Chlamydial Infection-Dependent Synthesis of Sphingomyelin as a Novel Anti-Chlamydial Target of Ceramide Mimetic Compounds
Abstract
:1. Introduction
2. Results
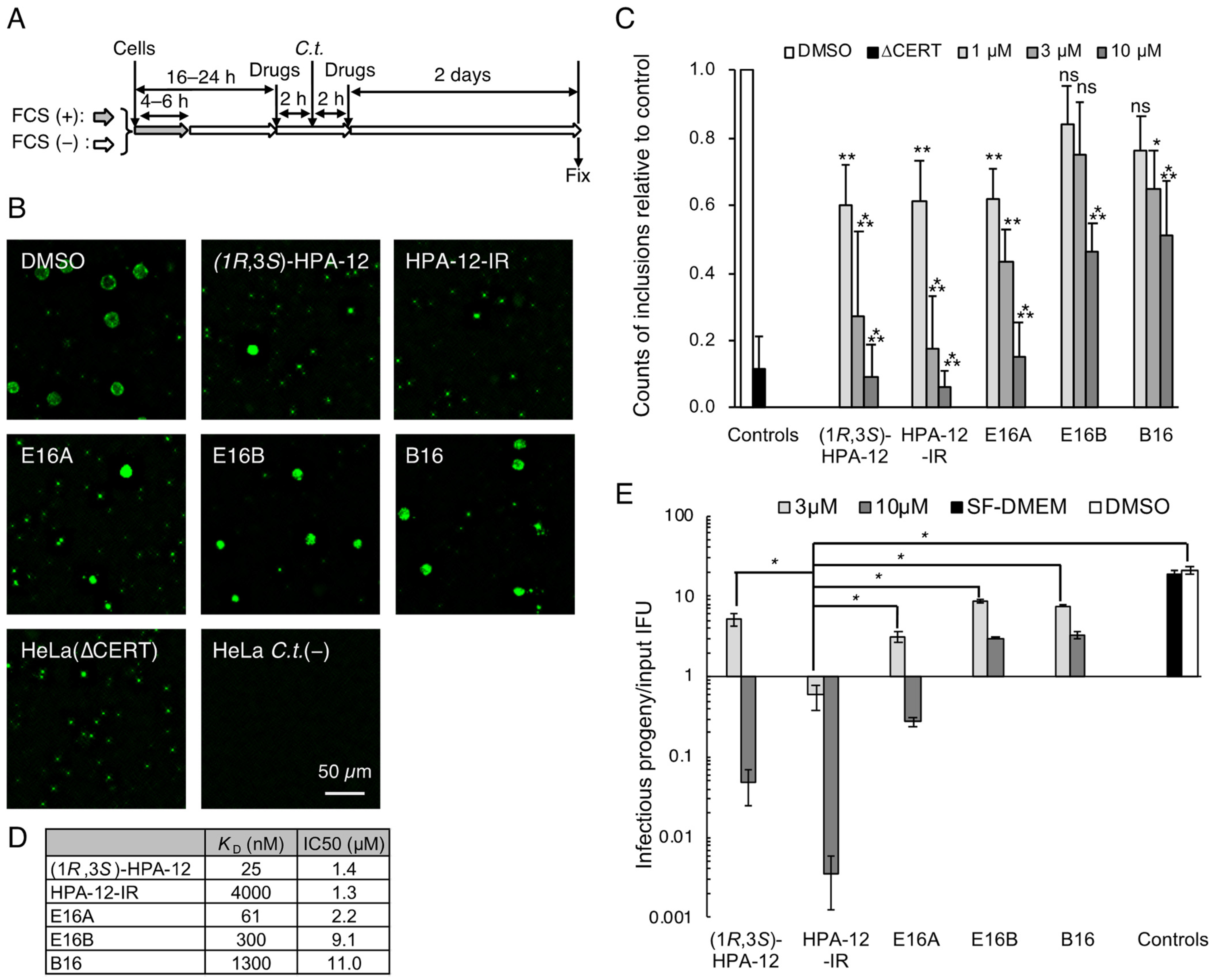
2.1. Repression of C. trachomatis Proliferation in HeLa Cells by CERT Inhibitors
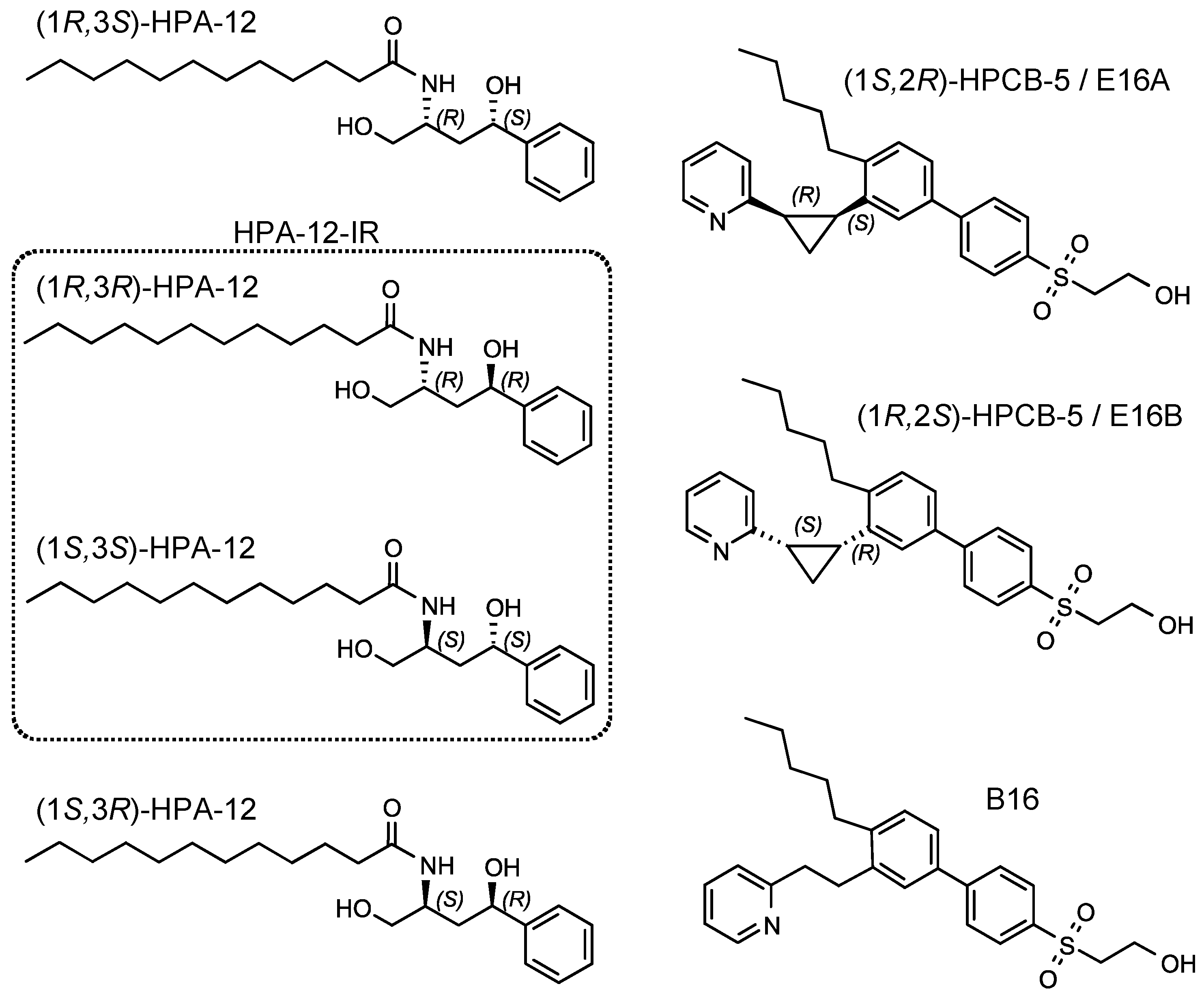
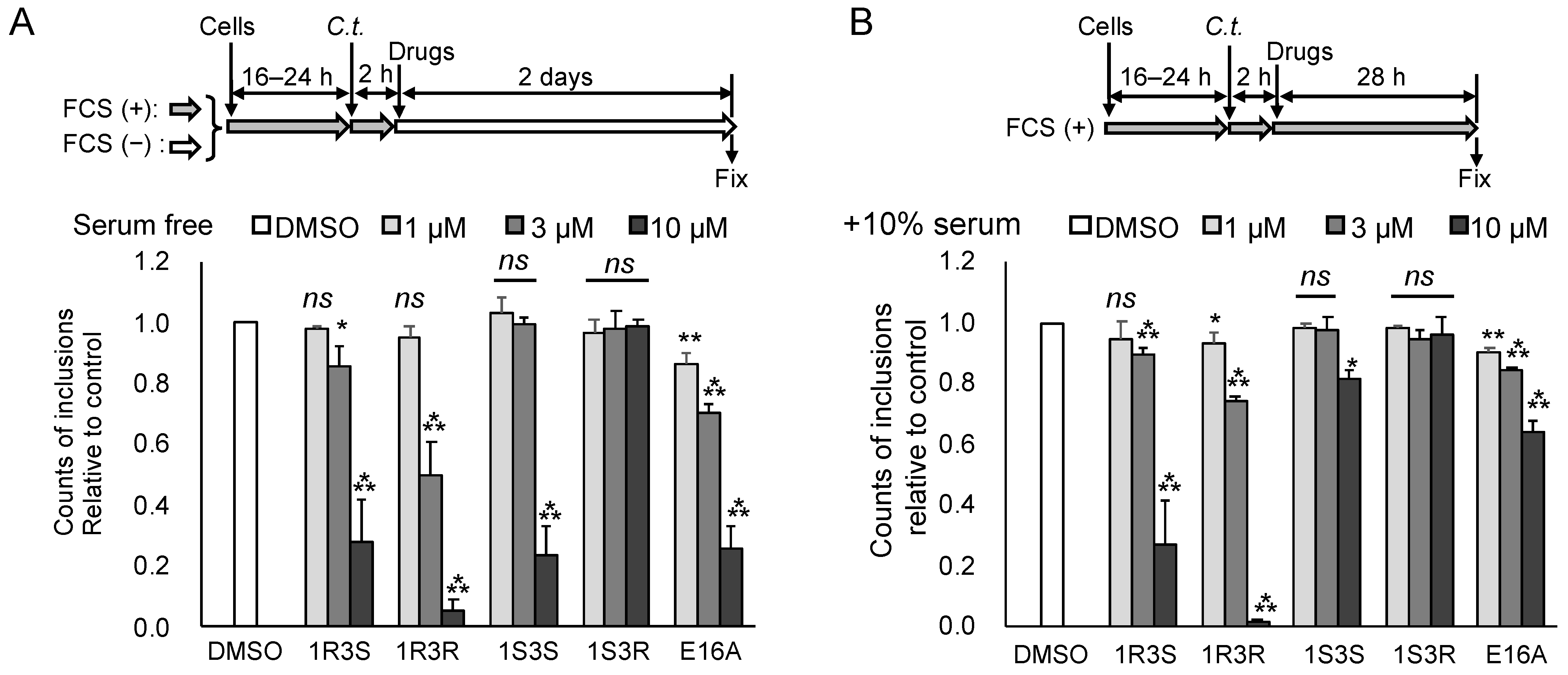
2.2. Repression of C. trachomatis Proliferation by HPA-12 Isomers Which Lack CERT Inhibitory Activity
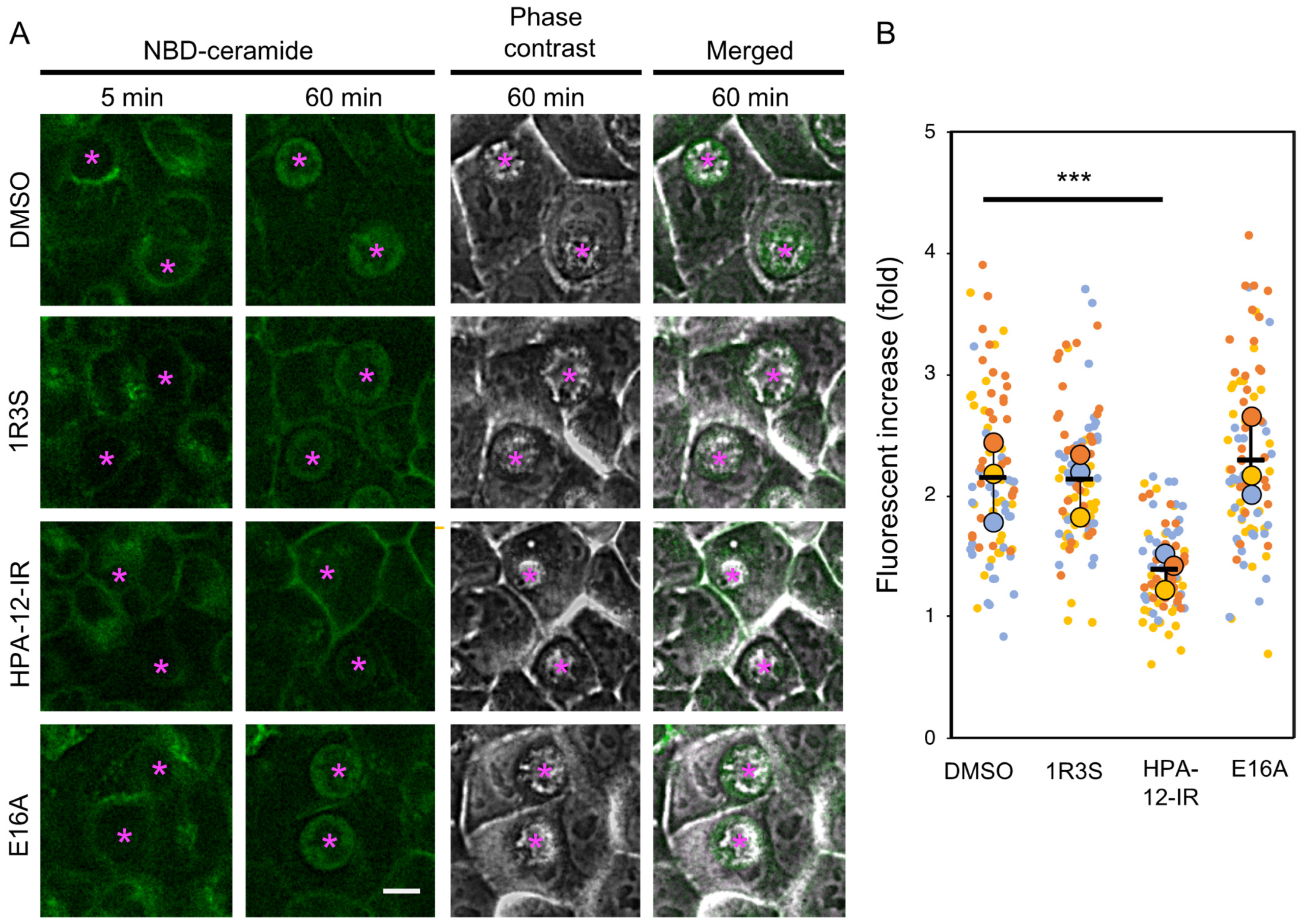
2.3. (1R,3R)-HPA-12 Inhibited the Redistribution of a Fluorescence-Labeled Ceramide in the Inclusions
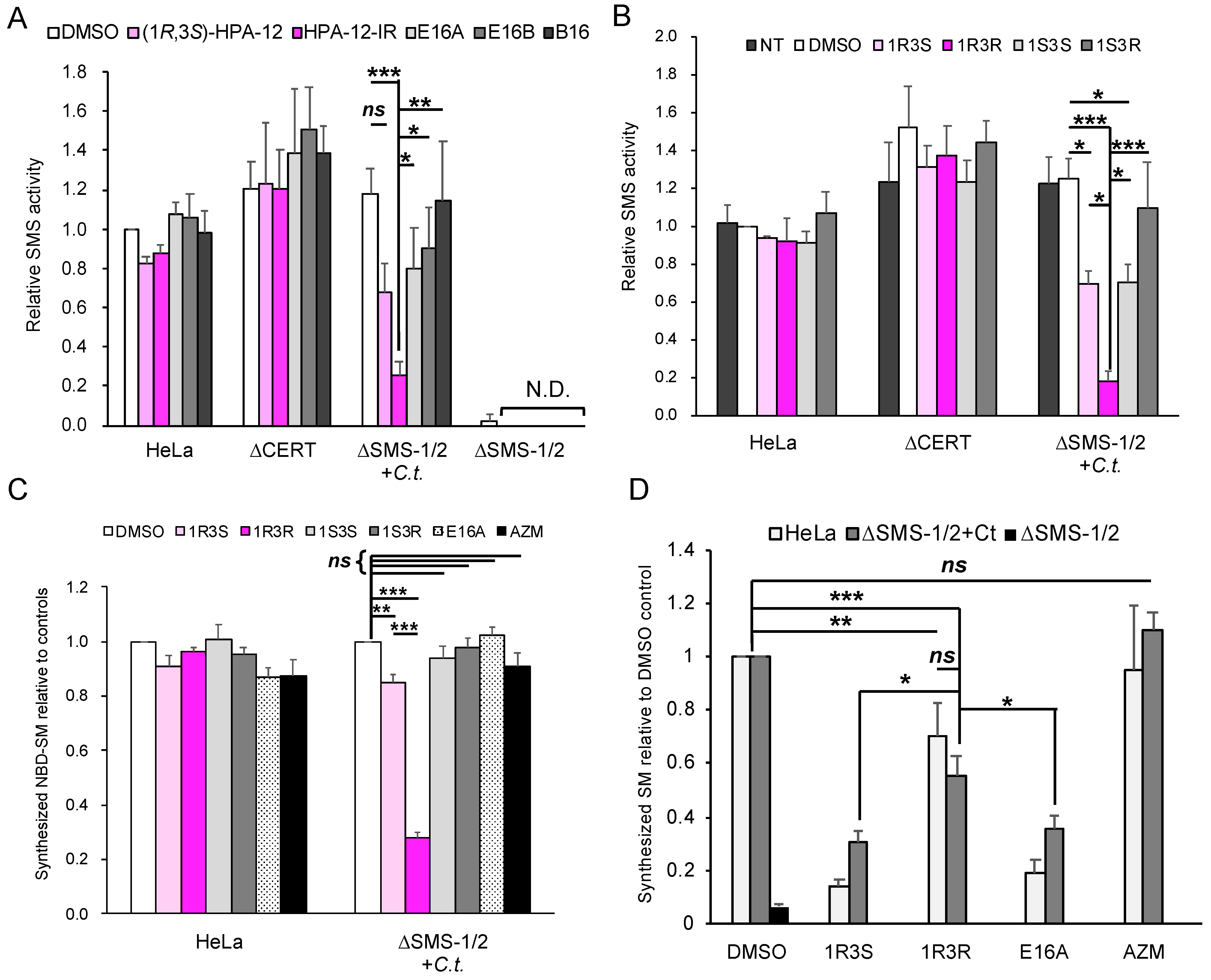
2.4. (1R,3R)-HPA-12 Is an Inhibitor of cidSM-Synthesis
2.5. The Anti-Chlamydial Effect of (1R,3R)-HPA-12 Is Partially Correlated with Inhibition of cidSM-Synthesis
2.6. Phospholcholine-Conjugated (1R,3R)-HPA-12, Produced by the cidSM-Synthesis Pathway, Is Related to Anti-Chlamydial Activity
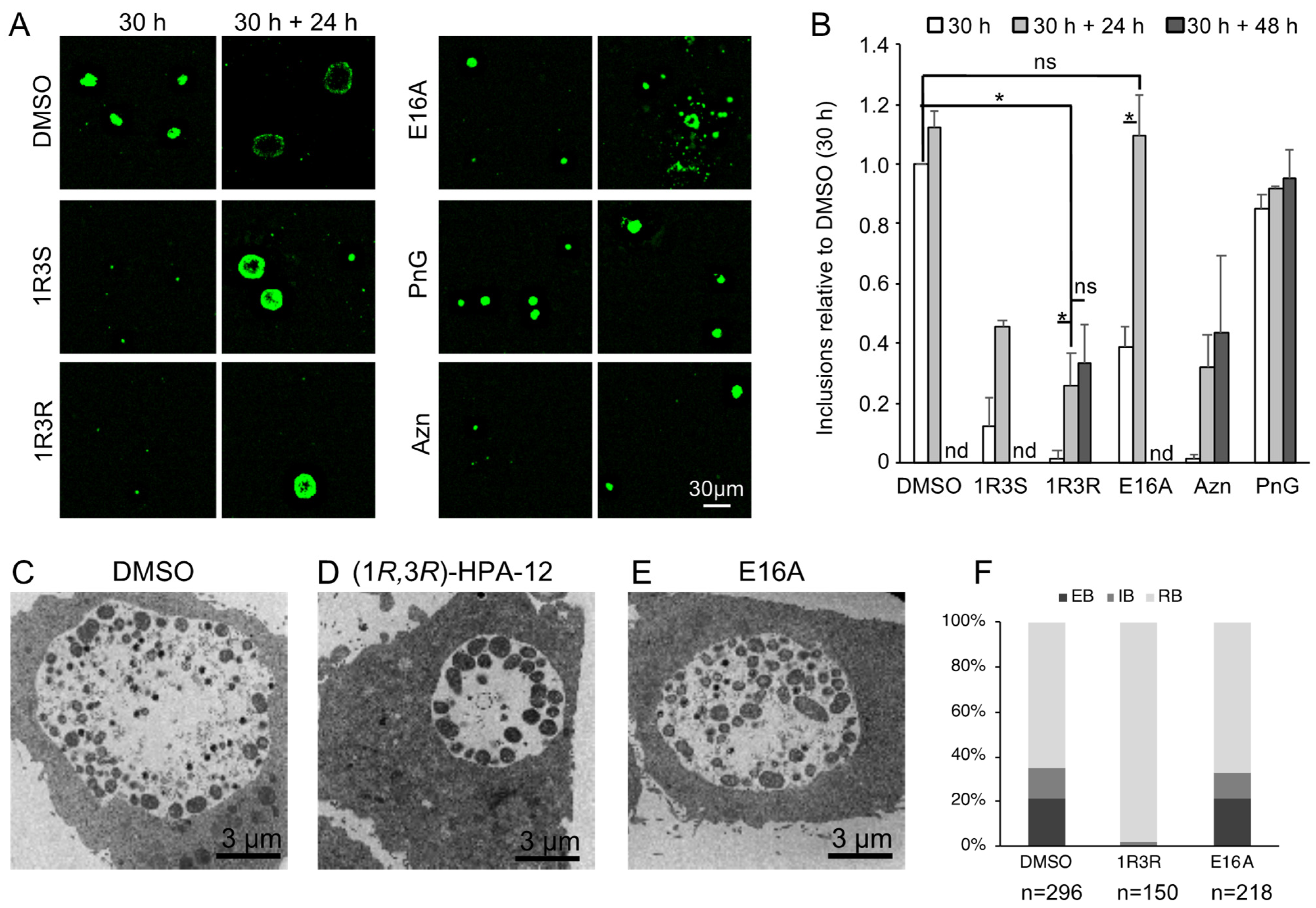
2.7. Retardation of RB-to-EB Maturation Induced by (1R,3R)-HPA-12 Treatment
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Propagation of C. trachomatis
4.3. Plasmids
4.4. Primary Inclusion Formation Assay
4.5. Progeny Formation Assay
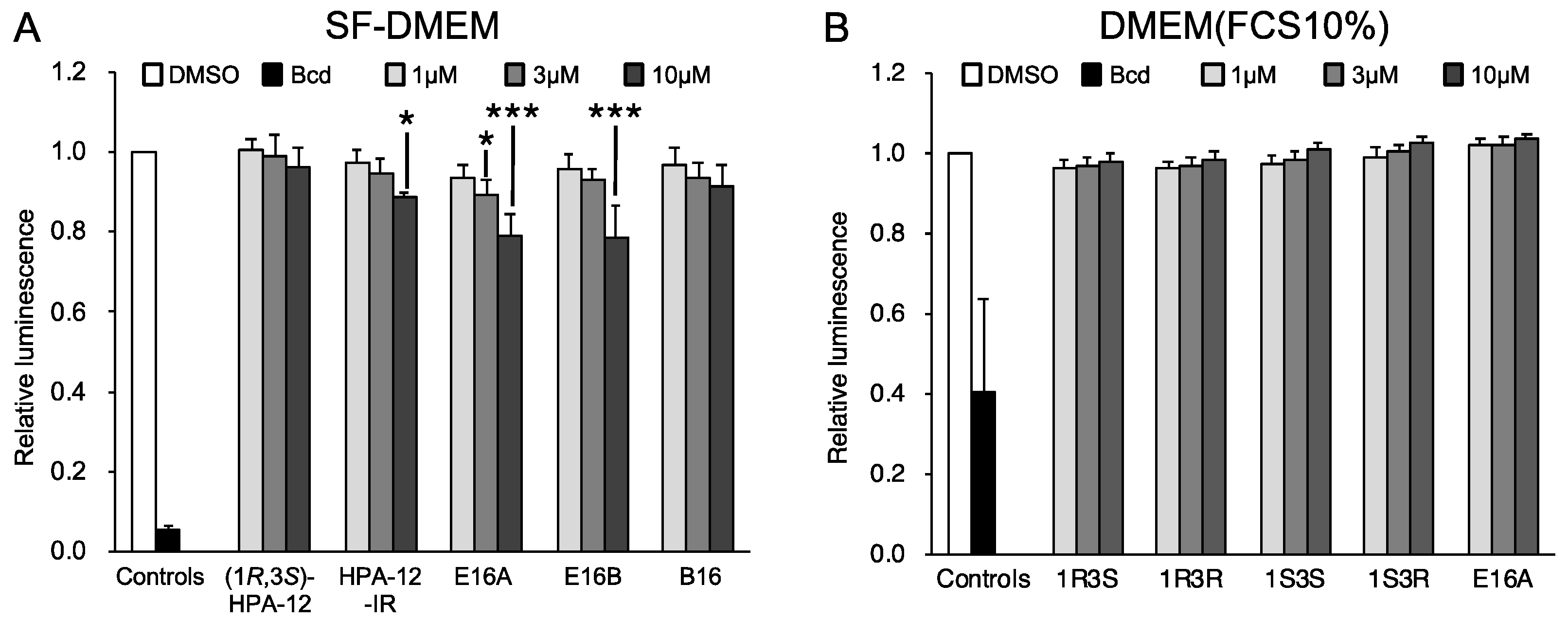
4.6. Cell Growth Assay
4.7. SMS Assay
4.8. Metabolic Labeling and Live-Cell Imaging with C6-NBD-Ceramide
4.9. Metabolic Labeling with [14C]Serine
4.10. LC-MS Analysis
4.11. TEM Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandell, G.L.; Bennett, J.E.; Dolin, R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 7th ed.; Churchill Livingstone/Elsevier: Philadelphia, PA, USA, 2010; ISBN 9781437720600. [Google Scholar]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia Cell Biology and Pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef]
- Bastidas, R.J.; Elwell, C.A.; Engel, J.N.; Valdivia, R.H. Chlamydial Intracellular Survival Strategies. Cold Spring Harb. Perspect. Med. 2013, 3, a010256. [Google Scholar] [CrossRef] [Green Version]
- Hybiske, K.; Stephens, R.S. Mechanisms of Host Cell Exit by the Intracellular Bacterium Chlamydia. Proc. Natl. Acad. Sci. USA 2007, 104, 11430–11435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanada, K. Co-Evolution of Sphingomyelin and the Ceramide Transport Protein CERT. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 704–719. [Google Scholar] [CrossRef] [PubMed]
- Wylie, J.L.; Hatch, G.M.; Mcclarty, G. Host Cell Phospholipids Are Trafficked to and Then Modified by Chlamydia trachomatis. J. Bacteriol. 1997, 179, 7233–7242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.; Cherian, P.T.; Frank, M.W.; Rock, C.O. Chlamydia trachomatis Relies on Autonomous Phospholipid Synthesis for Membrane Biogenesis. J. Biol. Chem. 2015, 290, 18874–18888. [Google Scholar] [CrossRef] [Green Version]
- Huitema, K.; Van Den Dikkenberg, J.; Brouwers, J.F.H.M.; Holthuis, J.C.M. Identification of a Family of Animal Sphingomyelin Synthases. EMBO J. 2004, 23, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Derré, I.; Swiss, R.; Agaisse, H. The Lipid Transfer Protein CERT Interacts with the Chlamydia Inclusion Protein IncD and Participates to ER-Chlamydia Inclusion Membrane Contact Sites. PLoS Pathog. 2011, 7, e1002092. [Google Scholar] [CrossRef] [PubMed]
- Tachida, Y.; Kumagai, K.; Sakai, S.; Ando, S.; Yamaji, T.; Hanada, K. Chlamydia trachomatis-Infected Human Cells Convert Ceramide to Sphingomyelin without Sphingomyelin Synthases 1 and 2. FEBS Lett. 2020, 594, 519–529. [Google Scholar] [CrossRef]
- Elwell, C.A.; Jiang, S.; Kim, J.H.; Lee, A.; Wittmann, T.; Hanada, K.; Melancon, P.; Engel, J.N. Chlamydia trachomatis Co-Opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development. PLoS Pathog. 2011, 7, e1002198. [Google Scholar] [CrossRef] [PubMed]
- Koch-Edelmann, S.; Banhart, S.; Saied, E.M.; Rose, L.; Aeberhard, L.; Laue, M.; Doellinger, J.; Arenz, C.; Heuer, D. The Cellular Ceramide Transport Protein CERT Promotes Chlamydia psittaci Infection and Controls Bacterial Sphingolipid Uptake. Cell. Microbiol. 2017, 19, e12752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelrahman, Y.; Ouellette, S.P.; Belland, R.J.; Cox, J.V. Polarized Cell Division of Chlamydia trachomatis. PLoS Pathog. 2016, 12, e1005866. [Google Scholar] [CrossRef]
- Götz, R.; Kunz, T.C.; Fink, J.; Solger, F.; Schlegel, J.; Seibel, J.; Kozjak-Pavlovic, V.; Rudel, T.; Sauer, M. Nanoscale Imaging of Bacterial Infections by Sphingolipid Expansion Microscopy. Nat. Commun. 2020, 11, 6173. [Google Scholar] [CrossRef] [PubMed]
- Hackstadt, T.; Scidmore, M.A.; Rockey, D.D. Lipid Metabolism in Chlamydia trachomatis-Infected Cells: Directed Trafficking of Golgi-Derived Sphingolipids to the Chlamydial Inclusion. Proc. Natl. Acad. Sci. USA 1995, 92, 4877–4881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, S.; Kitagawa, H.; Ueno, M.; Ishitani, H.; Fukasawa, M.; Nishijima, M.; Kobayashi, S.; Hanada, K. A Novel Inhibitor of Ceramide Trafficking from the Endoplasmic Reticulum to the Site of Sphingomyelin Synthesis. J. Biol. Chem. 2001, 276, 43994–44002. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Matsubara, R.; Kitagawa, H.; Kobayashi, S.; Kumagai, K.; Yasuda, S.; Hanada, K. Stereoselective Synthesis and Structure–Activity Relationship of Novel Ceramide Trafficking Inhibitors. (1R,3R)-N-(3-Hydroxy-1-hydroxymethyl-3-phenylpropyl)dodecanamide and Its Analogues. J. Med. Chem. 2003, 46, 3688–3695. [Google Scholar] [CrossRef]
- Nakao, N.; Ueno, M.; Sakai, S.; Egawa, D.; Hanzawa, H.; Kawasaki, S.; Kumagai, K.; Suzuki, M.; Kobayashi, S.; Hanada, K. Natural Ligand-Nonmimetic Inhibitors of the Lipid-Transfer Protein CERT. Commun. Chem. 2019, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Ďuriš, A.; Wiesenganger, T.; Moravčíková, D.; Baran, P.; Kožíšek, J.; Daïch, A.; Berkeš, D. Expedient and Practical Synthesis of CERT-Dependent Ceramide Trafficking Inhibitor HPA-12 and Its Analogues. Org. Lett. 2011, 13, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Huang, Y.-Y.; Yamano, A.; Kobayashi, S. Revised Stereochemistry of Ceramide-Trafficking Inhibitor HPA-12 by X-Ray Crystallography Analysis. Org. Lett. 2013, 15, 2869–2871. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Sakai, S.; Kumagai, K. Natural Ligand-Mimetic and Nonmimetic Inhibitors of the Ceramide Transport Protein CERT. Int. J. Mol. Sci. 2022, 23, 2098. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Kitagawa, H.; Ishitani, H.; Yasuda, S.; Hanada, K.; Kobayashi, S. Catalytic Enantioselective Synthesis of a Novel Inhibitor of Ceramide Trafficking, (1R,3R)-N-(3-Hydroxy-1-hydroxymethyl-3-phenylpropyl)dodecanamide (HPA-12). Tetrahedron Lett. 2001, 42, 7863–7865. [Google Scholar] [CrossRef]
- Carabeo, R.A.; Mead, D.J.; Hackstadt, T. Golgi-Dependent Transport of Cholesterol to the Chlamydia trachomatis Inclusion. Proc. Natl. Acad. Sci. USA 2003, 100, 6771–6776. [Google Scholar] [CrossRef] [Green Version]
- Engström, P.; Bergström, M.; Alfaro, A.C.; Syam Krishnan, K.; Bahnan, W.; Almqvist, F.; Bergström, S. Expansion of the Chlamydia trachomatis Inclusion Does Not Require Bacterial Replication. Int. J. Med. Microbiol. 2015, 305, 378–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagano, R.E.; Martin, O.C. A Series of Fluorescent N-Acylsphingosines: Synthesis, Physical Properties, and Studies in Cultured Cells. Biochemistry 1988, 27, 4439–4445. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Enciso, G.A.; Boassa, D.; Chander, C.N.; Lou, T.H.; Pairawan, S.S.; Guo, M.C.; Wan, F.Y.M.; Ellisman, M.H.; Sütterlin, C.; et al. Replication-Dependent Size Reduction Precedes Differentiation in Chlamydia trachomatis. Nat. Commun. 2018, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pütz, U.; Schwarzmann, G. Golgi Staining by Two Fluorescent Ceramide Analogues in Cultured Fibroblasts Requires Metabolism. Eur. J. Cell Biol. 1995, 68, 113–121. [Google Scholar]
- Banhart, S.; Saied, E.M.; Martini, A.; Koch, S.; Aeberhard, L.; Madela, K.; Arenz, C.; Heuer, D. Improved Plaque Assay Identifies a Novel Anti-Chlamydia Ceramide Derivative with Altered Intracellular Localization. Antimicrob. Agents Chemother. 2014, 58, 5537–5546. [Google Scholar] [CrossRef] [Green Version]
- Lord, S.J.; Velle, K.B.; Mullins, R.D.; Fritz-Laylin, L.K. SuperPlots: Communicating Reproducibility and Variability in Cell Biology. J. Cell Biol. 2020, 219, e202001064. [Google Scholar] [CrossRef] [PubMed]
- Rosenwald, A.G.; Pagano, R.E. Inhibition of Glycoprotein Traffic through the Secretory Pathway by Ceramide. J. Biol. Chem. 1993, 268, 4577–4579. [Google Scholar] [CrossRef]
- Bai, J.; Pagano, R.E. Measurement of Spontaneous Transfer and Transbilayer Movement of BODIPY-Labeled Lipids in Lipid Vesicles. Biochemistry 1997, 36, 8840–8848. [Google Scholar] [CrossRef]
- Fukasawa, M.; Nishijima, M.; Hanada, K. Genetic Evidence for ATP-Dependent Endoplasmic Reticulum-to-Golgi Apparatus Trafficking of Ceramide for Sphingomyelin Synthesis in Chinese Hamster Ovary Cells. J. Cell Biol. 1999, 144, 673–685. [Google Scholar] [CrossRef]
- Welsh, L.E.; Gaydos, C.A.; Quinn, T.C. In Vitro Evaluation of Activities of Azithromycin, Erythromycin, and Tetracycline against Chlamydia trachomatis and Chlamydia pneumoniae. Antimicrob. Agents Chemother. 1992, 36, 291–294. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, K.; Yasuda, S.; Okemoto, K.; Nishijima, M.; Kobayashi, S.; Hanada, K. CERT Mediates Intermembrane Transfer of Various Molecular Species of Ceramides. J. Biol. Chem. 2005, 280, 6488–6495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crivelli, S.M.; Paulus, A.; Markus, J.; Bauwens, M.; Berkes, D.; De Vries, H.E.; Mulder, M.T.; Walter, J.; Mottaghy, F.M.; Losen, M.; et al. Synthesis, Radiosynthesis, and Preliminary In Vitro and In Vivo Evaluation of the Fluorinated Ceramide Trafficking Inhibitor (HPA-12) for Brain Applications. J. Alzheimer’s Dis. 2017, 60, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, S.M.; van Kruining, D.; Luo, Q.; Stevens, J.A.A.; Giovagnoni, C.; Paulus, A.; Bauwens, M.; Berkes, D.; de Vries, H.E.; Mulder, M.T.; et al. Ceramide Analog [18F]F-HPA-12 Detects Sphingolipid Disbalance in the Brain of Alzheimer’s Disease Transgenic Mice by Functioning as a Metabolic Probe. Sci. Rep. 2020, 10, 19354. [Google Scholar] [CrossRef] [PubMed]
- Hatch, G.M.; McClarty, G. Phospholipid Composition of Purified Chlamydia trachomatis Mimics That of the Eucaryotic Host Cell. Infect. Immun. 1998, 66, 3727–3735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaji, T.; Hanada, K. Establishment of HeLa Cell Mutants Deficient in Sphingolipid-Related Genes Using TALENs. PLoS ONE 2014, 9, e88124. [Google Scholar] [CrossRef] [Green Version]
- Satoh, M.; Ogawa, M.; Saijo, M.; Ando, S. Multilocus VNTR Analysis-OmpA Typing of Venereal Isolates of Chlamydia trachomatis in Japan. J. Infect. Chemother. 2014, 20, 656–659. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Vacaru, A.M.; Tafesse, F.G.; Ternes, P.; Kondylis, V.; Hermansson, M.; Brouwers, J.F.H.M.; Somerharju, P.; Rabouille, C.; Holthuis, J.C.M. Sphingomyelin Synthase-Related Protein SMSr Controls Ceramide Homeostasis in the ER. J. Cell Biol. 2009, 185, 1013–1027. [Google Scholar] [CrossRef] [Green Version]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software ‘EZR’ for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumagai, K.; Sakai, S.; Ueno, M.; Kataoka, M.; Kobayashi, S.; Hanada, K. Chlamydial Infection-Dependent Synthesis of Sphingomyelin as a Novel Anti-Chlamydial Target of Ceramide Mimetic Compounds. Int. J. Mol. Sci. 2022, 23, 14697. https://doi.org/10.3390/ijms232314697
Kumagai K, Sakai S, Ueno M, Kataoka M, Kobayashi S, Hanada K. Chlamydial Infection-Dependent Synthesis of Sphingomyelin as a Novel Anti-Chlamydial Target of Ceramide Mimetic Compounds. International Journal of Molecular Sciences. 2022; 23(23):14697. https://doi.org/10.3390/ijms232314697
Chicago/Turabian StyleKumagai, Keigo, Shota Sakai, Masaharu Ueno, Michiyo Kataoka, Shu Kobayashi, and Kentaro Hanada. 2022. "Chlamydial Infection-Dependent Synthesis of Sphingomyelin as a Novel Anti-Chlamydial Target of Ceramide Mimetic Compounds" International Journal of Molecular Sciences 23, no. 23: 14697. https://doi.org/10.3390/ijms232314697




