRPV1 antago. (%) | hTRPV3 antago. (%) | hTRPA1 ago. (%) | hTRPA1 Antago. (%) | ASIC3 Antago. (%) | Binding hCGRPR (%) | Binding hCB2 (%) | Binding hM3 (%) |
|---|---|---|---|---|---|---|---|---|---|
| RGM8-51 | 31.3 ± 3.6 | 19.4 ± 1.7 | −6.3 ± 5.9 | 0.4 ± 0.3 | 4.3 ± 1.1 | 6.0 ± 0.9 | −1.0 ± 4.3 | 7.3 ± 0.3 | −4.1 ± 3.1 |
| Subsite | Location | % of Docking Solutions (Estimated Binding Energies, kcal/mol) |
|---|---|---|
| 1 | Inner pore, S5S6, S5 | 58% (8.39) |
| 2 | Internal mouth pore | 12% (8.44) |
| 3 | External pore, S3S4, S6 | 16% (6.63) |
| 4 | External loops | 8% (6.65) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Escura, C.; Medina-Peris, A.; Spear, L.A.; de la Torre Martínez, R.; Olivos-Oré, L.A.; Barahona, M.V.; González-Rodríguez, S.; Fernández-Ballester, G.; Fernández-Carvajal, A.; Artalejo, A.R.; et al. β–Lactam TRPM8 Antagonist RGM8-51 Displays Antinociceptive Activity in Different Animal Models. Int. J. Mol. Sci. 2022, 23, 2692. https://doi.org/10.3390/ijms23052692
Martín-Escura C, Medina-Peris A, Spear LA, de la Torre Martínez R, Olivos-Oré LA, Barahona MV, González-Rodríguez S, Fernández-Ballester G, Fernández-Carvajal A, Artalejo AR, et al. β–Lactam TRPM8 Antagonist RGM8-51 Displays Antinociceptive Activity in Different Animal Models. International Journal of Molecular Sciences. 2022; 23(5):2692. https://doi.org/10.3390/ijms23052692
Chicago/Turabian StyleMartín-Escura, Cristina, Alicia Medina-Peris, Luke A. Spear, Roberto de la Torre Martínez, Luis A. Olivos-Oré, María Victoria Barahona, Sara González-Rodríguez, Gregorio Fernández-Ballester, Asia Fernández-Carvajal, Antonio R. Artalejo, and et al. 2022. "β–Lactam TRPM8 Antagonist RGM8-51 Displays Antinociceptive Activity in Different Animal Models" International Journal of Molecular Sciences 23, no. 5: 2692. https://doi.org/10.3390/ijms23052692







