Synergistic In Vitro Anticancer Toxicity of Pulsed Electric Fields and Glutathione
Abstract
1. Introduction
2. Results
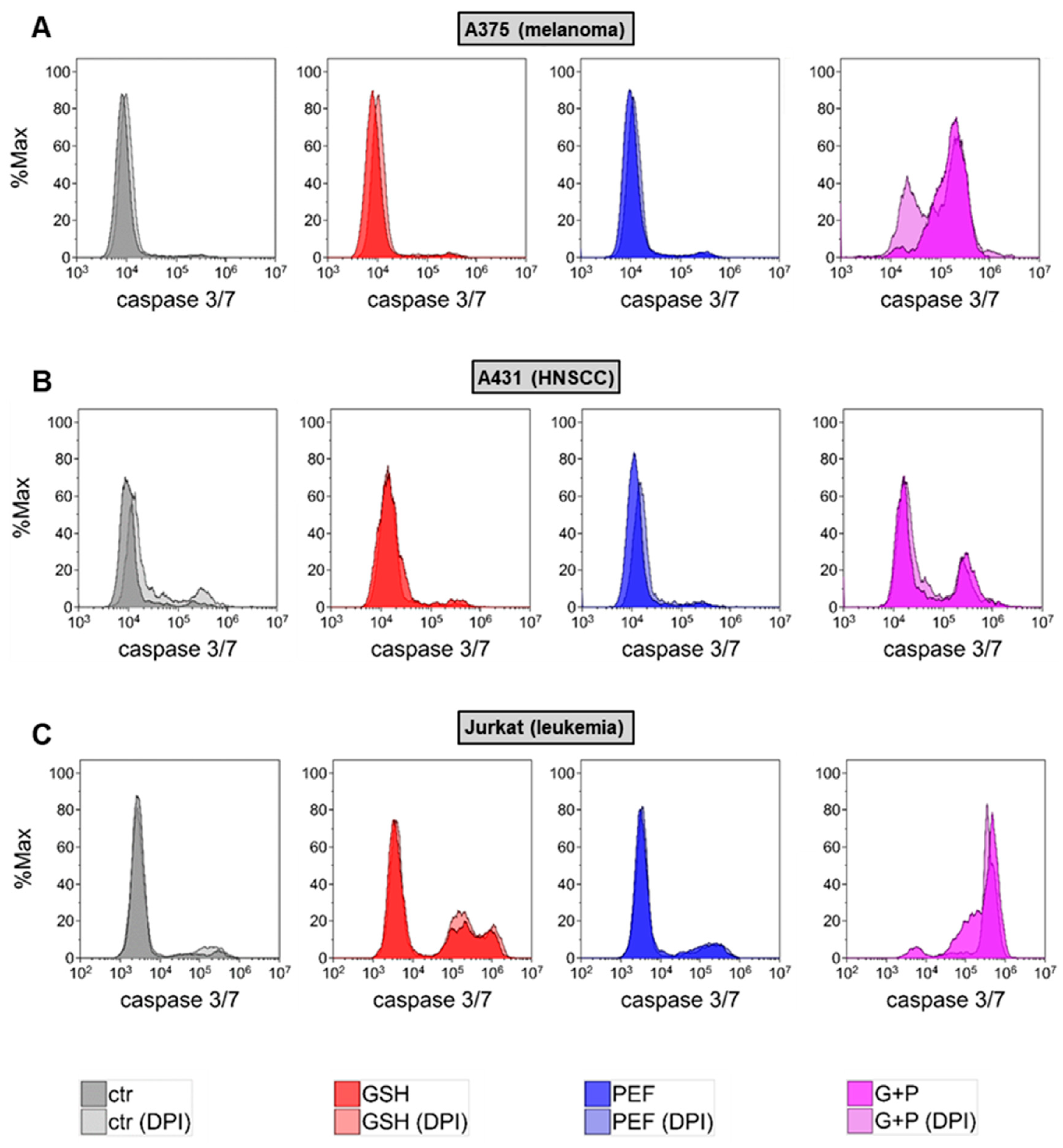
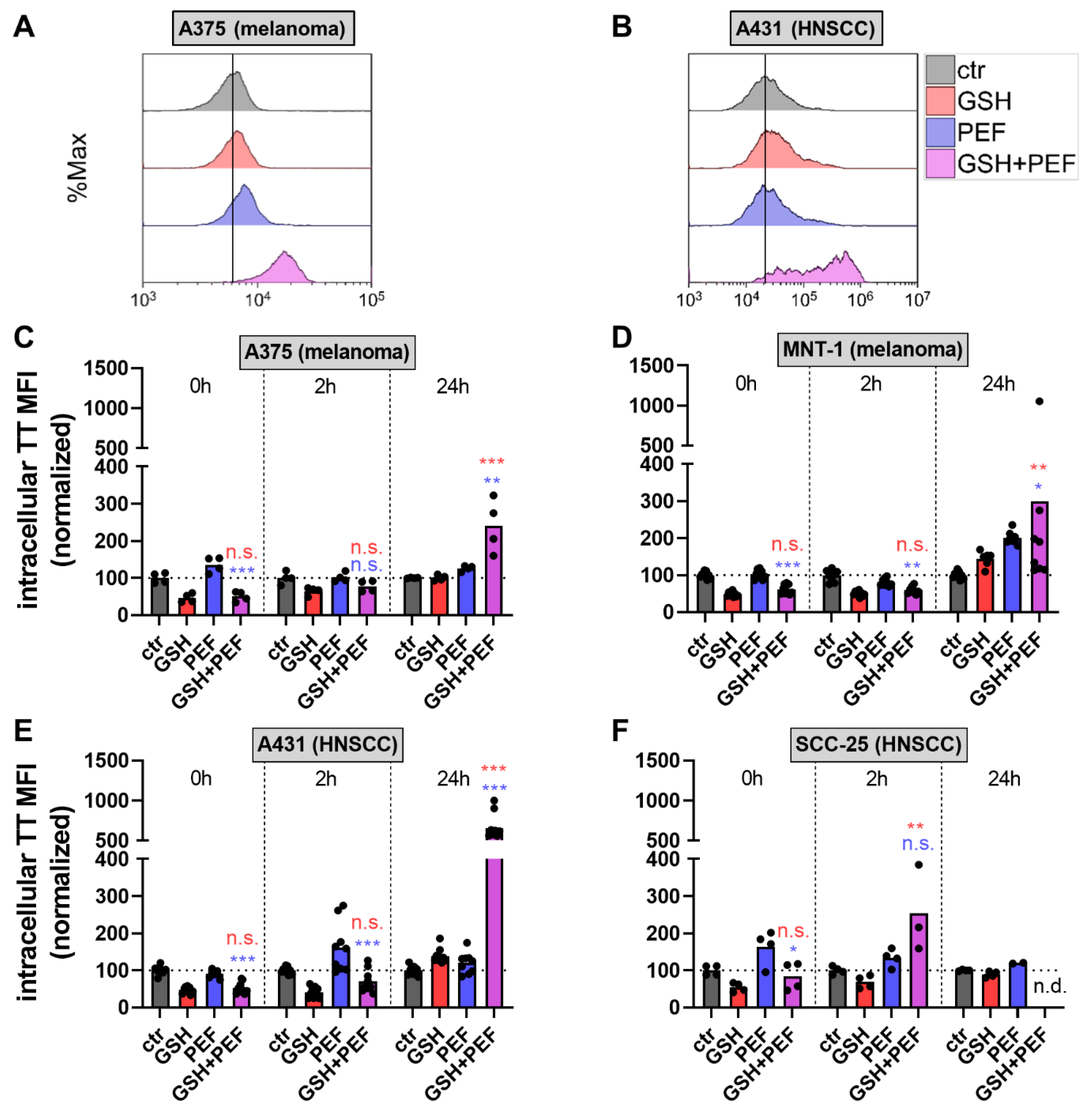
2.1. Synergistic GSH and PEF Treatment Shows Striking Anticancer Toxicity
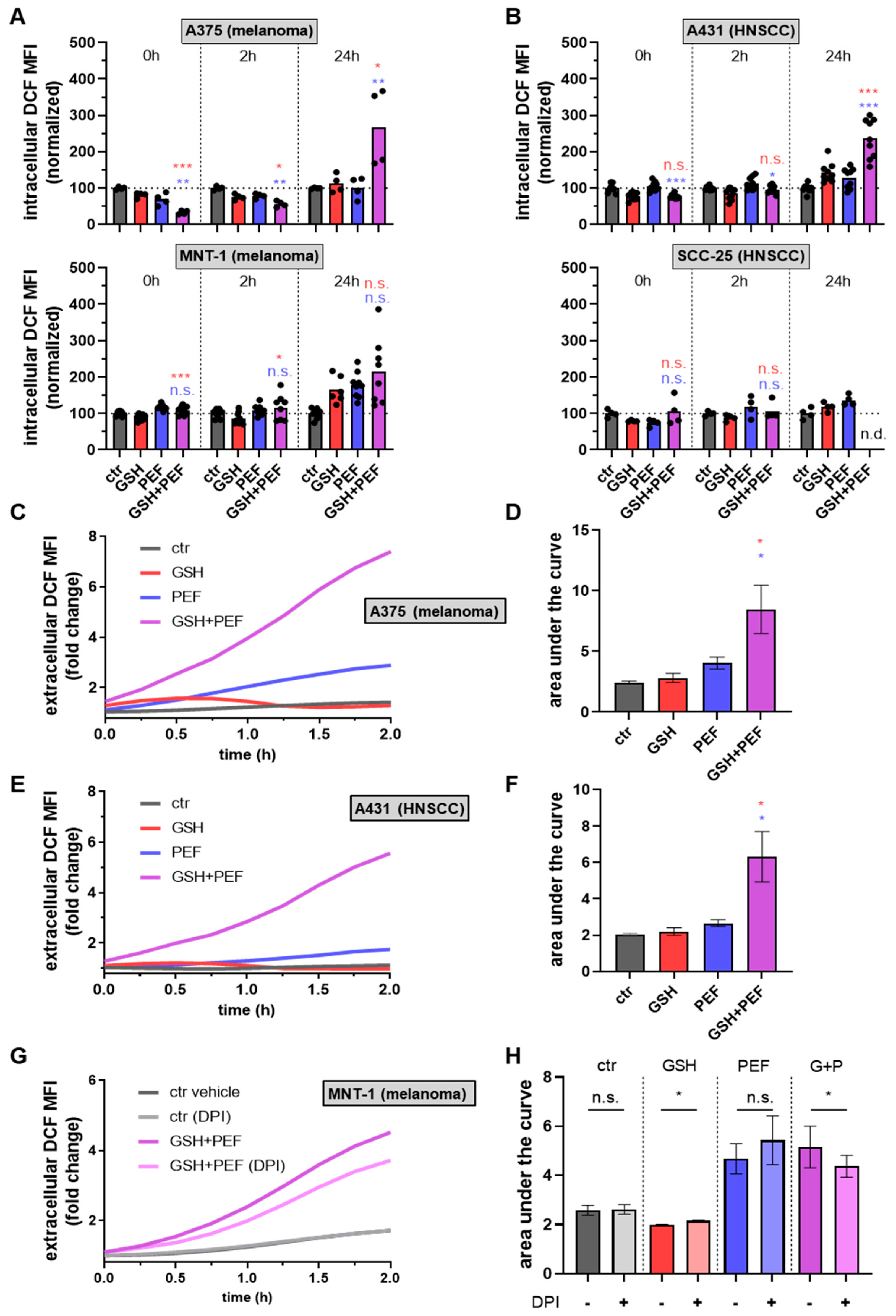
2.2. Presence and Roles of Reactive Oxygen Species in Combined GSH + PEF Toxicity
3. Discussion
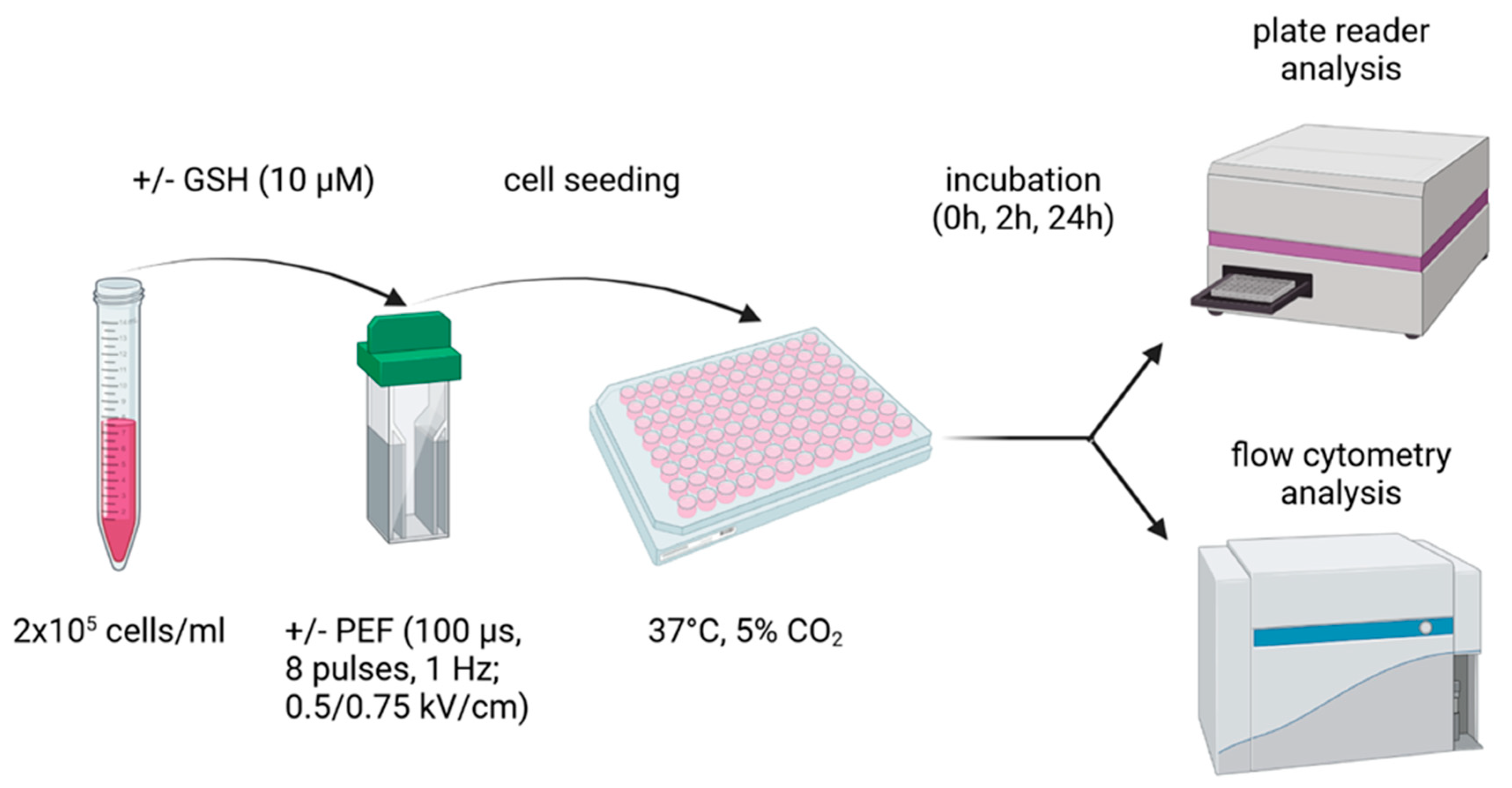
4. Materials and Methods
4.1. Cell Culture
4.2. Pulsed Electric Fields (PEF) Application
4.3. Metabolic Activity
4.4. Cell Viability
4.5. Intracellular Glutathione
4.6. Intracellular and Extracellular Reactive Oxygen Species
4.7. Software and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The changing therapeutic landscape of head and neck cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Fisher, D.E. Treatment of Advanced Melanoma in 2020 and Beyond. J. Investig. Dermatol. 2020, 141, 23–31. [Google Scholar] [CrossRef]
- Reinhold, U. Electrochemotherapy for primary skin cancer and skin metastasis related to other malignancies. Anti-Cancer Drugs 2011, 22, 711–718. [Google Scholar] [CrossRef]
- Landström, F.J.; Nilsson, C.O.; Crafoord, S.; Reizenstein, J.A.; Adamsson, G.-B.M.; Löfgren, L.A. Electroporation Therapy of Skin Cancer in the Head and Neck Area. Dermatol. Surg. 2010, 36, 1245–1250. [Google Scholar] [CrossRef]
- Bertino, G.; Sersa, G.; De Terlizzi, F.; Occhini, A.; Plaschke, C.C.; Groselj, A.; Langdon, C.; Grau, J.J.; McCaul, J.A.; Heuveling, D.; et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results of the treatment of skin cancer. Eur. J. Cancer 2016, 63, 41–52. [Google Scholar] [CrossRef]
- Mali, B.; Jarm, T.; Snoj, M.; Sersa, G.; Miklavcic, D. Antitumor effectiveness of electrochemotherapy: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2013, 39, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Wolff, C.M.; Steuer, A.; Stoffels, I.; von Woedtke, T.; Weltmann, K.-D.; Bekeschus, S.; Kolb, J.F. Combination of cold plasma and pulsed electric fields—A rationale for cancer patients in palliative care. Clin. Plasma Med. 2019, 16, 100096. [Google Scholar] [CrossRef]
- Radzevičiūtė, E.; Malyško-Ptašinskė, V.; Kulbacka, J.; Rembiałkowska, N.; Novickij, J.; Girkontaitė, I.; Novickij, V. Nanosecond electrochemotherapy using bleomycin or doxorubicin: Influence of pulse amplitude, duration and burst frequency. Bioelectrochemistry 2022, 148, 108251. [Google Scholar] [CrossRef]
- Łapińska, Z.; Dębiński, M.; Szewczyk, A.; Choromańska, A.; Kulbacka, J.; Saczko, J. Electrochemotherapy with Calcium Chloride and 17β-Estradiol Modulated Viability and Apoptosis Pathway in Human Ovarian Cancer. Pharmaceutics 2021, 13, 19. [Google Scholar] [CrossRef]
- Ágoston, D.; Baltás, E.; Ócsai, H.; Rátkai, S.; Lázár, P.G.; Korom, I.; Varga, E.; Németh, I.B.; Viharosné, D.-R.; Gehl, J.; et al. Evaluation of Calcium Electroporation for the Treatment of Cutaneous Metastases: A Double Blinded Randomised Controlled Phase II Trial. Cancers 2020, 12, 179. [Google Scholar] [CrossRef]
- Daeschlein, G.; Scholz, S.; Lutze, S.; Arnold, A.; Von Podewils, S.; Kiefer, T.; Tueting, T.; Hardt, O.; Haase, H.; Grisk, O.; et al. Comparison between cold plasma, electrochemotherapy and combined therapy in a melanoma mouse model. Exp. Dermatol. 2013, 22, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Mozzillo, N.; Simeone, E.; Benedetto, L.; Curvietto, M.; Giannarelli, D.; Gentilcore, G.; Camerlingo, R.; Capone, M.; Madonna, G.; Festino, L.; et al. Assessing a novel immuno-oncology-based combination therapy: Ipilimumab plus electrochemotherapy. OncoImmunology 2015, 4, e1008842. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Sandhu, J.K.; Harper, M.-E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Ricotti, F.; Giuliodori, K.; Cataldi, I.; Campanati, A.; Ganzetti, G.; Ricotti, G.; Offidani, A. Electrochemotherapy: An effective local treatment of cutaneous and subcutaneous melanoma metastases. Dermatol. Ther. 2013, 27, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Sloot, S.; Rashid, O.; Sarnaik, A.A.; Zager, J.S. Developments in Intralesional Therapy for Metastatic Melanoma. Cancer Control 2016, 23, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Raththagala, M.; Root, P.D.; Spence, D.M. Dynamic Monitoring of Glutathione in Erythrocytes, without a Separation Step, in the Presence of an Oxidant Insult. Anal. Chem. 2006, 78, 8556–8560. [Google Scholar] [CrossRef]
- White, J.A.; Pliquett, U.; Blackmore, P.F.; Joshi, R.P.; Schoenbach, K.H.; Kolb, J.F. Plasma membrane charging of Jurkat cells by nanosecond pulsed electric fields. Eur. Biophys. J. 2011, 40, 947–957. [Google Scholar] [CrossRef]
- Huang, F.; Fang, Z.; Mast, J.; Chen, W. Comparison of membrane electroporation and protein denature in response to pulsed electric field with different durations. Bioelectromagnetics 2013, 34, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.R.; Regen, S.L. The Structural Role of Cholesterol in Cell Membranes: From Condensed Bilayers to Lipid Rafts. Acc. Chem. Res. 2014, 47, 3512–3521. [Google Scholar] [CrossRef] [PubMed]
- Hanna, H.; Denzi, A.; Liberti, M.; André, F.M.; Mir, L.M. Electropermeabilization of Inner and Outer Cell Membranes with Microsecond Pulsed Electric Fields: Quantitative Study with Calcium Ions. Sci. Rep. 2017, 7, 13079. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Gutscher, M.; Pauleau, A.-L.; Marty, L.; Brach, T.; Wabnitz, G.H.; Samstag, Y.; Meyer, A.J.; Dick, T.P. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods 2008, 5, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.J.; Reaume, R.J.; Pradhan, A.K. Visual Detection of Denatured Glutathione Peptides: A Facile Method to Visibly Detect Heat Stressed Biomolecules. Sci. Rep. 2017, 7, 2604. [Google Scholar] [CrossRef] [PubMed]
- Boyault, C.; Zhang, Y.; Fritah, S.; Caron, C.; Gilquin, B.; Kwon, S.H.; Garrido, C.; Yao, T.-P.; Vourc’H, C.; Matthias, P.; et al. HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes Dev. 2007, 21, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Bucciantini, M.; Calloni, G.; Chiti, F.; Formigli, L.; Nosi, D.; Dobson, C.M.; Stefani, M. Prefibrillar Amyloid Protein Aggregates Share Common Features of Cytotoxicity. J. Biol. Chem. 2004, 279, 31374–31382. [Google Scholar] [CrossRef] [PubMed]
- Tanori, M.; Casciati, A.; Zambotti, A.; Pinto, R.; Gianlorenzi, I.; Pannicelli, A.; Giardullo, P.; Benassi, B.; Marino, C.; Mancuso, M.; et al. Microsecond Pulsed Electric Fields: An Effective Way to Selectively Target and Radiosensitize Medulloblastoma Cancer Stem Cells. Int. J. Radiat. Oncol. 2021, 109, 1495–1507. [Google Scholar] [CrossRef]
- Yoo, S.K.; Huttenlocher, A. Spatiotemporal photolabeling of neutrophil trafficking during inflammation in live zebrafish. J. Leukoc. Biol. 2011, 89, 661–667. [Google Scholar] [CrossRef]
- Goswami, I.; Perry, J.B.; Allen, M.E.; Brown, D.A.; von Spakovsky, M.R.; Verbridge, S.S. Influence of Pulsed Electric Fields and Mitochondria-Cytoskeleton Interactions on Cell Respiration. Biophys. J. 2018, 114, 2951–2964. [Google Scholar] [CrossRef]
- Liu, J.; Shen, H.M.; Ong, C.N. Role of intracellular thiol depletion, mitochondrial dysfunction and reactive oxygen species in Salvia miltiorrhiza-induced apoptosis in human hepatoma HepG2 cells. Life Sci. 2001, 69, 1833–1850. [Google Scholar] [CrossRef]
- Koppula, P.; Zhang, Y.; Shi, J.; Li, W.; Gan, B. The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. J. Biol. Chem. 2017, 292, 14240–14249. [Google Scholar] [CrossRef]
- Wang, Y.; Yen, F.S.; Zhu, X.G.; Timson, R.C.; Weber, R.; Xing, C.; Liu, Y.; Allwein, B.; Luo, H.; Yeh, H.-W.; et al. SLC25A39 is necessary for mitochondrial glutathione import in mammalian cells. Nature 2021, 599, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.L.; Mena, S.; Estrela, J.M. Glutathione in Cancer Cell Death. Cancers 2011, 3, 1285–1310. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Wang, Y.; Lin, S.; Zhao, P.; Jones, G. A novel application of pulsed electric field (PEF) processing for improving glutathione (GSH) antioxidant activity. Food Chem. 2014, 161, 361–366. [Google Scholar] [CrossRef]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, D.; et al. Electrochemotherapy—An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]


| A375 | MNT-1 | A431 | SSC-25 | THP-1 | Jurkat | |
|---|---|---|---|---|---|---|
| cell viability | 0.08 | 0.56 | 0.33 | 0.12 | 0.56 | 0.04 |
| metabolic activity | 0.09 | 0.29 | 0.16 | 0.55 | 0.05 | 0/n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolff, C.M.; Bekeschus, S. Synergistic In Vitro Anticancer Toxicity of Pulsed Electric Fields and Glutathione. Int. J. Mol. Sci. 2022, 23, 14772. https://doi.org/10.3390/ijms232314772
Wolff CM, Bekeschus S. Synergistic In Vitro Anticancer Toxicity of Pulsed Electric Fields and Glutathione. International Journal of Molecular Sciences. 2022; 23(23):14772. https://doi.org/10.3390/ijms232314772
Chicago/Turabian StyleWolff, Christina M., and Sander Bekeschus. 2022. "Synergistic In Vitro Anticancer Toxicity of Pulsed Electric Fields and Glutathione" International Journal of Molecular Sciences 23, no. 23: 14772. https://doi.org/10.3390/ijms232314772
APA StyleWolff, C. M., & Bekeschus, S. (2022). Synergistic In Vitro Anticancer Toxicity of Pulsed Electric Fields and Glutathione. International Journal of Molecular Sciences, 23(23), 14772. https://doi.org/10.3390/ijms232314772






