A Micro-Immunotherapy Sequential Medicine MIM-seq Displays Immunomodulatory Effects on Human Macrophages and Anti-Tumor Properties towards In Vitro 2D and 3D Models of Colon Carcinoma and in an In Vivo Subcutaneous Xenograft Colon Carcinoma Model
Abstract
:1. Introduction
2. Results
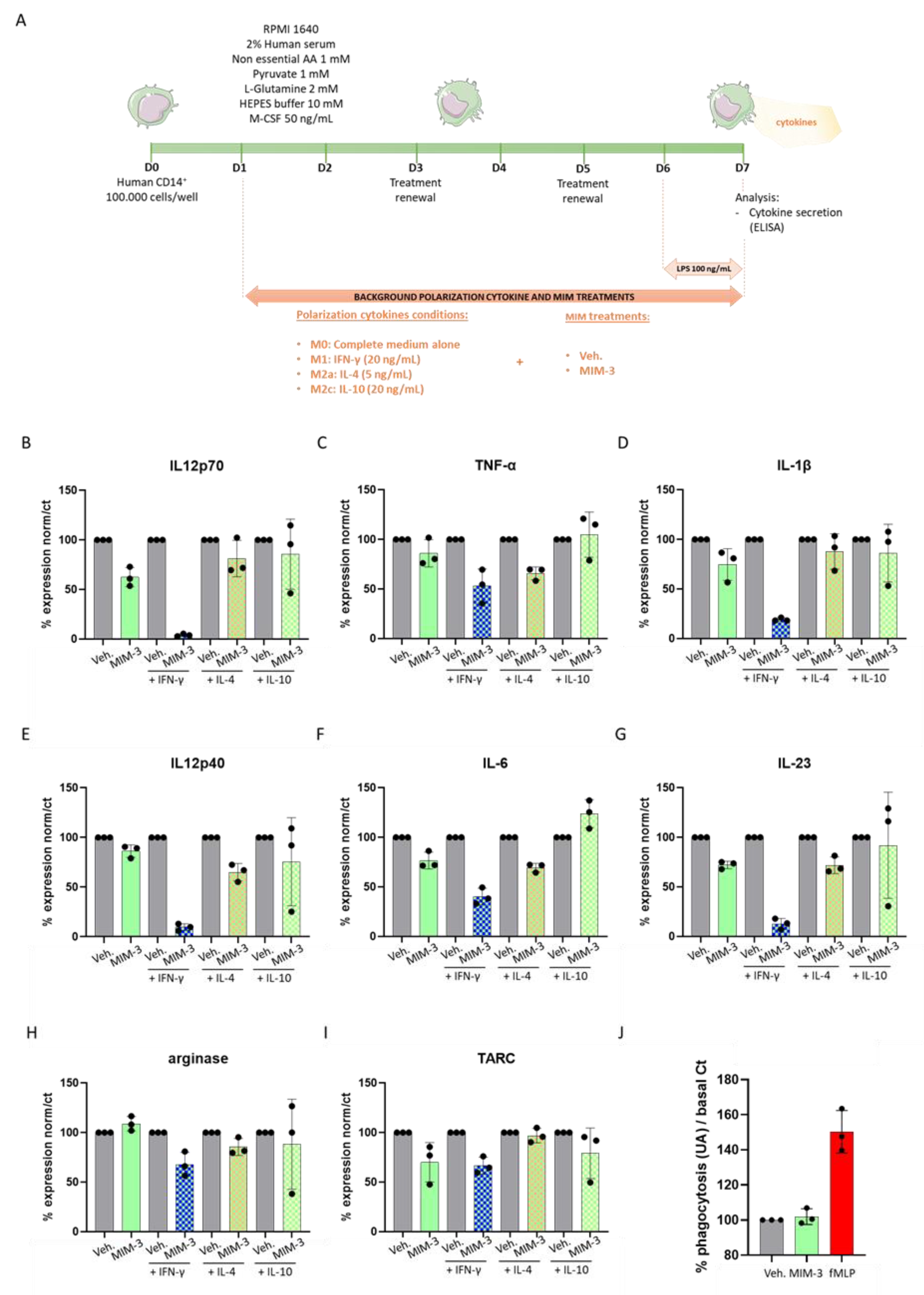
2.1. MIM-3 Acts as an Immune-Modulator of Cytokines’ Secretion in Macrophages Cultivated under Various Immune Contexts
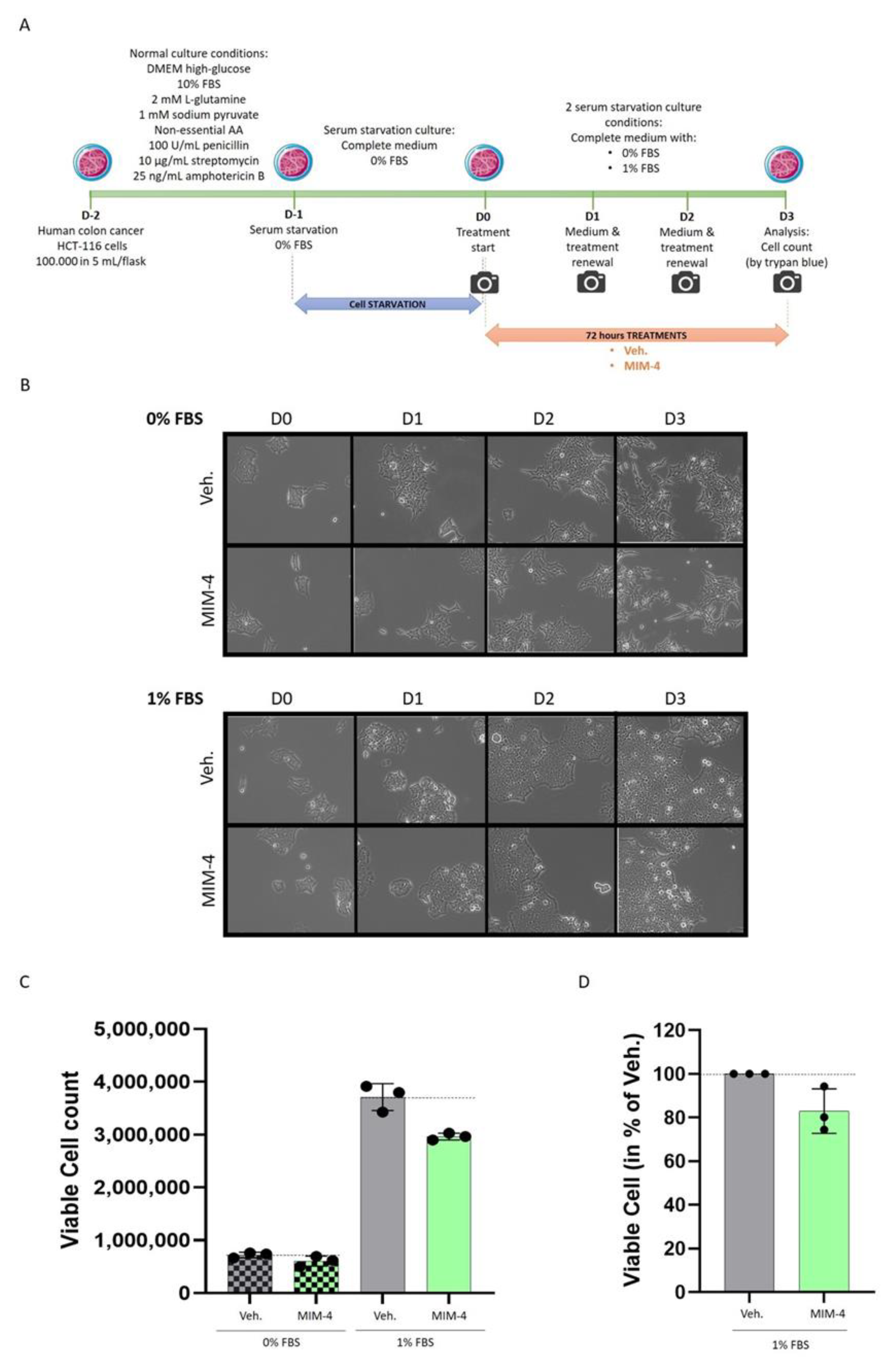
2.2. MIM-4 Displays Slight Anti-Proliferative Effects on Colon Carcinoma Cells Cultivated in 2-Dimensions, under Starvation
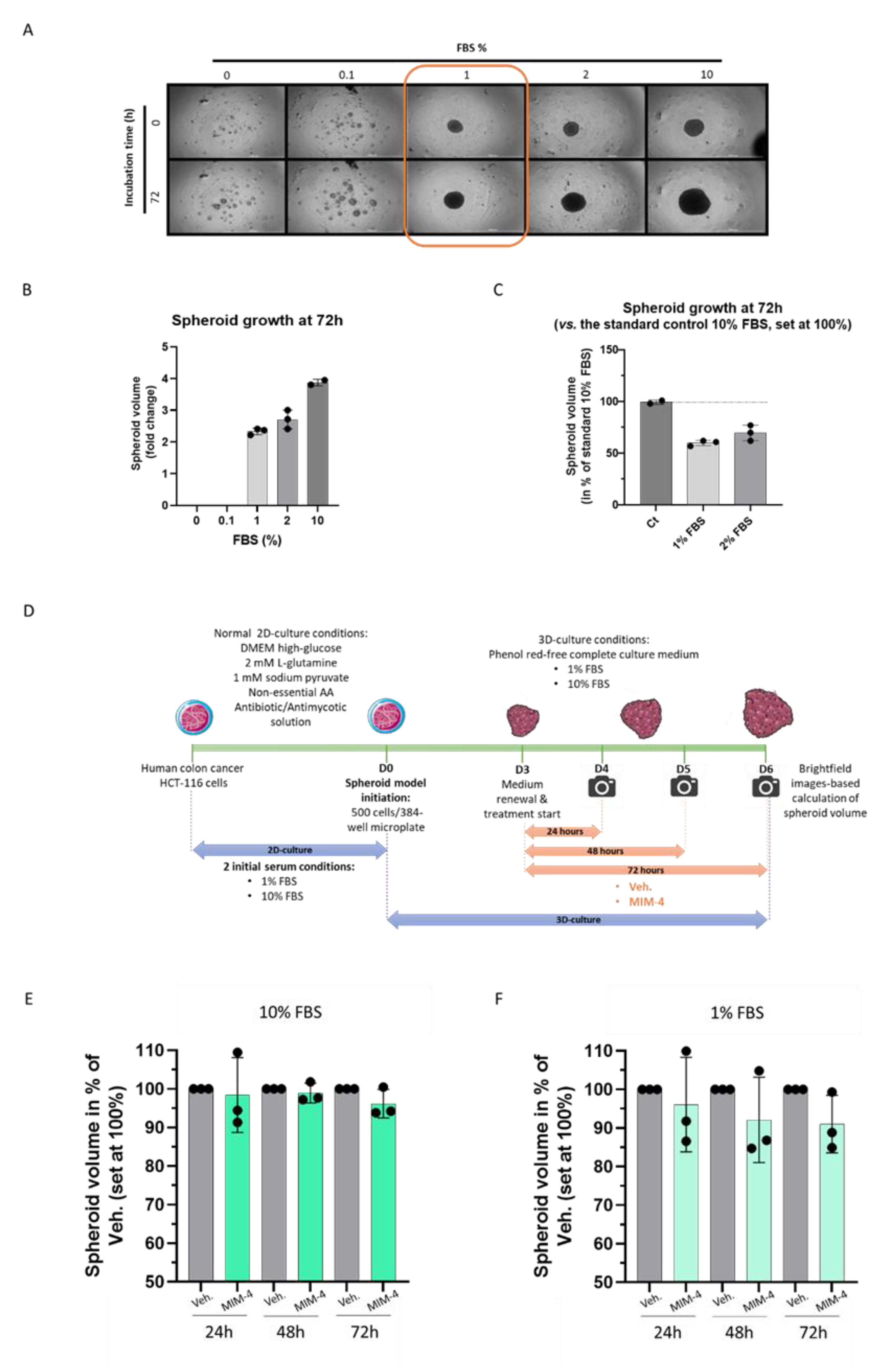
2.3. MIM-4 Displays Moderate Anti-Proliferative Effects on Colon Carcinoma Cells Cultivated in a 3-Dimensions-Spheroids’ Model
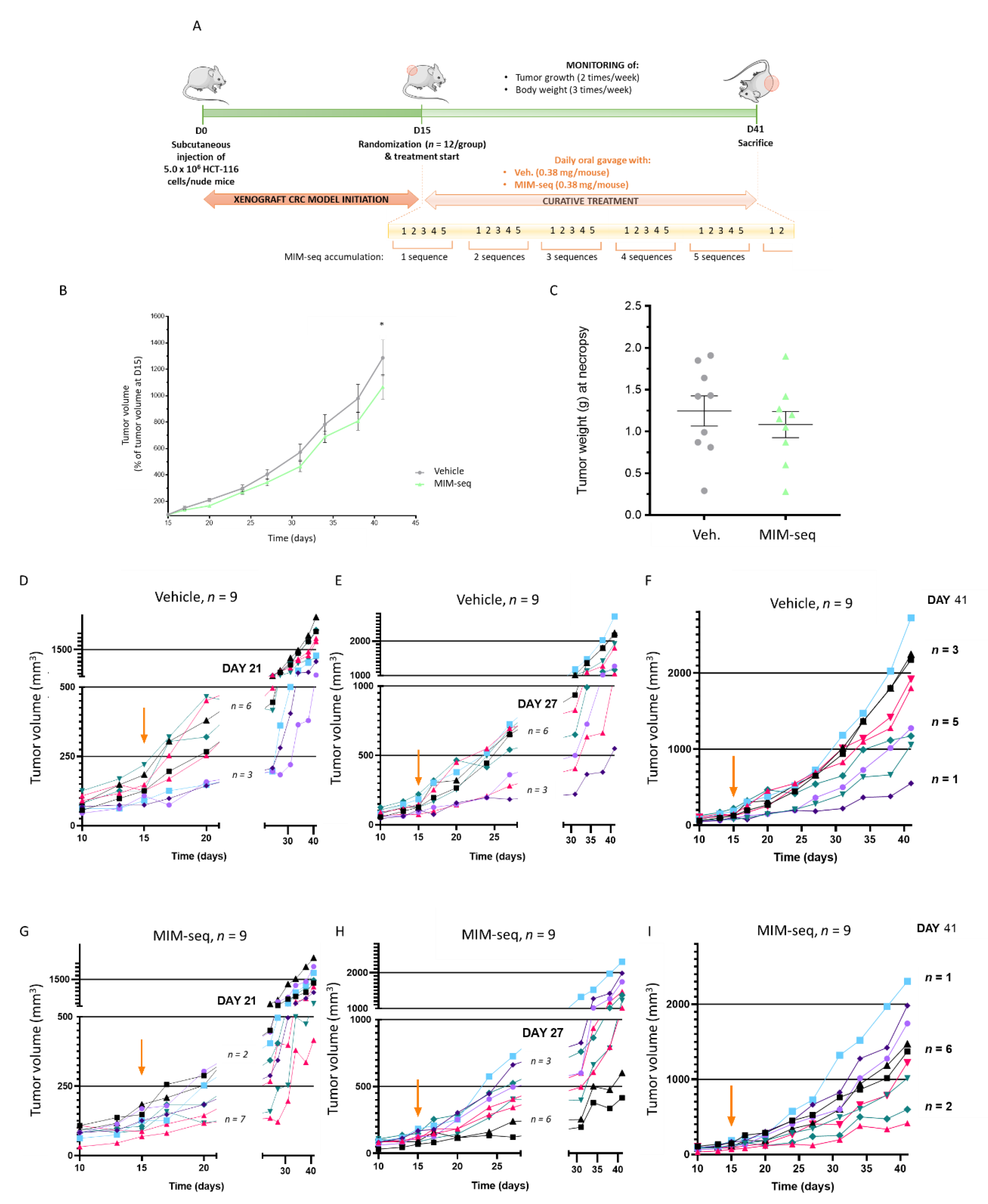
2.4. MIM-seq Displays Slight Anti-Tumor Effects in an In Vivo Subcutaneous Xenograft Model of Colon Carcinoma
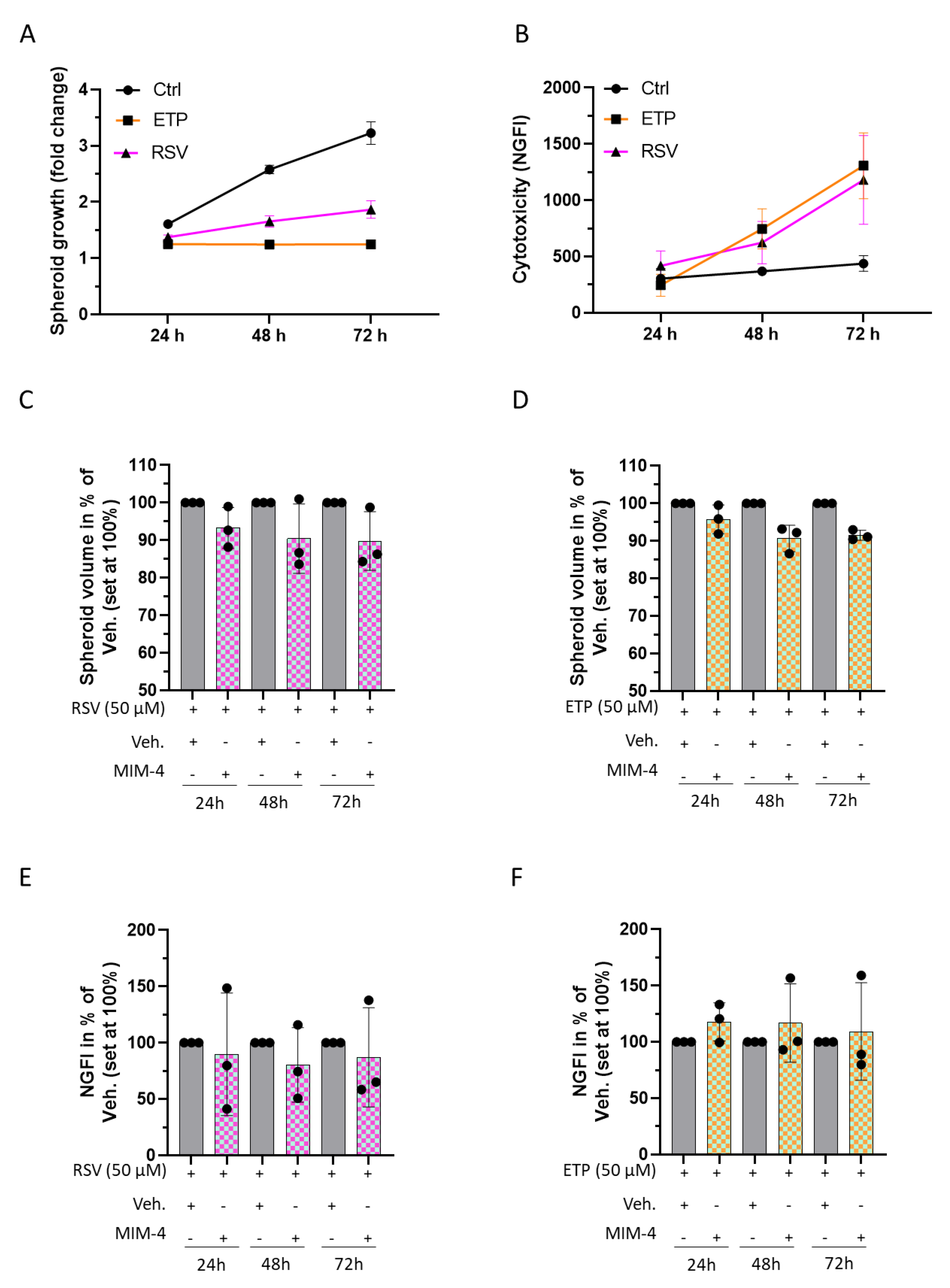
2.5. MIM-4 Could Act as an Adjuvant When Associated with Etoposide or Resveratrol, in an In Vitro 3D-Spheroid Model of Colorectal Cancer
3. Discussion
4. Materials and Methods
4.1. Tested Item and Experimental Control
4.2. Phagocytosis
4.3. Macrophage Cytokine Secretion Evaluation
4.4. Colon Cancer In Vitro Models
4.4.1. Experiments Performed on Classical Monolayer Cultured HCT-116 Cells
4.4.2. Initiation of the Spheroid Model
4.4.3. Kinetics of 3D Spheroid Growth and Cytotoxicity
4.4.4. Data Analysis
4.5. Colon Cancer Animal Model
4.5.1. Animal Housing and General Experimental Conditions
4.5.2. Cell Culture for the Generation of the In Vivo Model
4.5.3. In Vivo Study Design
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D: | 2-dimensions |
| 3D: | 3-dimensions; |
| AA: | amino acids; |
| AMPK: | AMP-activated protein kinase; |
| BSA: | bovine serum albumin; |
| CH: | centesimal Hahnemannian |
| COX: | cyclooxygenase |
| CRC: | colorectal cancer; |
| CTLs: | cytotoxic T lymphocytes; |
| DNA: | deoxyribonucleic acid |
| EGF: | epidermal growth factor; |
| EGFR: | epidermal growth factor receptor |
| ELISA: | enzyme linked immunosorbent assay; |
| EMA: | European Medicines Agency; |
| ETP: | etoposide; |
| FBS: | fetal bovine serum; |
| FCS: | fetal calf serum; |
| FDA: | Food and Drug Administration; |
| FGF2: | fibroblast growth factor 2; |
| fMLP: | N-formyl methionyl-leucyl-phenylalanine; |
| GFI: | green fluorescence integral; |
| GFP: | green fluorescent protein; |
| GM-CSF: | granulocyte-macrophage colony-stimulating factor; |
| H&E: | hematoxylin and eosin; |
| HLA: | human leukocyte antigen; |
| hr: | human recombinant; |
| IFN: | interferon; |
| IHC: | immunohistochemistry; |
| ILs: | interleukins; |
| K: | Korsakovian dilution; |
| LD: | low dose; |
| LPS: | lipopolysaccharide; |
| M1: | M1 macrophage; |
| MCH: | major histocompatibility complex; |
| M-CSF: | macrophage colony-stimulating factor; |
| MI: | micro-immunotherapy; |
| MIM: | micro-immunotherapy medicine; |
| NF-κB: | nuclear factor kappa-light-chain-enhancer of activated B cells; |
| NGFI: | normalized green fluorescence intensity; |
| NK: | natural killer; |
| NMRI: | naval medical research institute; |
| NO: | nitric oxide; |
| PBMCs: | peripheral blood mononuclear cells; PBS: Phosphate buffer saline; |
| PFA: | Paraformaldehyde; |
| P/S: | penicillin/streptomycin; |
| RNA: | ribonucleic acid; |
| ROS: | reactive oxygen species; |
| RSV: | resveratrol; |
| RT: | room temperature; |
| SIRT1: | silent mating type information regulator two homolog 1; |
| SKP: | serial kinetic process; |
| SNA®: | specific nucleic acids; |
| TAMs: | tumor-associated macrophages; TARC: thymus and activation-regulated chemokine; |
| TGF-β: | transforming growth factor-β; |
| TGFBR: | transforming growth factor receptor; |
| TLR: | toll-like receptor; |
| TNF-α: | tumor necrosis factor-α; |
| TUNEL: | terminal deoxynucleotidyl transferase dUTP nick end labeling; |
| ULD: | ultra-low dose; |
| VEGF: | vascular endothelial growth factor; |
| Veh: | vehicle; |
| Wnt: | wingless-related integration site; |
References
- Recio-Boiles, A.; Cagir, B. Colon Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK470380/ (accessed on 8 February 2022).
- Schmoll, H.J.; Van Cutsem, E.; Stein, A.; Valentini, V.; Glimelius, B.; Haustermans, K.; Nordlinger, B.; van de Velde, C.J.; Balmana, J.; Regula, J.; et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann. Oncol. 2012, 23, 2479–2516. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Golshani, G.; Zhang, Y. Advances in immunotherapy for colorectal cancer: A review. Ther. Adv. Gastroenterol. 2020, 13, 1756284820917527. [Google Scholar] [CrossRef] [PubMed]
- Johdi, N.A.; Sukor, N.F. Colorectal Cancer Immunotherapy: Options and Strategies. Front Immunol. 2020, 11, 1624. Available online: https://www.frontiersin.org/article/10.3389/fimmu.2020.01624 (accessed on 4 February 2022). [CrossRef]
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; Schreiber, R.D. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001, 410, 1107–1111. [Google Scholar] [CrossRef]
- Wrangle, J.M.; Patterson, A.; Johnson, C.B.; Neitzke, D.J.; Mehrotra, S.; Denlinger, C.E.; Paulos, C.M.; Li, Z.; Cole, D.J.; Rubinstein, M.P. IL-2 and Beyond in Cancer Immunotherapy. J. Interferon. Cytokine Res. 2018, 38, 45–68. [Google Scholar] [CrossRef]
- Khan, W.A. Autoantibodies and Cytokines; BoD—Books on Demand: Norderstedt, Germany, 2019; 216p. [Google Scholar]
- García-Martínez, E.; Smith, M.; Buqué, A.; Aranda, F.; de la Peña, F.A.; Ivars, A.; Cánovas, M.S.; Conesa, M.A.V.; Fucikova, J.; Spisek, R.; et al. Trial Watch: Immunostimulation with recombinant cytokines for cancer therapy. Oncoimmunology 2018, 7, e1433982. [Google Scholar] [CrossRef]
- Vacchelli, E.; Aranda, F.; Bloy, N.; Buqué, A.; Cremer, I.; Eggermont, A.; Fridman, W.H.; Fucikova, J.; Galon, J.; Spisek, R.; et al. Trial Watch—Immunostimulation with cytokines in cancer therapy. OncoImmunology 2016, 5, e1115942. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Huang, L.; Zhao, H.; Yan, Y.; Lu, J. The Role of Interleukins in Colorectal Cancer. Int. J. Biol. Sci. 2020, 16, 2323–2339. [Google Scholar] [CrossRef]
- Marabondo, S.; Kaufman, H.L. High-dose interleukin-2 (IL-2) for the treatment of melanoma: Safety considerations and future directions. Expert Opin. Drug. Saf. 2017, 16, 1347–1357. [Google Scholar] [CrossRef]
- Wolkenstein, P.; Chosidow, O.; Wechsler, J.; Guillaume, J.C.; Lescs, M.C.; Brandely, M.; Avril, M.F.; Revuz, J. Cutaneous side effects associated with interleukin 2 administration for metastatic melanoma. J. Am. Acad. Dermatol. 1993, 28, 66–70. [Google Scholar] [CrossRef]
- Kirkwood, J.M.; Resnick, G.D.; Cole, B.F. Efficacy, safety, and risk-benefit analysis of adjuvant interferon alfa-2b in melanoma. Semin. Oncol. 1997, 24, S16–S23. [Google Scholar] [PubMed]
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M.; et al. High-Dose Recombinant Interleukin 2 Therapy for Patients with Metastatic Melanoma: Analysis of 270 Patients Treated Between 1985 and 1993. J. Clin. Oncol. 1999, 17, 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Papiernik, M.; de Moraes, M.L.; Pontoux, C.; Vasseur, F.; Pénit, C. Regulatory CD4 T cells: Expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int. Immunol. 1998, 10, 371–378. [Google Scholar] [CrossRef]
- Tuomisto, A.E.; Mäkinen, M.J.; Väyrynen, J.P. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J. Gastroenterol. 2019, 25, 4383–4404. [Google Scholar] [CrossRef]
- Mármol, I.; Sánchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [Green Version]
- Floris, I.; Appel, K.; Rose, T.; Lejeune, B. 2LARTH®, a micro-immunotherapy medicine, exerts anti-inflammatory effects in vitro and reduces TNF-α and IL-1β secretion. J. Inflamm. Res. 2018, 11, 397–405. [Google Scholar] [CrossRef] [Green Version]
- Ferrà-Cañellas, M.D.M.; Munar-Bestard, M.; Garcia-Sureda, L.; Lejeune, B.; Ramis, J.M.; Monjo, M. BMP4 micro-immunotherapy increases collagen deposition and reduces PGE2 release in human gingival fibroblasts and increases tissue viability of engineered 3D gingiva under inflammatory conditions. J. Periodontol. 2021, 92, 1448–1459. [Google Scholar] [CrossRef]
- Lilli, N.L.; Révy, D.; Volteau, C.; Robelet, S.; Lejeune, B. Effect of 2LMISEN® on Long-Term Hippocampal Neurons Culture as a Screening Senescent Cells Model: p16INK4A and Caspase 3 Quantification. Adv. Aging Res. 2019, 8, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Lilli, N.L.; Révy, D.; Robelet, S.; Lejeune, B. Effect of the micro-immunotherapy medicine 2LPARK® on rat primary dopaminergic neurons after 6-OHDA injury: Oxidative stress and survival evaluation in an in vitro model of Parkinson’s disease. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efficacy of 2LPAPI®, A Micro-Immunotherapy Drug, In Patients with High-Risk Papillomavirus Genital Infection. Available online: https://www.scirp.org/journal/paperinformation.aspx?paperid=64702 (accessed on 24 February 2022).
- Floris, I.; Chenuet, P.; Togbe, D.; Volteau, C.; Lejeune, B. Potential Role of the Micro-Immunotherapy Medicine 2LALERG in the Treatment of Pollen-Induced Allergic Inflammation. Dose-Response 2020, 18, 1559325820914092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pro-Inflammatory Cytokines at Ultra-Low Dose Exert Anti-Inflammatory Effect In Vitro: A Possible Mode of Action Involving Sub-Micron Particles? Available online: https://pubmed.ncbi.nlm.nih.gov/33633511/ (accessed on 7 March 2022).
- Floris, I.; García-González, V.; Palomares, B.; Appel, K.; Lejeune, B. The Micro-Immunotherapy Medicine 2LARTH® Reduces Inflammation and Symptoms of Rheumatoid Arthritis In Vivo. Int. J. Rheumatol. 2020, 2020, 1594573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacques, C.; Chatelais, M.; Fekir, K.; Fauconnier, L.; Mellier, M.; Togbe, D.; Floris, I. The Micro-Immunotherapy Medicine 2LEID Exhibits an Immunostimulant Effect by Boosting Both Innate and Adaptive Immune Responses. Int. J. Mol. Sci. 2021, 23, 110. [Google Scholar] [CrossRef]
- Jacques, C.; Chatelais, M.; Fekir, K.; Brulefert, A.; Floris, I. The Unitary Micro-Immunotherapy Medicine Interferon-γ (4 CH) Displays Similar Immunostimulatory and Immunomodulatory Effects than Those of Biologically Active Human Interferon-γ on Various Cell Types. Int. J. Mol. Sci. 2022, 23, 2314. [Google Scholar] [CrossRef]
- Jacques, C.; Floris, I.; Lejeune, B. Ultra-Low Dose Cytokines in Rheumatoid Arthritis, Three Birds with One Stone as the Rationale of the 2LARTH® Micro-Immunotherapy Treatment. Int. J. Mol. Sci. 2021, 22, 6717. [Google Scholar] [CrossRef]
- Hovav, A.-H. Dendritic cells of the oral mucosa. Mucosal Immunol. 2014, 7, 27–37. [Google Scholar] [CrossRef]
- Wu, R.-Q.; Zhang, D.-F.; Tu, E.; Chen, Q.-M.; Chen, W. The mucosal immune system in the oral cavity-an orchestra of T cell diversity. Int. J. Oral Sci. 2014, 6, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Vishnyakova, P.; Poltavets, A.; Karpulevich, E.; Maznina, A.; Vtorushina, V.; Mikhaleva, L.; Kananykhina, E.; Lokhonina, A.; Kovalchuk, S.; Makarov, A.; et al. The response of two polar monocyte subsets to inflammation. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 139, 111614. [Google Scholar] [CrossRef]
- Jiang, L.N.; Yao, C.Y.; Jin, Q.L.; He, W.X.; Li, B.Q. The Enhanceing effect of IL-12 on phagocytosis and killing of Mycobacterium tuberculosis by neutrophils in tuberculosis patients. Chin. J. Cell. Mol. Immunol. 2011, 27, 1191–1194. [Google Scholar]
- Rollag, H.; Degré, M.; Sonnenfeld, G. Effects of Interferon-α/β and Interferon-γ Preparations on Phagocytosis by Mouse Peritoneal Macrophages. Scand. J. Immunol. 1984, 20, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Duineveld, L.A.M.; van Asselt, K.M.; Bemelman, W.A.; Smits, A.B.; Tanis, P.J.; van Weert, H.C.P.M.; Wind, J. Symptomatic and Asymptomatic Colon Cancer Recurrence: A Multicenter Cohort Study. Ann. Fam. Med. 2016, 14, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzè, E.; Laudisi, F.; Di Grazia, A.; Marônek, M.; Bellato, V.; Sica, G.; Monteleone, G. Macrophages produce and functionally respond to interleukin-34 in colon cancer. Cell Death Discov. 2020, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Cortese, N.; Donadon, M.; Rigamonti, A.; Marchesi, F. Macrophages at the crossroads of anticancer strategies. Front. Biosci. Landmark 2019, 24, 1271–1283. [Google Scholar] [CrossRef]
- Forssell, J.; Oberg, A.; Henriksson, M.L.; Stenling, R.; Jung, A.; Palmqvist, R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin. Cancer Res. 2007, 13, 1472–1479. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W.; Chang, H.J.; Yeo, H.Y.; Han, N.; Kim, B.C.; Kong, S.-Y.; Kim, J.; Oh, J.H. The relationships between systemic cytokine profiles and inflammatory markers in colorectal cancer and the prognostic significance of these parameters. Br. J. Cancer 2020, 123, 610–618. [Google Scholar] [CrossRef]
- Mehrotra, A.; D’Angelo, J.A.; Romney-Vanterpool, A.; Chu, T.; Bertoletti, A.; Janssen, H.L.A.; Gehring, A.J. IFN-α Suppresses Myeloid Cytokine Production, Impairing IL-12 Production and the Ability to Support T-Cell Proliferation. J. Infect. Dis. 2020, 222, 148–157. [Google Scholar] [CrossRef]
- Wolters, T.L.C.; Netea, M.G.; Hermus, A.R.M.M.; Smit, J.W.A.; Netea-Maier, R.T. IGF1 potentiates the pro-inflammatory response in human peripheral blood mononuclear cells via MAPK. J. Mol. Endocrinol. 2017, 59, 129–139. [Google Scholar] [CrossRef]
- Garg, A.D.; Romano, E.; Rufo, N.; Agostinis, P. Immunogenic versus tolerogenic phagocytosis during anticancer therapy: Mechanisms and clinical translation. Cell Death Differ. 2016, 23, 938–951. [Google Scholar] [CrossRef] [Green Version]
- Jin, G.F.; Guo, Y.S.; Ball, C.; Houston, C.W. Insulin-like growth factors enhance phagocytosis by human neutrophils in vitro. Regul. Pept. 1993, 49, 125–131. [Google Scholar] [CrossRef]
- Ji, K.-Y.; Kim, K.M.; Kim, Y.H.; Shim, K.-S.; Lee, J.Y.; Kim, T.; Chae, S. Serum Starvation Sensitizes Anticancer Effect of Anemarrhena asphodeloides via p38/JNK-Induced Cell Cycle Arrest and Apoptosis in Colorectal Cancer Cells. Am. J. Chin. Med. 2021, 49, 1001–1016. [Google Scholar] [CrossRef] [PubMed]
- Yao, G. Modelling mammalian cellular quiescence. Interface Focus 2014, 4, 20130074. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koveitypour, Z.; Panahi, F.; Vakilian, M.; Peymani, M.; Seyed Forootan, F.; Nasr Esfahani, M.H.; Ghaedi, K. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019, 9, 97. [Google Scholar] [CrossRef] [Green Version]
- Spano, J.P.; Fagard, R.; Soria, J.-C.; Rixe, O.; Khayat, D.; Milano, G. Epidermal growth factor receptor signaling in colorectal cancer: Preclinical data and therapeutic perspectives. Ann. Oncol. 2005, 16, 189–194. [Google Scholar] [CrossRef]
- Mitsudomi, T.; Yatabe, Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010, 277, 301–308. [Google Scholar] [CrossRef]
- Cho, Y.B.; Hong, H.K.; Choi, Y.-L.; Oh, E.; Joo, K.M.; Jin, J.; Nam, D.-H.; Ko, Y.-H.; Lee, W.Y. Colorectal cancer patient-derived xenografted tumors maintain characteristic features of the original tumors. J. Surg. Res. 2014, 187, 502–509. [Google Scholar] [CrossRef]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef]
- Huot, J.R.; Novinger, L.J.; Pin, F.; Bonetto, A. HCT116 colorectal liver metastases exacerbate muscle wasting in a mouse model for the study of colorectal cancer cachexia. Dis. Model Mech. 2020, 13, dmm043166. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, K.; Fujii, G.; Sakurai, H.; Yoshitome, H.; Mutoh, M.; Wada, M. Intestinal Peyer’s patches prevent tumorigenesis in ApcMin/+ mice. J. Clin. Biochem. Nutr. 2015, 56, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, W.; Leong, X.; Engleman, E. Orthotopic Mouse Model of Colorectal Cancer. J. Vis. Exp. 2007, 10, e484. [Google Scholar] [CrossRef] [PubMed]
- Szadvari, I.; Krizanova, O.; Babula, P. Athymic nude mice as an experimental model for cancer treatment. Physiol. Res. 2016, 65, S441–S453. [Google Scholar] [CrossRef] [PubMed]
- Budzynski, W.; Radzikowski, C. Cytotoxic cells in immunodeficient athymic mice. Immunopharmacol. Immunotoxicol. 1994, 16, 319–346. [Google Scholar] [CrossRef]
- De Jong, M.; Essers, J.; van Weerden, W.M. Imaging preclinical tumour models: Improving translational power. Nat. Rev. Cancer 2014, 14, 481–493. [Google Scholar] [CrossRef]
- Tachibana, T.; Oyama, T.G.; Yoshii, Y.; Hihara, F.; Igarashi, C.; Tsuji, A.B.; Higashi, T.; Taguchi, M. Establishment of an In Vivo Xenograft Mouse Model of a Subcutaneous Submillimeter HT-29 Tumor Formed from a Single Spheroid Transplanted Using Radiation-Crosslinked Gelatin Hydrogel Microwell. Appl. Sci. 2021, 11, 7031. [Google Scholar] [CrossRef]
- Atzpodien, J.; Kirchner, H.; Hänninen, E.L.; Deckert, M.; Fenner, M.; Poliwoda, H. Interleukin-2 in combination with interferon-alpha and 5-fluorouracil for metastatic renal cell cancer. Eur. J. Cancer 1993, 29A (Suppl. S5), S6–S8. [Google Scholar] [CrossRef]
- Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers 2021, 13, 167. [Google Scholar] [CrossRef]
- Colombo, M.P.; Trinchieri, G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002, 13, 155–168. [Google Scholar] [CrossRef]
- Zaharoff, D.A.; Hance, K.W.; Rogers, C.J.; Schlom, J.; Greiner, J.W. Intratumoral immunotherapy of established solid tumors with chitosan/IL-12. J. Immunother. 2010, 33, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Coca, S.; Enrech, S.; Moreno García, V.; Sáez, M.A.; Gutiérrez, C.; Colmenarejo, A.; Hernández, J.M.; Pérez Piqueras, J. Evaluation of the antitumor activity of interleukin-12 in an experimental murine model of colorectal cancer induced by 1,2 dimethyl-hydrazine (DMH). Rev. Esp. Enferm. Dig. 2005, 97, 619–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hande, K.R. Etoposide: Four decades of development of a topoisomerase II inhibitor. Eur. J. Cancer 1998, 34, 1514–1521. [Google Scholar] [CrossRef]
- Burden, D.A.; Kingma, P.S.; Froelich-Ammon, S.J.; Bjornsti, M.-A.; Patchan, M.W.; Thompson, R.B.; Osheroff, N. Topoisomerase II·Etoposide Interactions Direct the Formation of Drug-induced Enzyme-DNA Cleavage Complexes. J. Biol. Chem. 1996, 271, 29238–29244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.; Oh, S.H.; Yun, J.H.; Jho, E.H.; Kang, J.-H.; Batsuren, D.; Tunsag, J.; Park, K.H.; Kim, M.; Nho, C.W. A Novel Topoisomerase Inhibitor, Daurinol, Suppresses Growth of HCT116 Cells with Low Hematological Toxicity Compared to Etoposide. Neoplasia 2011, 13, 1043–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiduschka, G.; Bigenzahn, J.; Brunner, M.; Thurnher, D. Resveratrol synergistically enhances the effect of etoposide in HNSCC cell lines. Acta Otolaryngol. 2014, 134, 1071–1078. [Google Scholar] [CrossRef]
- Vernousfaderani, E.K.; Akhtari, N.; Rezaei, S.; Rezaee, Y.; Shiranirad, S.; Mashhadi, M.; Hashemi, A.; Khankandi, H.P.; Behzad, S. Resveratrol and Colorectal Cancer: A Molecular Approach to Clinical Researches. Curr. Top. Med. Chem. 2021, 21, 2634–2646. [Google Scholar] [CrossRef]
- Athar, M.; Back, J.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch. Biochem. Biophys. 2009, 486, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Schwager, J.; Richard, N.; Widmer, F.; Raederstorff, D. Resveratrol distinctively modulates the inflammatory profiles of immune and endothelial cells. BMC Complement. Altern. Med. 2017, 17, 309. [Google Scholar] [CrossRef] [Green Version]
- Buttari, B.; Profumo, E.; Segoni, L.; D’Arcangelo, D.; Rossi, S.; Facchiano, F.; Saso, L.; Businaro, R.; Iuliano, L.; Riganò, R. Resveratrol Counteracts Inflammation in Human M1 and M2 Macrophages upon Challenge with 7-Oxo-Cholesterol: Potential Therapeutic Implications in Atherosclerosis. Oxid. Med. Cell. Longev. 2014, 2014, 257543. [Google Scholar] [CrossRef]
- Sheng, J.; Sun, H.; Yu, F.-B.; Li, B.; Zhang, Y.; Zhu, Y.-T. The Role of Cyclooxygenase-2 in Colorectal Cancer. Int. J. Med. Sci. 2020, 17, 1095–1101. [Google Scholar] [CrossRef]
- Szewczuk, L.M.; Forti, L.; Stivala, L.A.; Penning, T.M. Resveratrol is a peroxidase-mediated inactivator of COX-1 but not COX-2: A mechanistic approach to the design of COX-1 selective agents. J. Biol. Chem. 2004, 279, 22727–22737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salla, M.; Pandya, V.; Bhullar, K.S.; Kerek, E.; Wong, Y.F.; Losch, R.; Ou, J.; Aldawsari, F.S.; Velazquez-Martinez, C.; Thiesen, A.; et al. Resveratrol and Resveratrol-Aspirin Hybrid Compounds as Potent Intestinal Anti-Inflammatory and Anti-Tumor Drugs. Molecules 2020, 25, 3849. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berardi, V.; Ricci, F.; Castelli, M.; Galati, G.; Risuleo, G. Resveratrol exhibits a strong cytotoxic activity in cultured cells and has an antiviral action against polyomavirus: Potential clinical use. J. Exp. Clin. Cancer Res. 2009, 28, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornienko, J.S.; Smirnova, I.S.; Pugovkina, N.A.; Ivanova, J.S.; Shilina, M.A.; Grinchuk, T.M.; Shatrova, A.N.; Aksenov, N.D.; Zenin, V.V.; Nikolsky, N.N.; et al. High doses of synthetic antioxidants induce premature senescence in cultivated mesenchymal stem cells. Sci. Rep. 2019, 9, 1296. [Google Scholar] [CrossRef] [Green Version]
- Koch, J.; Mönch, D.; Maaß, A.; Gromoll, C.; Hehr, T.; Leibold, T.; Schlitt, H.J.; Dahlke, M.-H.; Renner, P. Three dimensional cultivation increases chemo- and radioresistance of colorectal cancer cell lines. PLoS ONE 2021, 16, e0244513. [Google Scholar] [CrossRef]
- Obinu, A.; Rassu, G.; Corona, P.; Maestri, M.; Riva, F.; Miele, D.; Giunchedi, P.; Gavini, E. Poly (ethyl 2-cyanoacrylate) nanoparticles (PECA-NPs) as possible agents in tumor treatment. Colloids Surf. B Biointerfaces 2019, 177, 520–528. [Google Scholar] [CrossRef]
- Fiorentino, F.P.; Marchesi, I.; Schröder, C.; Schmidt, R.; Yokota, J.; Bagella, L. BET-Inhibitor I-BET762 and PARP-Inhibitor Talazoparib Synergy in Small Cell Lung Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9595. [Google Scholar] [CrossRef]
| MIM-seq Composition | MIM-3 Composition | MIM-4 Composition |
|---|---|---|
| hr-IL-1β (9 CH) | - | - |
| hr-IL-2 (3 CH) | hr-IL-2 (3 CH) | - |
| hr-IL-6 (9 CH) | - | - |
| hr-IL-7 (4 CH) | - | - |
| hr-IL-10 (27 CH) | hr-IL-10 (27 CH) | - |
| hr-IL-12 (4 CH) | hr-IL-12 (4 CH) | hr-IL-12 (4 CH) |
| hr-IFN-α (3 CH) | hr-IFN-α (3 CH) | - |
| hr-IFN-γ (6 CH) | hr-IFN-γ (6 CH) | - |
| hr-TNF-α (5 CH) | - | hr-TNF-α (5 CH) |
| h-EGF (27 CH) | - | h-EGF (27 CH) |
| h-bFGF (27 CH) | - | - |
| hr-IGF-1 (27 CH) | hr-IGF-1 (27 CH) | - |
| hr-TGF-β (27 CH) | - | hr-TGF-β (27 CH) |
| DNA (6-12-30-200 K) | - | DNA (6–12 K) |
| RNA (6-12-30-200 K) | RNA (30–200 K) | - |
| SNA®-C1a01 (18 CH) | - | SNA®-C1a01 (18 CH) |
| SNA®-C1b01 (18 CH) | - | SNA®-C1b01 (18 CH) |
| SNA®-C1c01 (18 CH) | - | SNA®-C1c01 (18 CH) |
| SNA®-HLA I (18 CH) | SNA®-HLA I (18 CH) | - |
| SNA®-HLA II (18 CH) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacques, C.; Marchesi, I.; Fiorentino, F.P.; Chatelais, M.; Lilli, N.L.; Appel, K.; Lejeune, B.; Floris, I. A Micro-Immunotherapy Sequential Medicine MIM-seq Displays Immunomodulatory Effects on Human Macrophages and Anti-Tumor Properties towards In Vitro 2D and 3D Models of Colon Carcinoma and in an In Vivo Subcutaneous Xenograft Colon Carcinoma Model. Int. J. Mol. Sci. 2022, 23, 6059. https://doi.org/10.3390/ijms23116059
Jacques C, Marchesi I, Fiorentino FP, Chatelais M, Lilli NL, Appel K, Lejeune B, Floris I. A Micro-Immunotherapy Sequential Medicine MIM-seq Displays Immunomodulatory Effects on Human Macrophages and Anti-Tumor Properties towards In Vitro 2D and 3D Models of Colon Carcinoma and in an In Vivo Subcutaneous Xenograft Colon Carcinoma Model. International Journal of Molecular Sciences. 2022; 23(11):6059. https://doi.org/10.3390/ijms23116059
Chicago/Turabian StyleJacques, Camille, Irene Marchesi, Francesco Paolo Fiorentino, Mathias Chatelais, Nicoletta Libera Lilli, Kurt Appel, Beatrice Lejeune, and Ilaria Floris. 2022. "A Micro-Immunotherapy Sequential Medicine MIM-seq Displays Immunomodulatory Effects on Human Macrophages and Anti-Tumor Properties towards In Vitro 2D and 3D Models of Colon Carcinoma and in an In Vivo Subcutaneous Xenograft Colon Carcinoma Model" International Journal of Molecular Sciences 23, no. 11: 6059. https://doi.org/10.3390/ijms23116059







