Associations between Maternal Risk Factors and Intrinsic Placental and Fetal Brain Functional Properties in Congenital Heart Disease
Abstract
:1. Introduction
2. Methods
2.1. Subject Population
2.2. Clinical Data
2.3. Maternal Risk Factors
2.4. Placental Pathology
2.5. MRI Image Acquisition
2.6. Fetal Brain Imaging
2.7. Image Analysis
2.7.1. Image Pre-Processing
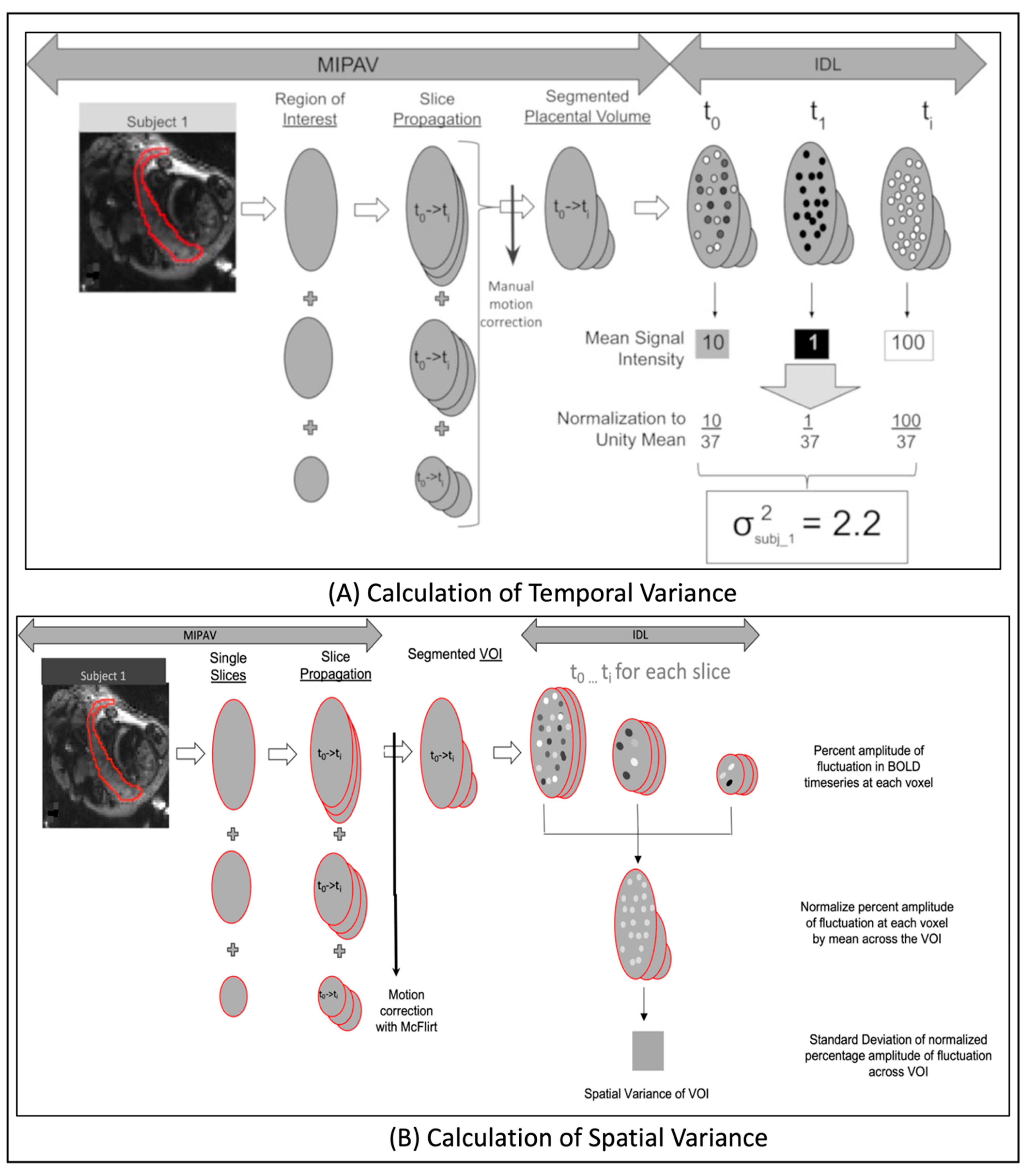
2.7.2. Computation of Temporal Variance
2.7.3. Computation of Spatial Variance
2.8. Statistical Analysis
3. Results
3.1. Recruitment and Patient Characteristics
3.2. Influence of Maternal Risk Factors
3.2.1. Placenta
3.2.2. Fetal Brain
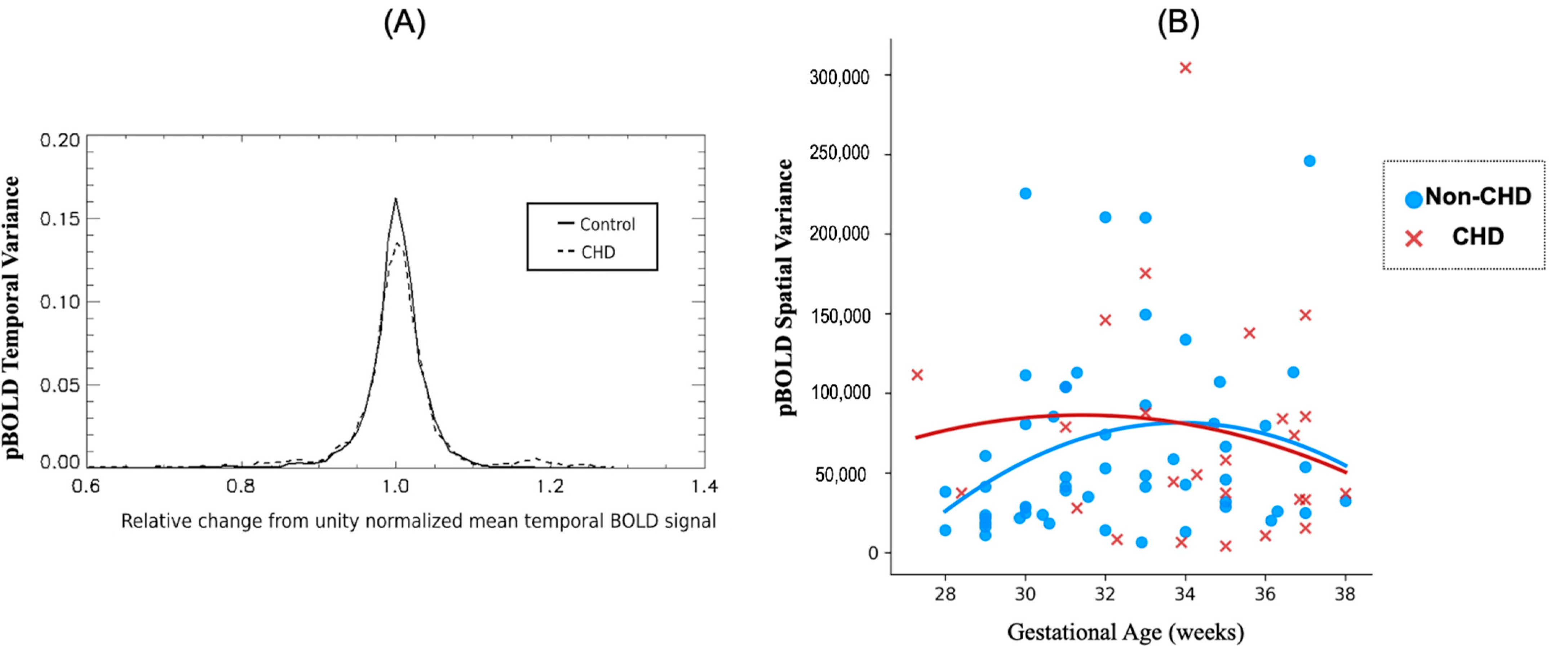
3.3. Differences between CHDs and Non-CHD Groups
3.3.1. Placenta
3.3.2. Fetal Brain
3.4. Interaction of MRFs and CHD
3.4.1. Placenta
3.4.2. Fetal Brain
4. Discussion
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRI | magnetic resonance imaging |
| CHD | congenital heart disease |
| MRF | maternal risk factors |
| pBOLD | placental Blood Oxygen Level Dependent MRI |
| brain BOLD | fetal brain resting Blood Oxygen Level Dependent MRI |
| GA | gestational age |
References
- Howell, K.R.; Powell, T.L. Effects of maternal obesity on placental function and fetal development. Reproduction 2017, 153, R97–R108. [Google Scholar] [CrossRef] [Green Version]
- Vambergue, A.; Fajardy, I. Consequences of gestational and pregestational diabetes on placental function and birth weight. World J. Diabetes 2011, 2, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Veerbeek, J.; Nikkels, P.; Torrance, H.; Gravesteijn, J.; Uiterweer, E.P.; Derks, J.; Koenen, S.; Visser, G.; Van Rijn, B.; Franx, A. Placental pathology in early intrauterine growth restriction associated with maternal hypertension. Placenta 2014, 35, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Rivera, H.M.; Christiansen, K.J.; Sullivan, E.L. The role of maternal obesity in the risk of neuropsychiatric disorders. Front. Neurosci. 2015, 9, 194. [Google Scholar] [CrossRef] [Green Version]
- Edlow, A.G. Maternal metabolic disease and offspring neurodevelopment—An evolving public health crisis. JAMA Netw. Open 2021, 4, e2129674. [Google Scholar] [CrossRef] [PubMed]
- Tuovinen, S.; Eriksson, J.G.; Kajantie, E.; Räikkönen, K. Maternal hypertensive pregnancy disorders and cognitive functioning of the offspring: A systematic review. J. Am. Soc. Hypertens. 2014, 8, 832–847.e831. [Google Scholar] [CrossRef]
- Andescavage, N.N.; Limperopoulos, C. Placental abnormalities in congenital heart disease. Transl. Pediatr. 2021, 10, 2148. [Google Scholar] [CrossRef]
- Andescavage, N.; Yarish, A.; Donofrio, M.; Bulas, D.; Evangelou, I.; Vezina, G.; McCarter, R.; duPlessis, A.; Limperopoulos, C. 3-D volumetric MRI evaluation of the placenta in fetuses with complex congenital heart disease. Placenta 2015, 36, 1024–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hare, C.B.; Mangin-Heimos, K.S.; Gu, H.; Edmunds, M.; Bebbington, M.; Lee, C.K.; He, M.; Ortinau, C.M. Placental Delayed Villous Maturation is Associated with Fetal Congenital Heart Disease. Am. J. Obstet. Gynecol. 2022. [Google Scholar] [CrossRef]
- Leon, R.L.; Sharma, K.; Mir, I.N.; Herrera, C.L.; Brown, S.L.; Spong, C.Y.; Chalak, L.F. Placental vascular malperfusion lesions in fetal congenital heart disease. Am. J. Obstet. Gynecol. 2022, 227, 620.e1–620.e8. [Google Scholar] [CrossRef]
- Leon, R.L.; Mir, I.N.; Herrera, C.L.; Sharma, K.; Spong, C.Y.; Twickler, D.M.; Chalak, L.F. Neuroplacentology in congenital heart disease: Placental connections to neurodevelopmental outcomes. Pediatr. Res. 2022, 91, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.W.; Moldenhauer, J.S.; Rychik, J.; Burnham, N.B.; Zullo, E.; Parry, S.I.; Simmons, R.A.; Elovitz, M.A.; Nicolson, S.C.; Linn, R.L.; et al. Damaging variants in proangiogenic genes impair growth in fetuses with cardiac defects. J. Pediatr. 2019, 213, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, J.W.; Parry, S.; Moldenhauer, J.S.; Simmons, R.A.; Rychik, J.; Ittenbach, R.F.; Russell, W.W.; Zullo, E.; Ward, J.L.; Nicolson, S.C.; et al. The impact of the maternal–foetal environment on outcomes of surgery for congenital heart disease in neonates. Eur. J. Cardio-Thorac. Surg. 2018, 54, 348–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, O.; Ruiz-Romero, A.; Domínguez, C.; Ferrer, Q.; Ribera, I.; Rodríguez-Sureda, V.; Alijotas, J.; Arévalo, S.; Carreras, E.; Cabero, L.; et al. Brain angiogenic gene expression in fetuses with congenital heart disease. Ultrasound Obstet. Gynecol. 2018, 52, 734–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savla, J.J.; Putt, M.E.; Huang, J.; Parry, S.; Moldenhauer, J.S.; Reilly, S.; Youman, O.; Rychik, J.; Mercer-Rosa, L.; Gaynor, J.W.; et al. Impact of Maternal–Fetal Environment on Mortality in Children With Single Ventricle Heart Disease. J. Am. Heart Assoc. 2022, 11, e020299. [Google Scholar] [CrossRef] [PubMed]
- Nealon, E.; Phelps, C.; Krawczeski, C.; Alexander, R.; Stiver, C.; Ball, M.K.; Carrillo, S.; Texter, K. Impact of Maternal-Fetal Environment on Outcomes following the Hybrid Procedure in the Single Ventricle Population; Research Square: Durham, NC, USA, 2022. [Google Scholar]
- Limperopoulos, C.; Wessel, D.L.; du Plessis, A.J. Understanding the maternal-fetal environment and the birth of prenatal pediatrics. J. Am. Heart Assoc. 2022, 11, e023807. [Google Scholar] [CrossRef]
- Tseng, S.Y.; Anderson, S.; DeFranco, E.; Rossi, R.; Divanovic, A.A.; Cnota, J.F. Severe maternal morbidity in pregnancies complicated by fetal congenital heart disease. JACC Adv. 2022, 1, 1–10. [Google Scholar] [CrossRef]
- Steinweg, J.K.; Hui, G.T.Y.; Pietsch, M.; Ho, A.; van Poppel, M.P.; Lloyd, D.; Colford, K.; Simpson, J.M.; Razavi, R.; Pushparajah, K.; et al. T2* placental MRI in pregnancies complicated with fetal congenital heart disease. Placenta 2021, 108, 23–31. [Google Scholar] [CrossRef]
- You, W.; Andescavage, N.N.; Kapse, K.; Donofrio, M.T.; Jacobs, M.; Limperopoulos, C. Hemodynamic responses of the placenta and brain to maternal hyperoxia in fetuses with congenital heart disease by using blood oxygen-level dependent MRI. Radiology 2020, 294, 141–148. [Google Scholar] [CrossRef]
- Rasanen, J.; Wood, D.C.; Debbs, R.H.; Cohen, J.; Weiner, S.; Huhta, J.C. Reactivity of the human fetal pulmonary circulation to maternal hyperoxygenation increases during the second half of pregnancy: A randomized study. Circulation 1998, 97, 257–262. [Google Scholar] [CrossRef]
- Sorensen, A.V.; Hutter, J.M.; Grant, E.P.; Seed, M.; Gowland, P. T2* weighted placental MRI: Basic research tool or an emerging clinical test of placental dysfunction? Ultrasound Obstet. Gynecol. 2020, 55, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Bilardo, C.; Snijders, R.; Campbell, S.; Nicolaides, K. Doppler study of the fetal circulation during long-term maternal hyperoxygenation for severe early onset intrauterine growth retardation. Ultrasound Obstet. Gynecol. 1991, 1, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, C.; Artini, P.G.; D’Ambrogio, G.; Galli, P.A.; Segre, A.; Genazzani, A.R. Maternal hyperoxygenation in the treatment of intrauterine growth retardation. Am. J. Obstet. Gynecol. 1992, 167, 430–435. [Google Scholar] [CrossRef]
- Sinding, M.; Peters, D.A.; Poulsen, S.S.; Frøkjær, J.B.; Christiansen, O.B.; Petersen, A.; Uldbjerg, N.; Sørensen, A. Placental baseline conditions modulate the hyperoxic BOLD-MRI response. Placenta 2018, 61, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yuan, L.-X.; Jia, X.-Z.; Zhou, X.-F.; Deng, X.-P.; He, H.-J.; Zhong, J.; Wang, J.; Zang, Y.-F. Intra-and inter-scanner reliability of voxel-wise whole-brain analytic metrics for resting state fMRI. Front. Neuroinformatics 2018, 12, 54. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.Z.; Sun, J.W.; Ji, G.J.; Liao, W.; Lv, Y.T.; Wang, J.; Wang, Z.; Zhang, H.; Liu, D.-Q.; Zang, Y.F. Percent amplitude of fluctuation: A simple measure for resting-state fMRI signal at single voxel level. PLoS ONE 2020, 15, e0227021. [Google Scholar] [CrossRef] [Green Version]
- Sutton, M.S.J.; Theard, M.A.; Bhatia, S.J.; Plappert, T.; Saltzman, D.H.; Doubilet, P. Changes in placental blood flow in the normal human fetus with gestational age. Pediatr. Res. 1990, 28, 383–387. [Google Scholar] [CrossRef] [Green Version]
- Romero-Garcia, R.; Hart, M.G.; Bethlehem, R.A.; Mandal, A.; Assem, M.; Crespo-Facorro, B.; Gorriz, J.M.; Burke, G.A.A.; Price, S.J.; Santarius, T.; et al. BOLD Coupling between Lesioned and Healthy Brain Is Associated with Glioma Patients’ Recovery. Cancers 2021, 13, 5008. [Google Scholar] [CrossRef]
- Yang, G.J.; Murray, J.D.; Repovs, G.; Cole, M.W.; Savic, A.; Glasser, M.F.; Pittenger, C.; Krystal, J.H.; Wang, X.-J.; Pearlson, G.D. Altered global brain signal in schizophrenia. Proc. Natl. Acad. Sci. USA 2014, 111, 7438–7443. [Google Scholar] [CrossRef] [Green Version]
- Melbourne, A.; Aughwane, R.; Sokolska, M.; Owen, D.; Kendall, G.; Flouri, D.; Bainbridge, A.; Atkinson, D.; Deprest, J.; Vercauteren, T.; et al. Separating fetal and maternal placenta circulations using multiparametric MRI. Magn. Reson. Med. 2019, 81, 350–361. [Google Scholar] [CrossRef]
- Pietryga, M.; Brazert, J.; Wender-Ozegowska, E.; Dubiel, M.; Gudmundsson, S. Placental Doppler velocimetry in gestational diabetes mellitus. J. Perinat. Med. 2006, 34, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, T.; Kaplin, A.; Goncharov, I.Y.; Malyutina, E.; Zemlyanskaya, L.; Nesterov, A. Uteroplacental blood flow in maternal diabetes mellitus. Arkhiv Patol. 2021, 83, 25–30. [Google Scholar] [CrossRef]
- Bedell, S.; Hutson, J.; de Vrijer, B.; Eastabrook, G. Effects of maternal obesity and gestational diabetes mellitus on the placenta: Current knowledge and targets for therapeutic interventions. Curr. Vasc. Pharmacol. 2021, 19, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.M. Perinatal implications of maternal hypertension. Semin. Pediatric Neurol. 2001, 8, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Khalil, R.A. Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia. Am. J. Physiol.-Heart Circ. Physiol. 2020, 319, H661–H681. [Google Scholar] [CrossRef] [PubMed]
- Remuzzi, G.; Ruggenenti, P. Prevention and treatment of pregnancy-associated hypertension: What have we learned in the last 10 years? Am. J. Kidney Dis. 1991, 18, 285–305. [Google Scholar] [CrossRef]
- Torres-Espínola, F.J.; Berglund, S.K.; Garcia, S.; Pérez-García, M.; Catena, A.; Rueda, R.; Sáez, J.A.; Campoy, C. Visual evoked potentials in offspring born to mothers with overweight, obesity and gestational diabetes. PLoS ONE 2018, 13, e0203754. [Google Scholar] [CrossRef] [Green Version]
- Shook, L.L.; Kislal, S.; Edlow, A.G. Fetal brain and placental programming in maternal obesity: A review of human and animal model studies. Prenat. Diagn. 2020, 40, 1126–1137. [Google Scholar] [CrossRef]
- Edlow, A.G. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat. Diagn. 2017, 37, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Wade, M.; Jenkins, J.M. Pregnancy hypertension and the risk for neuropsychological difficulties across early development: A brief report. Child Neuropsychol. 2016, 22, 247–254. [Google Scholar] [CrossRef]
- Linder, K.; Schleger, F.; Kiefer-Schmidt, I.; Fritsche, L.; Kümmel, S.; Heni, M.; Weiss, M.; Häring, H.-U.; Preissl, H.; Fritsche, A. Gestational diabetes impairs human fetal postprandial brain activity. J. Clin. Endocrinol. Metab. 2015, 100, 4029–4036. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Brown, C.; Hains, S.; Kisilevsky, B. Fetal development: Voice processing in normotensive and hypertensive pregnancies. Biol. Res. Nurs. 2007, 8, 272–282. [Google Scholar] [CrossRef]
- Zun, Z.; Zaharchuk, G.; Andescavage, N.N.; Donofrio, M.T.; Limperopoulos, C. Non-Invasive Placental Perfusion Imaging in Pregnancies Complicated by Fetal Heart Disease Using Velocity-Selective Arterial Spin Labeled MRI. Sci. Rep. 2017, 7, 16126. [Google Scholar] [CrossRef] [Green Version]
- Miremberg, H.; Gindes, L.; Schreiber, L.; Raucher Sternfeld, A.; Bar, J.; Kovo, M. The association between severe fetal congenital heart defects and placental vascular malperfusion lesions. Prenat. Diagn. 2019, 39, 962–967. [Google Scholar] [CrossRef]
- Ozcan, T.; Ravishankar, S.; Kikano, S.; Plummer, S.; Strainic, J. 162: Congenital cardiac defects and placental vascular malperfusion. Am. J. Obstet. Gynecol. 2020, 222, S117–S118. [Google Scholar] [CrossRef]
- Yu, Y.; Arah, O.A.; Liew, Z.; Cnattingius, S.; Olsen, J.; Sørensen, H.T.; Qin, G.; Li, J. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: Population based cohort study with 40 years of follow-up. BMJ 2019, 367, l6398. [Google Scholar] [CrossRef] [Green Version]
- Rowland, T.W.; Hubbell, J.P., Jr.; Nadas, A.S. Congenital heart disease in infants of diabetic mothers. J. Pediatr. 1973, 83, 815–820. [Google Scholar] [CrossRef]
- Peyvandi, S.; Xu, D.; Wang, Y.; Hogan, W.; Moon-Grady, A.; Barkovich, A.J.; Glenn, O.; McQuillen, P.; Liu, J. Fetal cerebral oxygenation is impaired in congenital heart disease and shows variable response to maternal hyperoxia. J. Am. Heart Assoc. 2021, 10, e018777. [Google Scholar] [CrossRef]
- Lauridsen, M.H.; Uldbjerg, N.; Henriksen, T.B.; Petersen, O.B.; Stausbøl-Grøn, B.; Matthiesen, N.B.; Peters, D.A.; Ringgaard, S.; Hjortdal, V.E. Cerebral Oxygenation Measurements by Magnetic Resonance Imaging in Fetuses with and without Heart Defects. Circ. Cardiovasc. Imaging 2017, 10, e006459. [Google Scholar] [CrossRef] [Green Version]
- You, W.; Donofrio, M.; Wessel, D.; Zun, Z.; De Asis-Cruz, J.; Vezina, G.; Bulas, D.; Jonas, R.; du Plessis, A.; Limperopoulos, C. Maternal Hyperoxia Increases Cerebral Oxygenation in Fetuses With Complex Congenital Heart Disease: A Functional MRI Study. Circulation 2015, 132, A19532. [Google Scholar] [CrossRef]
- Sun, L.; Macgowan, C.K.; Sled, J.G.; Yoo, S.J.; Manlhiot, C.; Porayette, P.; Grosse-Wortmann, L.; Jaeggi, E.; McCrindle, B.W.; Kingdom, J.; et al. Reduced Fetal Cerebral Oxygen Consumption is Associated With Smaller Brain Size in Fetuses With Congenital Heart Disease. Circulation 2015, 131, 1313–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di, X.; Kim, E.H.; Huang, C.-C.; Tsai, S.-J.; Lin, C.-P.; Biswal, B.B. The influence of the amplitude of low-frequency fluctuations on resting-state functional connectivity. Front. Hum. Neurosci. 2013, 7, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, Y.; Meng, C.; Yang, Y.; Zhu, C.; Wang, L.; Yan, Q.; Lin, C.; Yu, C. Resting-state brain activity in adult males who stutter. PLoS ONE 2012, 7, e30570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermans, T.; Thewissen, L.; Gewillig, M.; Cools, B.; Jansen, K.; Pillay, K.; De Vos, M.; Van Huffel, S.; Naulaers, G.; Dereymaeker, A. Functional brain maturation and sleep organisation in neonates with congenital heart disease. Eur. J. Paediatr. Neurol. 2022, 36, 115–122. [Google Scholar] [CrossRef]
- Sadhwani, A.; Wypij, D.; Rofeberg, V.; Gholipour, A.; Mittleman, M.; Rohde, J.; Velasco-Annis, C.; Calderon, J.; Friedman, K.G.; Tworetzky, W.; et al. Fetal brain volume predicts neurodevelopment in congenital heart disease. Circulation 2022, 145, 1108–1119. [Google Scholar] [CrossRef]
- Schabdach, J.; Ceschin, R.; Schmithorst, V.; Tisdall, M.D.; Alexander-Bloch, A.; Panigrahy, A. A Descriptive Review of the Impact of Patient Motion in Early Childhood Resting-State Functional Magnetic Resonance Imaging. Diagnostics 2022, 12, 1032. [Google Scholar] [CrossRef]
- Brombach, C.; Tong, W.; Giussani, D.A. Maternal obesity: New placental paradigms unfolded. Trends Mol. Med. 2022, 28, 823–835. [Google Scholar] [CrossRef]
- Desoye, G. The human placenta in diabetes and obesity: Friend or foe? The 2017 Norbert Freinkel award lecture. Diabetes Care 2018, 41, 1362–1369. [Google Scholar] [CrossRef]

| Demographics and Clinical Characteristics | Non-CHD (n = 114) | CHD (n = 58) | p -Value |
|---|---|---|---|
| GA at MRI (wks) | 32.27 (3.01) | 33.62 (3.10) | 0.0136 |
| Mean maternal age at delivery—years (SD) | 30.69 (4.57) | 29.22 (5.94) | 0.2156 |
| Sex, n (%) | 0.0668 | ||
| Male | 48 (42.11) | 33 (56.90) | |
| Female | 66 (57.89) | 25 (43.10) | |
| Race, n (%) | 0.4461 | ||
| White | 87 (76.32) | 44 (75.86) | |
| Black | 18 (15.79) | 11 (18.97) | |
| Other or Unknown | 9 (7.89) | 3 (5.17) | |
| Cardiac Lesion, n (%) | |||
| Hypoplastic left heart syndrome | . | 14 (24.14) | |
| Transposition of the great arteries | . | 13 (22.41) | |
| Double outlet right ventricle | . | 9 (15.52) | |
| Tetralogy of fallot | . | 9 (15.52) | |
| Complex single ventricle (TA, PA) | . | 11 (18.97) | |
| Coarctation of aorta | . | 5 (8.62) | |
| Other (AVC, VSD, ASD) | . | 33 (56.90) |
| Maternal Risk Factors | Non-CHD (n = 114) | CHD (n = 58) |
|---|---|---|
| Pregnancy Complications, n (%) | ||
| Any Risk | 65 (57.01) | 28 (48.27) |
| Maternal Obesity | 58 (50.88) | 28 (48.27) |
| Maternal Hypertension | 27 (23.69) | 6 (10.35) |
| Maternal Diabetes | 13 (11.40) | 5 (6.90) |
| Infant and Placental Characteristics | Non-CHD (n = 114) | CHD (n = 58) | p-Value |
|---|---|---|---|
| Birth Characteristics | |||
| Sex, n (%) | 0.0668 | ||
| Male | 48 (42.11) | 33 (56.90) | . |
| Female | 66 (57.89) | 25 (43.10) | . |
| Mean GA at birth —weeks (SD) | 38.8 (2.36) | 38.4 (1.30) | 0.2248 |
| Mean birthweight (SD) | 3225.4 (637.58) | 2972.0 (708.67) | 0.0216 |
| Mean birthweight percentile (SD) | 47.5 (26.50) | 39.6 (30.57) | 0.0876 |
| Mean length (SD) | 49.7 (4.64) | 48.0 (4.68) | 0.0262 |
| Mean length percentile (SD) | 58.0 (31.44) | 41.3 (32.50) | 0.0019 |
| Mean head circumference (SD) | 34.4 (3.69) | 33.2 (2.79) | 0.0289 |
| Mean head circumference percentile (SD) | 48.0 (30.63) | 37.6 (32.60) | 0.0497 |
| Mean number of days in NICU/CICU (SD) | 2.20 (8.58) | 35.86 (41.45) | <0.0001 |
| Placental Characteristics | |||
| Mean placental weight (SD) | 450.6 (104.39) | 428.2 (120.99) | 0.3651 |
| Main Effect * FDR Corrected | CHD Interaction | ||||
|---|---|---|---|---|---|
| Variable | Regression Parameter | One-Sided p | Regression Parameter | One-Sided p | |
| Placenta | ANY MATERNAL RISK FACTOR PRESENT | −59.05 | 0.276 | −430.58 | 0.047 * |
| MATERNAL DIABETES MELLITUS | −90.1 | 0.012 * | −70.43 | 0.258 | |
| MATERNAL HYPERTENSION | −36.44 | 0.046 * | −42.09 | 0.211 | |
| MATERNAL OBESITY | −5.26 | 0.172 | −5.47 | 0.359 | |
| Brain | ANY MATERNAL RISK FACTOR PRESENT | 44.75 | 0.226 | −21.79 | 0.398 |
| MATERNAL DIABETES MELLITUS | −66.19 | 0.05 * | 97.72 | 0.117 | |
| MATERNAL HYPERTENSION | −85.06 | 0.015 * | 93.27 | 0.062 | |
| MATERNAL OBESITY | 3.73 | 0.078 | −2.04 | 0.281 | |
| Main Effect * FDR Corrected | CHD Interaction | ||||
|---|---|---|---|---|---|
| Variable | Regression Parameter | One-Sided p | Regression Parameter | One-Sided p | |
| Placenta | ANY MATERNAL RISK FACTOR PRESENT | −134.051 | 0.49 | −270.47 | 0.0532 |
| MATERNAL DIABETES MELLITUS | −217.44 | 0.442 | 257.57 | 0.44 | |
| MATERNAL HYPERTENSION | −102.28 | 0.046 * | −877.62 | 0.148 | |
| MATERNAL OBESITY | −40.055 | 0.0173 * | −46.65 | 0.92 | |
| Brain | ANY MATERNAL RISK FACTOR PRESENT | −0.3814 | 0.0812 | 0.0081 | 0.987 |
| MATERNAL DIABETES MELLITUS | −0.7164 | 0.024 * | −0.0422 | 0.958 | |
| MATERNAL HYPERTENSION | −0.7920 | 0.008 * | 0.3228 | 0.636 | |
| MATERNAL OBESITY | 0.4037 | 0.297 | −0.9472 | 0.092 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajagopalan, V.; Schmithorst, V.; El-Ali, A.; Reynolds, W.; Lee, V.; Wallace, J.; Weinberg, J.; Johnson, J.; Votava-Smith, J.; Adibi, J.; et al. Associations between Maternal Risk Factors and Intrinsic Placental and Fetal Brain Functional Properties in Congenital Heart Disease. Int. J. Mol. Sci. 2022, 23, 15178. https://doi.org/10.3390/ijms232315178
Rajagopalan V, Schmithorst V, El-Ali A, Reynolds W, Lee V, Wallace J, Weinberg J, Johnson J, Votava-Smith J, Adibi J, et al. Associations between Maternal Risk Factors and Intrinsic Placental and Fetal Brain Functional Properties in Congenital Heart Disease. International Journal of Molecular Sciences. 2022; 23(23):15178. https://doi.org/10.3390/ijms232315178
Chicago/Turabian StyleRajagopalan, Vidya, Vanessa Schmithorst, Alexander El-Ali, William Reynolds, Vincent Lee, Julia Wallace, Jacqueline Weinberg, Jennifer Johnson, Jodie Votava-Smith, Jennifer Adibi, and et al. 2022. "Associations between Maternal Risk Factors and Intrinsic Placental and Fetal Brain Functional Properties in Congenital Heart Disease" International Journal of Molecular Sciences 23, no. 23: 15178. https://doi.org/10.3390/ijms232315178
APA StyleRajagopalan, V., Schmithorst, V., El-Ali, A., Reynolds, W., Lee, V., Wallace, J., Weinberg, J., Johnson, J., Votava-Smith, J., Adibi, J., & Panigrahy, A. (2022). Associations between Maternal Risk Factors and Intrinsic Placental and Fetal Brain Functional Properties in Congenital Heart Disease. International Journal of Molecular Sciences, 23(23), 15178. https://doi.org/10.3390/ijms232315178






