Role of Peroxisome Proliferator-Activated Receptors (PPARs) in Energy Homeostasis of Dairy Animals: Exploiting Their Modulation through Nutrigenomic Interventions
Abstract
:1. Introduction
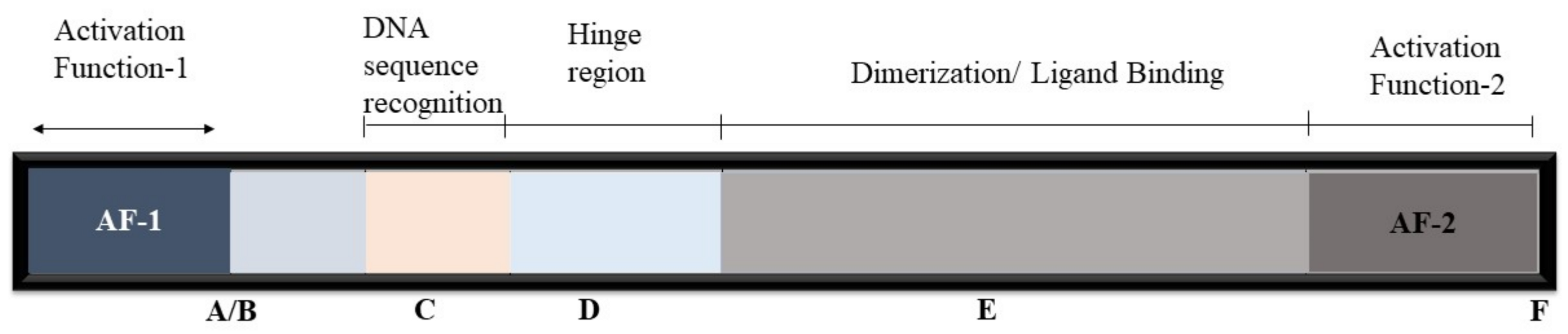
2. Nuclear Receptors’ Mode of Action
2.1. Transcriptional Activation
2.2. Nuclear Receptor Corepressor Binding
2.3. Nuclear Receptor Coactivator Binding
p160 and p300 Families
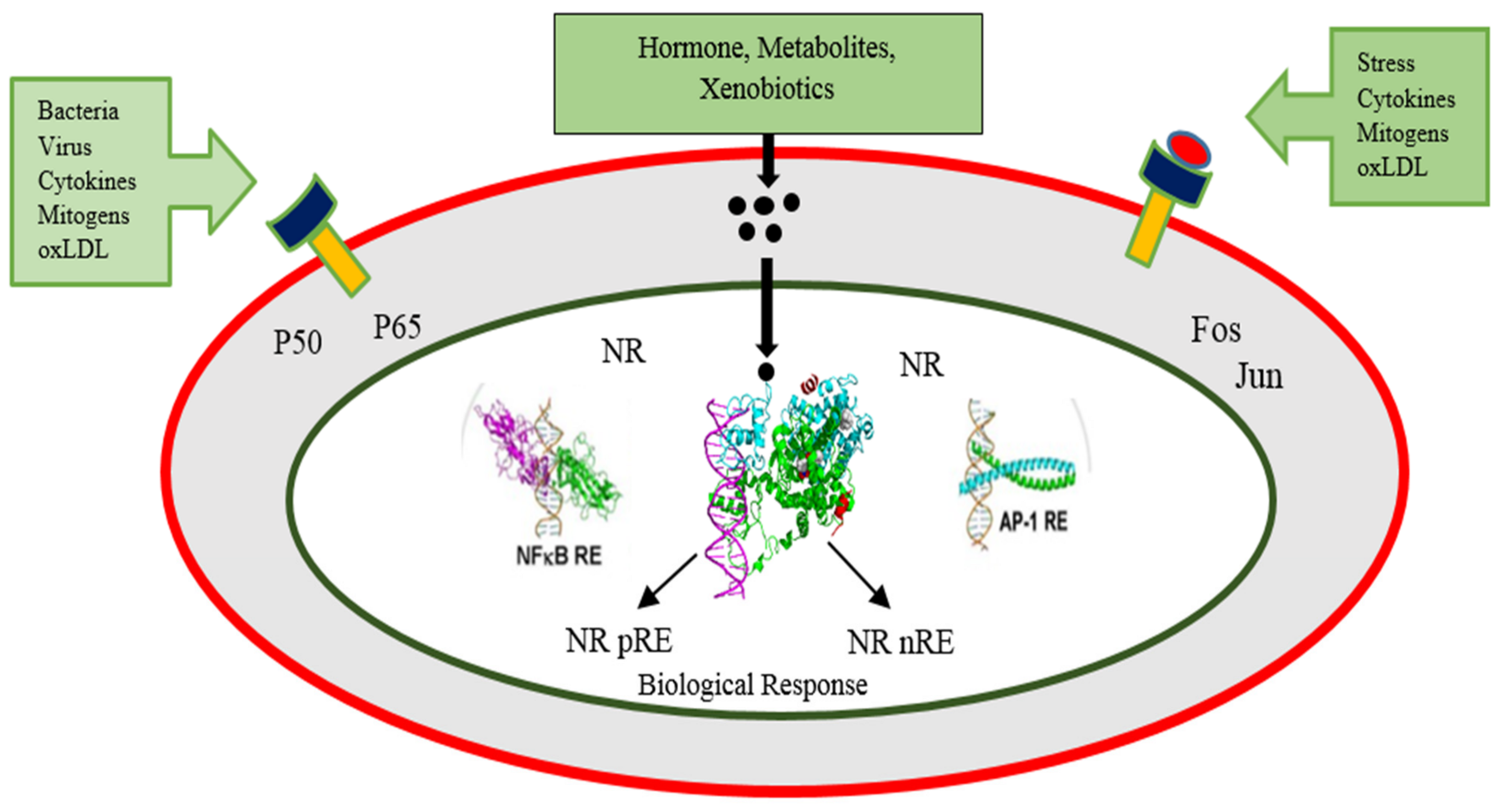
2.4. Transcriptional Repression
2.4.1. Transcriptional Repression by Unliganded Receptors
2.4.2. Direct Trans-Repression by Ligand Activated Receptors
2.4.3. Tethered Transrepression by Liganded Receptors
3. Role of PPARs and Coregulators in Energy Homeostasis
3.1. The ATP-Dependent Remodeling Complex SWI/SNF
3.2. The Mediator Complexes
3.3. PPARs Signaling in Different Body Parts
3.4. Energy Homeostasis through Co-Regulators of PPARs
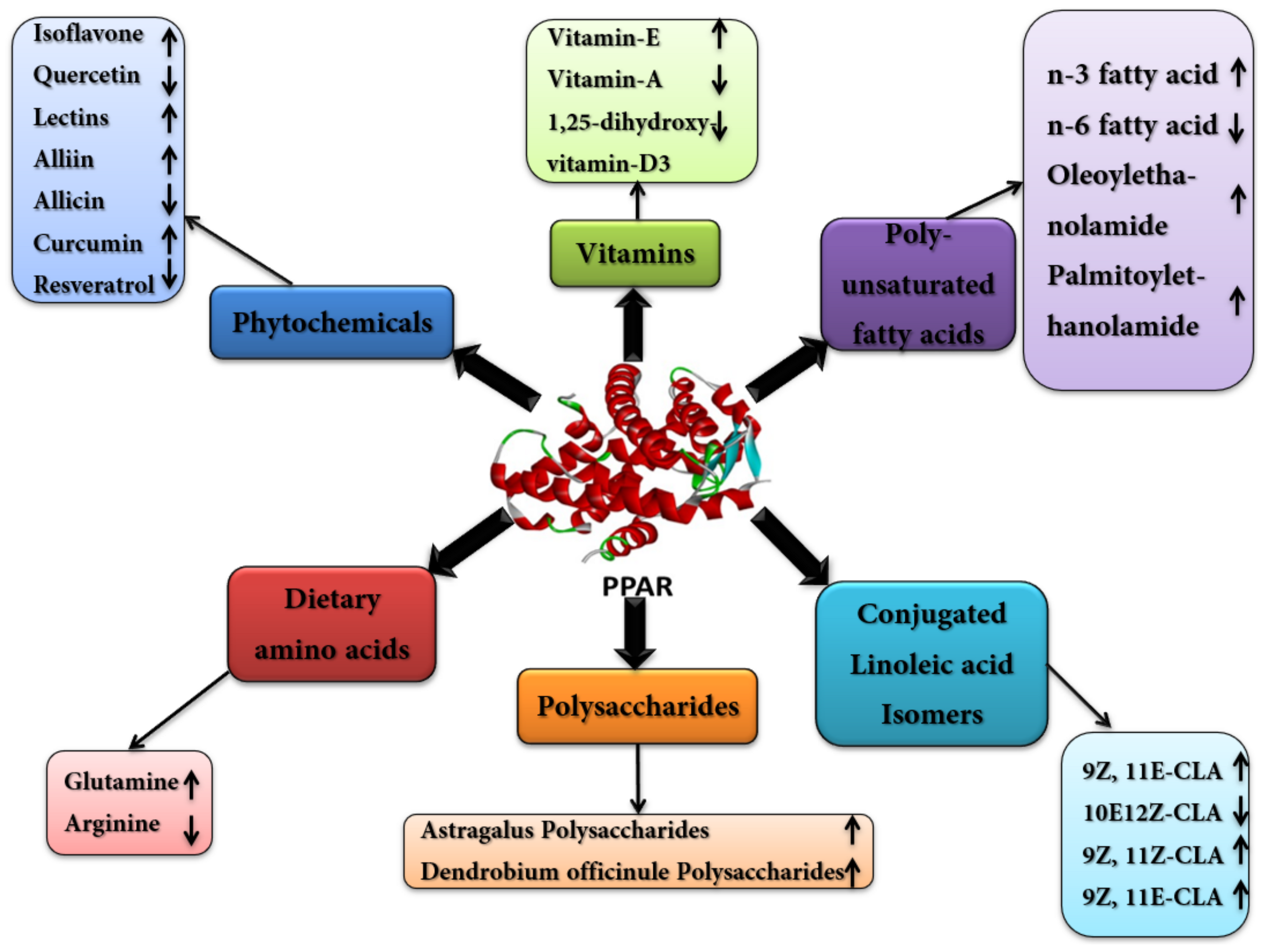
4. Nutritional Modulation of PPARs to Modify Gene Expression and Metabolic Networks
4.1. Poly Unsaturated Fatty Acids (PUFA)
4.2. Conjugated Linoleic-Acids (CLAs)
4.3. Dietary Amino Acids
4.4. Vitamins and Minerals
4.4.1. Beta Carotene, Vitamin A, and Its Derivatives
4.4.2. Vitamin E: Alpha Tocopherols and Tocotrienols
4.4.3. Retinoic Acid and 1,25-Dihydroxy Vitamin D3 (1,25(OH)2D3)
4.5. Phytochemicals
5. Biological Benefits of PPARs Modulation in Dairy Animals
5.1. Energy Metabolism and Lipid Oxidation in Various Organs
5.2. Adipogenesis and Milk Production
5.3. Controlling Inflammation
5.4. PPARs and Fatty Liver Syndrome of Dairy Animals
5.5. PPARs Interaction with Gut Microbiome and Animal Health
5.6. Other Benefits
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trevisi, E.; Amadori, M.; Archetti, I.; Lacetera, N.; Bertoni, G. Inflammatory response and acute phase proteins in the transition period of high-yielding dairy cows. Acute Phase Proteins Early Non-Specif. Biomark. Hum. Vet. Dis. 2011, 15, 355–379. [Google Scholar]
- Sordillo, L.M.; Contreras, G.; Aitken, S.L. Metabolic factors affecting the inflammatory response of periparturient dairy cows. Anim. Health Res. Rev. 2009, 10, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Liu, H.; Zhao, R. Nuclear Receptors in Hepatic Glucose and Lipid Metabolism During Neonatal and Adult Life. Curr. Protein Pept. Sci. 2017, 18, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Chambliss, K.; Umetani, M.; Mineo, C.; Shaul, P.W. Non-nuclear estrogen receptor signaling in the endothelium. J. Biol. Chem. 2011, 286, 14737–14743. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Xu, Y. Nuclear receptor coactivators (NCOAs) and corepressors (NCORs) in the brain. Endocrinology 2020, 161, bqaa083. [Google Scholar] [CrossRef]
- Burris, T.P.; Busby, S.A.; Griffin, P.R. Targeting orphan nuclear receptors for treatment of metabolic diseases and autoimmunity. Chem. Biol. 2012, 19, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Sever, R.; Glass, C.K. Signaling by nuclear receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a016709. [Google Scholar] [CrossRef] [Green Version]
- Pawlak, M.; Lefebvre, P.; Staels, B. General molecular biology and architecture of nuclear receptors. Curr. Top. Med. Chem. 2012, 12, 486–504. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, C.; Krey, G.; Keller, H.; Givel, F.; Helftenbein, G.; Wahli, W. Control of the peroxisomal β-oxidation pathway by a novel family of nuclear hormone receptors. Cell 1992, 68, 879–887. [Google Scholar] [CrossRef]
- Issemann, I.; Green, S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 1990, 347, 645–650. [Google Scholar] [CrossRef]
- Waku, T.; Shiraki, T.; Oyama, T.; Fujimoto, Y.; Maebara, K.; Kamiya, N.; Jingami, H.; Morikawa, K. Structural insight into PPARγ activation through covalent modification with endogenous fatty acids. J. Mol. Biol. 2009, 385, 188–199. [Google Scholar] [CrossRef]
- Forman, B.M.; Chen, J.; Evans, R.M. The Peroxisome Proliferator-activated Receptors: Ligands and Activators a. Ann. N. Y. Acad. Sci. 1996, 804, 266–275. [Google Scholar] [CrossRef]
- Bionaz, M.; Chen, S.; Khan, M.J.; Loor, J.J. Functional role of PPARs in ruminants: Potential targets for fine-tuning metabolism during growth and lactation. PPAR Res. 2013, 2013, 684159. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-X. PPARs: Diverse regulators in energy metabolism and metabolic diseases. Cell Res. 2010, 20, 124–137. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chinpaisal, C.; Wei, L.-N. Cloning and characterization of mouse RIP140, a corepressor for nuclear orphan receptor TR2. Mol. Cell. Biol. 1998, 18, 6745–6755. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, I.; Bastien, Y.; Wai, T.; Nygard, K.; Lin, R.; Cormier, O.; Lee, H.S.; Eng, F.; Bertos, N.R.; Pelletier, N. Ligand-dependent nuclear receptor corepressor LCoR functions by histone deacetylase-dependent and-independent mechanisms. Mol. Cell 2003, 11, 139–150. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Lazar, M.A. Determinants of CoRNR-dependent repression complex assembly on nuclear hormone receptors. Mol. Cell. Biol. 2001, 21, 1747–1758. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Lazar, M.A. The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature 1999, 402, 93–96. [Google Scholar] [CrossRef]
- Varlakhanova, N.; Snyder, C.; Jose, S.; Hahm, J.B.; Privalsky, M.L. Estrogen receptors recruit SMRT and N-CoR corepressors through newly recognized contacts between the corepressor N terminus and the receptor DNA binding domain. Mol. Cell. Biol. 2010, 30, 1434–1445. [Google Scholar] [CrossRef] [Green Version]
- Le Maire, A.; Teyssier, C.; Erb, C.; Grimaldi, M.; Alvarez, S.; De Lera, A.R.; Balaguer, P.; Gronemeyer, H.; Royer, C.A.; Germain, P. A unique secondary-structure switch controls constitutive gene repression by retinoic acid receptor. Nat. Struct. Mol. Biol. 2010, 17, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Phelan, C.A.; Gampe, R.T., Jr.; Lambert, M.H.; Parks, D.J.; Montana, V.; Bynum, J.; Broderick, T.M.; Hu, X.; Williams, S.P.; Nolte, R.T. Structure of Rev-erbα bound to N-CoR reveals a unique mechanism of nuclear receptor–co-repressor interaction. Nat. Struct. Mol. Biol. 2010, 17, 808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onate, S.A.; Tsai, S.Y.; Tsai, M.-J.; O’Malley, B.W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 1995, 270, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Lonard, D.M.; O’Malley, B.W. Multi-modulation of nuclear receptor coactivators through posttranslational modifications. Trends Endocrinol. Metab. 2009, 20, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasgupta, S.; Lonard, D.M.; O’Malley, B.W. Nuclear receptor coactivators: Master regulators of human health and disease. Annu. Rev. Med. 2014, 65, 279–292. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Huang, S.-M.; Stallcup, M.R. Synergistic, p160 coactivator-dependent enhancement of estrogen receptor function by CARM1 and p300. J. Biol. Chem. 2000, 275, 40810–40816. [Google Scholar] [CrossRef] [Green Version]
- Roth, S.Y.; Denu, J.M.; Allis, C.D. Histone acetyltransferases. Annu. Rev. Biochem. 2001, 70, 81–120. [Google Scholar] [CrossRef]
- Luger, K.; Mäder, A.W.; Richmond, R.K.; Sargent, D.F.; Richmond, T.J. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 1997, 389, 251–260. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Wang, J.; Nawaz, Z.; Liu, J.M.; Qin, J.; Wong, J. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J. 2000, 19, 4342–4350. [Google Scholar] [CrossRef] [Green Version]
- Perissi, V.; Jepsen, K.; Glass, C.K.; Rosenfeld, M.G. Deconstructing repression: Evolving models of co-repressor action. Nat. Rev. Genet. 2010, 11, 109–123. [Google Scholar] [CrossRef]
- Sakai, D.D.; Helms, S.; Carlstedt-Duke, J.; Gustafsson, J.-A.; Rottman, F.M.; Yamamoto, K.R. Hormone-mediated repression: A negative glucocorticoid response element from the bovine prolactin gene. Genes Dev. 1988, 2, 1144–1154. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, V.; Lee, J.-K.; Rentoumis, A.; Jameson, J.L. Negative regulation of the thyroid-stimulating hormone alpha gene by thyroid hormone: Receptor interaction adjacent to the TATA box. Proc. Natl. Acad. Sci. USA 1989, 86, 9114–9118. [Google Scholar] [CrossRef] [Green Version]
- Diamond, M.I.; Miner, J.N.; Yoshinaga, S.K.; Yamamoto, K.R. Transcription factor interactions: Selectors of positive or negative regulation from a single DNA element. Science 1990, 249, 1266–1272. [Google Scholar] [CrossRef]
- Subramaniam, N.; Cairns, W.; Okret, S. Glucocorticoids repress transcription from a negative glucocorticoid response element recognized by two homeodomain-containing proteins, Pbx and Oct-1. J. Biol. Chem. 1998, 273, 23567–23574. [Google Scholar] [CrossRef] [Green Version]
- Villa, A.; Santiago, J.; Belandia, B.; Pascual, A. A response unit in the first exon of the β-amyloid precursor protein gene containing thyroid hormone receptor and Sp1 binding sites mediates negative regulation by 3, 5, 3′-triiodothyronine. Mol. Endocrinol. 2004, 18, 863–873. [Google Scholar] [CrossRef] [Green Version]
- Surjit, M.; Ganti, K.P.; Mukherji, A.; Ye, T.; Hua, G.; Metzger, D.; Li, M.; Chambon, P. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell 2011, 145, 224–241. [Google Scholar] [CrossRef] [Green Version]
- Berghagen, H.; Ragnhildstveit, E.; Krogsrud, K.; Thuestad, G.; Apriletti, J.; Saatcioglu, F. Corepressor SMRT functions as a coactivator for thyroid hormone receptor T3Rα from a negative hormone response element. J. Biol. Chem. 2002, 277, 49517–49522. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, Y.; Murata, Y.; Sadow, P.; Hayashi, Y.; Seo, H.; Xu, J.; O’Malley, B.W.; Weiss, R.E.; Refetoff, S. Steroid receptor coactivator-1 deficiency causes variable alterations in the modulation of T3-regulated transcription of genes in vivo. Endocrinology 2002, 143, 1346–1352. [Google Scholar] [CrossRef]
- Weiss, R.E.; Xu, J.; Ning, G.; Pohlenz, J.; O’Malley, B.W.; Refetoff, S. Mice deficient in the steroid receptor co-activator 1 (SRC-1) are resistant to thyroid hormone. EMBO J. 1999, 18, 1900–1904. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Xia, X.; Weiss, R.E.; Refetoff, S.; Yen, P.M. Distinct and histone-specific modifications mediate positive versus negative transcriptional regulation of TSHα promoter. PLoS ONE 2010, 5, e9853. [Google Scholar] [CrossRef]
- Auphan, N.; DiDonato, J.A.; Rosette, C.; Helmberg, A.; Karin, M. Immunosuppression by glucocorticoids: Inhibition of NF-κB activity through induction of IκB synthesis. Science 1995, 270, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Saijo, K.; Collier, J.G.; Li, A.C.; Katzenellenbogen, J.A.; Glass, C.K. An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell 2011, 145, 584–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, M.J.; Eckert, A.; Lai, K.; Adelman, S.J.; Harnish, D.C. Reciprocal antagonism between estrogen receptor and NF-κB activity in vivo. Circ. Res. 2001, 89, 823–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, I.M.; Vanden Berghe, W.; Vermeulen, L.; Yamamoto, K.R.; Haegeman, G.; De Bosscher, K. Crosstalk in inflammation: The interplay of glucocorticoid receptor-based mechanisms and kinases and phosphatases. Endocr. Rev. 2009, 30, 830–882. [Google Scholar] [CrossRef]
- Ogawa, S.; Lozach, J.; Benner, C.; Pascual, G.; Tangirala, R.K.; Westin, S.; Hoffmann, A.; Subramaniam, S.; David, M.; Rosenfeld, M.G. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell 2005, 122, 707–721. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Pacheco, A.; Martínez-Iglesias, O.; Mendez-Pertuz, M.; Aranda, A. Residues K128, 132, and 134 in the thyroid hormone receptor-α are essential for receptor acetylation and activity. Endocrinology 2009, 150, 5143–5152. [Google Scholar] [CrossRef]
- Benkoussa, M.; Brand, C.; Delmotte, M.-H.; Formstecher, P.; Lefebvre, P. Retinoic acid receptors inhibit AP1 activation by regulating extracellular signal-regulated kinase and CBP recruitment to an AP1-responsive promoter. Mol. Cell. Biol. 2002, 22, 4522–4534. [Google Scholar] [CrossRef] [Green Version]
- Rauch, A.; Seitz, S.; Baschant, U.; Schilling, A.F.; Illing, A.; Stride, B.; Kirilov, M.; Takacz, A.; Schmidt-Ullrich, R.; Ostermay, S. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010, 11, 517–531. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Evans, R. PPARs and ERRs: Molecular mediators of mitochondrial metabolism. Curr. Opin. Cell Biol. 2015, 33, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Kota, B.P.; Huang, T.H.-W.; Roufogalis, B.D. An overview on biological mechanisms of PPARs. Pharm. Res. 2005, 51, 85–94. [Google Scholar] [CrossRef]
- Ahmed, W.; Ziouzenkova, O.; Brown, J.; Devchand, P.; Francis, S.; Kadakia, M.; Kanda, T.; Orasanu, G.; Sharlach, M.; Zandbergen, F. PPARs and their metabolic modulation: New mechanisms for transcriptional regulation? J. Intern. Med. 2007, 262, 184–198. [Google Scholar] [CrossRef]
- Wu, J.; Chen, L.; Zhang, D.; Huo, M.; Zhang, X.; Pu, D.; Guan, Y. Peroxisome proliferator-activated receptors and renal diseases. Front. Biosci. 2009, 14, 995–1009. [Google Scholar] [CrossRef] [Green Version]
- Dubois, V.; Eeckhoute, J.; Lefebvre, P.; Staels, B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J. Clin. Investig. 2017, 127, 1202–1214. [Google Scholar] [CrossRef] [Green Version]
- Kersten, S.; Desvergne, B.; Wahli, W. Roles of PPARs in health and disease. Nature 2000, 405, 421–424. [Google Scholar] [CrossRef]
- Ahmed, I.; Rehman, S.U.; Shahmohamadnejad, S.; Zia, M.A.; Ahmad, M.; Saeed, M.M.; Akram, Z.; Iqbal, H.; Liu, Q. Therapeutic Attributes of Endocannabinoid System against Neuro-Inflammatory Autoimmune Disorders. Molecules 2021, 26, 3389. [Google Scholar] [CrossRef]
- York, B.; O’Malley, B.W. Steroid receptor coactivator (SRC) family: Masters of systems biology. J. Biol. Chem. 2010, 285, 38743–38750. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Hayes, J.J. When push comes to shove: SWI/SNF uses a nucleosome to get rid of a nucleosome. Mol. Cell 2010, 38, 484–486. [Google Scholar] [CrossRef] [Green Version]
- Chiba, H.; Muramatsu, M.; Nomoto, A.; Kato, H. Two human homologues of Saccharomyces cerevisiae SWI2/SNF2 and Drosophila brahma are transcriptional coactivators cooperating with the estrogen receptor and the retinoic acid receptor. Nucleic Acids Res. 1994, 22, 1815–1820. [Google Scholar] [CrossRef] [Green Version]
- Link, K.A.; Burd, C.J.; Williams, E.; Marshall, T.; Rosson, G.; Henry, E.; Weissman, B.; Knudsen, K.E. BAF57 governs androgen receptor action and androgen-dependent proliferation through SWI/SNF. Mol. Cell. Biol. 2005, 25, 2200–2215. [Google Scholar] [CrossRef] [Green Version]
- Miao, J.; Fang, S.; Lee, J.; Comstock, C.; Knudsen, K.E.; Kemper, J.K. Functional specificities of Brm and Brg-1 Swi/Snf ATPases in the feedback regulation of hepatic bile acid biosynthesis. Mol. Cell. Biol. 2009, 29, 6170–6181. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, P.-W.; Fryer, C.J.; Trotter, K.W.; Wang, W.; Archer, T.K. BAF60a mediates critical interactions between nuclear receptors and the BRG1 chromatin-remodeling complex for transactivation. Mol. Cell. Biol. 2003, 23, 6210–6220. [Google Scholar] [CrossRef] [Green Version]
- Kemper, J.K.; Kim, H.; Miao, J.; Bhalla, S.; Bae, Y. Role of an mSin3A-Swi/Snf chromatin remodeling complex in the feedback repression of bile acid biosynthesis by SHP. Mol. Cell. Biol. 2004, 24, 7707–7719. [Google Scholar] [CrossRef] [Green Version]
- Underhill, C.; Qutob, M.S.; Yee, S.-P.; Torchia, J. A novel nuclear receptor corepressor complex, N-CoR, contains components of the mammalian SWI/SNF complex and the corepressor KAP-1. J. Biol. Chem. 2000, 275, 40463–40470. [Google Scholar] [CrossRef] [Green Version]
- Tao, W.; Chen, S.; Shi, G.; Guo, J.; Xu, Y.; Liu, C. SWItch/sucrose nonfermentable (SWI/SNF) complex subunit BAF60a integrates hepatic circadian clock and energy metabolism. Hepatology 2011, 54, 1410–1420. [Google Scholar] [CrossRef]
- Conaway, R.C.; Conaway, J.W. Origins and activity of the Mediator complex. Semin. Cell Dev. Biol. 2011, 22, 729–734. [Google Scholar] [CrossRef] [Green Version]
- Taatjes, D.J. The human Mediator complex: A versatile, genome-wide regulator of transcription. Trends Biochem. Sci. 2010, 35, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Jia, Y.; Viswakarma, N.; Huang, J.; Vluggens, A.; Wolins, N.E.; Jafari, N.; Rao, M.S.; Borensztajn, J.; Yang, G. Transcription coactivator mediator subunit MED1 is required for the development of fatty liver in the mouse. Hepatology 2011, 53, 1164–1174. [Google Scholar] [CrossRef] [Green Version]
- Ge, K.; Cho, Y.-W.; Guo, H.; Hong, T.B.; Guermah, M.; Ito, M.; Yu, H.; Kalkum, M.; Roeder, R.G. Alternative mechanisms by which mediator subunit MED1/TRAP220 regulates peroxisome proliferator-activated receptor γ-stimulated adipogenesis and target gene expression. Mol. Cell. Biol. 2008, 28, 1081–1091. [Google Scholar] [CrossRef] [Green Version]
- Ge, K.; Guermah, M.; Yuan, C.-X.; Ito, M.; Wallberg, A.E.; Spiegelman, B.M.; Roeder, R.G. Transcription coactivator TRAP220 is required for PPARγ2-stimulated adipogenesis. Nature 2002, 417, 563–567. [Google Scholar] [CrossRef]
- Pyper, S.R.; Viswakarma, N.; Yu, S.; Reddy, J.K. PPARα: Energy combustion, hypolipidemia, inflammation and cancer. Nucl. Recept. Signal. 2010, 8, nrs.08002. [Google Scholar] [CrossRef] [Green Version]
- Dressel, U.; Allen, T.L.; Pippal, J.B.; Rohde, P.R.; Lau, P.; Muscat, G.E. The peroxisome proliferator-activated receptor β/δ agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol. Endocrinol. 2003, 17, 2477–2493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefebvre, P.; Chinetti, G.; Fruchart, J.-C.; Staels, B. Sorting out the roles of PPARα in energy metabolism and vascular homeostasis. J. Clin. Investig. 2006, 116, 571–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feingold, K.R.; Wang, Y.; Moser, A.; Shigenaga, J.K.; Grunfeld, C. LPS decreases fatty acid oxidation and nuclear hormone receptors in the kidney. J. Lipid Res. 2008, 49, 2179–2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Birsoy, K.; Festuccia, W.T.; Laplante, M. A comparative perspective on lipid storage in animals. J. Cell Sci. 2013, 126, 1541–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tontonoz, P.; Hu, E.; Graves, R.A.; Budavari, A.I.; Spiegelman, B.M. mPPAR gamma 2: Tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994, 8, 1224–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef]
- Rosen, E.D.; Hsu, C.-H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPα induces adipogenesis through PPARγ: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Mullican, S.E.; DiSpirito, J.R.; Lazar, M.A. The orphan nuclear receptors at their 25-year reunion. J. Mol. Endocrinol. 2013, 51, T115–T140. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Huang, J.; Hou, Y.; Zhu, H.; Zhao, S.; Ding, B.; Yin, Y.; Yi, G.; Shi, J.; Fan, W. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br. J. Nutr. 2008, 100, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Luo, J.; Zhu, J.; Li, J.; Sun, Y.; Lin, X.; Zhang, L.; Yao, D.; Shi, H. PPARγ regulates genes involved in triacylglycerol synthesis and secretion in mammary gland epithelial cells of dairy goats. PPAR Res. 2013, 2013, 310948. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Zhang, C.; Zhao, W.; Luo, J.; Loor, J. Peroxisome proliferator-activated receptor delta facilitates lipid secretion and catabolism of fatty acids in dairy goat mammary epithelial cells. J. Dairy Sci. 2017, 100, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Manickam, R.; Wahli, W. Roles of Peroxisome Proliferator-Activated Receptor β/δ in skeletal muscle physiology. Biochimie 2017, 136, 42–48. [Google Scholar] [CrossRef]
- Cho, S.Y.; Jeong, H.W.; Sohn, J.H.; Seo, D.-B.; Kim, W.G.; Lee, S.-J. An ethanol extract of Artemisia iwayomogi activates PPARδ leading to activation of fatty acid oxidation in skeletal muscle. PLoS ONE 2012, 7, e33815. [Google Scholar] [CrossRef]
- Periasamy, M.; Herrera, J.L.; Reis, F.C. Skeletal muscle thermogenesis and its role in whole body energy metabolism. Diabetes Metab. J. 2017, 41, 327–336. [Google Scholar] [CrossRef]
- Gan, Z.; Burkart-Hartman, E.M.; Han, D.-H.; Finck, B.; Leone, T.C.; Smith, E.Y.; Ayala, J.E.; Holloszy, J.; Kelly, D.P. The nuclear receptor PPARβ/δ programs muscle glucose metabolism in cooperation with AMPK and MEF2. Genes Dev. 2011, 25, 2619–2630. [Google Scholar] [CrossRef] [Green Version]
- Lamichane, S.; Lamichane, B.D.; Kwon, S.-M. Pivotal Roles of Peroxisome Proliferator-Activated. PPARs Cell. Whole Body Energy Metab. 2019, 19, 382. [Google Scholar]
- Pérez-Schindler, J.; Svensson, K.; Vargas-Fernández, E.; Santos, G.; Wahli, W.; Handschin, C. The coactivator PGC-1α regulates skeletal muscle oxidative metabolism independently of the nuclear receptor PPARβ/δ in sedentary mice fed a regular chow diet. Diabetologia 2014, 57, 2405–2412. [Google Scholar] [CrossRef] [Green Version]
- Thach, T.T.; Lee, C.-K.; woo Park, H.; Lee, S.-J.; Lee, S.-J. Syringaresinol induces mitochondrial biogenesis through activation of PPARβ pathway in skeletal muscle cells. Bioorgan. Med. Chem. Lett. 2016, 26, 3978–3983. [Google Scholar] [CrossRef]
- Vrins, C.L.; van der Velde, A.E.; van den Oever, K.; Levels, J.H.; Huet, S.; Elferink, R.P.O.; Kuipers, F.; Groen, A.K. Peroxisome proliferator-activated receptor delta activation leads to increased transintestinal cholesterol efflux. J. Lipid Res. 2009, 50, 2046–2054. [Google Scholar] [CrossRef] [Green Version]
- Higashimura, Y.; Naito, Y.; TAkAGI, T.; Uchiyama, K.; Mizushima, K.; YOSHIkAWA, T. Propionate promotes fatty acid oxidation through the up-regulation of peroxisome proliferator-activated receptor α in intestinal epithelial cells. J. Nutri. Sci. Vitaminol. 2015, 61, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Korecka, A.; de Wouters, T.; Cultrone, A.; Lapaque, N.; Pettersson, S.; Doré, J.; Blottière, H.M.; Arulampalam, V. ANGPTL4 expression induced by butyrate and rosiglitazone in human intestinal epithelial cells utilizes independent pathways. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 304, G1025–G1037. [Google Scholar] [CrossRef] [Green Version]
- Azari, E.K.; Leitner, C.; Jaggi, T.; Langhans, W.; Mansouri, A. Possible role of intestinal fatty acid oxidation in the eating-inhibitory effect of the PPAR-α agonist Wy-14643 in high-fat diet fed rats. PLoS ONE 2013, 8, e74869. [Google Scholar]
- Bünger, M.; de Groot, P.J.; Bosch-Vermeulen, H.; Hooiveld, G.J.; Müller, M. PPARalpha-mediated effects of dietary lipids on intestinal barrier gene expression. BMC Genom. 2008, 9, 231. [Google Scholar]
- Van den Bosch, H.M.; Bünger, M.; de Groot, P.J.; van der Meijde, J.; Hooiveld, G.J.; Müller, M. Gene expression of transporters and phase I/II metabolic enzymes in murine small intestine during fasting. BMC Genom. 2007, 8, 267. [Google Scholar] [CrossRef] [Green Version]
- Takei, K.; Nakagawa, Y.; Wang, Y.; Han, S.-i.; Satoh, A.; Sekiya, M.; Matsuzaka, T.; Shimano, H. Effects of K-877, a novel selective PPARα modulator, on small intestine contribute to the amelioration of hyperlipidemia in low-density lipoprotein receptor knockout mice. J. Pharmacol. Sci. 2017, 133, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Lempradl, A.; Pospisilik, J.A.; Penninger, J.M. Exploring the emerging complexity in transcriptional regulation of energy homeostasis. Nat. Rev. Genet. 2015, 16, 665–681. [Google Scholar] [CrossRef]
- Yamamoto, H.; Williams, E.G.; Mouchiroud, L.; Canto, C.; Fan, W.; Downes, M.; Héligon, C.; Barish, G.D.; Desvergne, B.; Evans, R.M. NCoR1 is a conserved physiological modulator of muscle mass and oxidative function. Cell 2011, 147, 827–839. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Jimenez, C.P.; Kyrmizi, I.; Cardot, P.; Gonzalez, F.J.; Talianidis, I. Hepatocyte nuclear factor 4α coordinates a transcription factor network regulating hepatic fatty acid metabolism. Mol. Cell. Biol. 2010, 30, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236. [Google Scholar] [CrossRef] [PubMed]
- Krey, G.; Braissant, O.; L’Horset, F.; Kalkhoven, E.; Perroud, M.; Parker, M.G.; Wahli, W. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol. Endocrinol. 1997, 11, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Fairall, L.; Amin, K.; Inaba, Y.; Szanto, A.; Balint, B.L.; Nagy, L.; Yamamoto, K.; Schwabe, J.W. Structural basis for the activation of PPARγ by oxidized fatty acids. Nat. Struct. Mol. Biol. 2008, 15, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.E.; Lambert, M.H.; Montana, V.G.; Parks, D.J.; Blanchard, S.G.; Brown, P.J.; Sternbach, D.D.; Lehmann, J.M.; Wisely, G.B.; Willson, T.M. Molecular recognition of fatty acids by peroxisome proliferator–activated receptors. Mol. Cell 1999, 3, 397–403. [Google Scholar] [CrossRef]
- Cavalieri, D.; Calura, E.; Romualdi, C.; Marchi, E.; Radonjic, M.; Van Ommen, B.; Müller, M. Filling gaps in PPAR-alpha signaling through comparative nutrigenomics analysis. BMC Genom. 2009, 10, 596. [Google Scholar] [CrossRef] [Green Version]
- Sanderson, L.M.; de Groot, P.J.; Hooiveld, G.J.; Koppen, A.; Kalkhoven, E.; Müller, M.; Kersten, S. Effect of synthetic dietary triglycerides: A novel research paradigm for nutrigenomics. PLoS ONE 2008, 3, e1681. [Google Scholar] [CrossRef]
- Yoon, J.C.; Puigserver, P.; Chen, G.; Donovan, J.; Wu, Z.; Rhee, J.; Adelmant, G.; Stafford, J.; Kahn, C.R.; Granner, D.K. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 2001, 413, 131–138. [Google Scholar] [CrossRef]
- Rakhshandehroo, M.; Knoch, B.; Müller, M.; Kersten, S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 2010, 2010, 612089. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, G.; Ecker, J. The opposing effects of n− 3 and n− 6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef]
- Khan, S.A.; Heuvel, J.P.V. Reviews: Current topicsrole of nuclear receptors in the regulation of gene expression by dietary fatty acids. J. Nutr. Biochem. 2003, 14, 554–567. [Google Scholar] [CrossRef]
- Power, G.W.; Newsholme, E.A. Dietary fatty acids influence the activity and metabolic control of mitochondrial carnitine palmitoyltransferase I in rat heart and skeletal muscle. J. Nutr. 1997, 127, 2142–2150. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Bayona, W.; Kallen, C.B.; Harding, H.P.; Ravera, C.P.; McMahon, G.; Brown, M.; Lazar, M.A. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J. Biol. Chem. 1995, 270, 23975–23983. [Google Scholar] [CrossRef] [Green Version]
- Borrelli, F.; Izzo, A.A. Role of acylethanolamides in the gastrointestinal tract with special reference to food intake and energy balance. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 33–49. [Google Scholar] [CrossRef]
- O’Sullivan, S. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br. J. Pharmacol. 2007, 152, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Huber, J.; Löffler, M.; Bilban, M.; Reimers, M.; Kadl, A.; Todoric, J.; Zeyda, M.; Geyeregger, R.; Schreiner, M.; Weichhart, T. Prevention of high-fat diet-induced adipose tissue remodeling in obese diabetic mice by n-3 polyunsaturated fatty acids. Int. J. Obesity 2007, 31, 1004–1013. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Shin, H.-J.; Kim, S.Y.; Kim, J.H.; Lee, Y.S.; Kim, D.-H.; Lee, M.-O. Genistein enhances expression of genes involved in fatty acid catabolism through activation of PPARα. Mol. Cell. Endocrinol. 2004, 220, 51–58. [Google Scholar] [CrossRef]
- Ricketts, M.-L.; Moore, D.D.; Banz, W.J.; Mezei, O.; Shay, N.F. Molecular mechanisms of action of the soy isoflavones includes activation of promiscuous nuclear receptors. A review. J. Nutr. Biochem. 2005, 16, 321–330. [Google Scholar] [CrossRef]
- Mezei, O.; Li, Y.; Mullen, E.; Ross-Viola, J.S.; Shay, N.F. Dietary isoflavone supplementation modulates lipid metabolism via PPARα-dependent and-independent mechanisms. Physiol. Genom. 2006, 26, 8–14. [Google Scholar] [CrossRef]
- Yeh, S.-L.; Yeh, C.-L.; Chan, S.-T.; Chuang, C.-H. Plasma rich in quercetin metabolites induces G2/M arrest by upregulating PPAR-γ expression in human A549 lung cancer cells. Planta Med. 2011, 77, 992–998. [Google Scholar] [CrossRef]
- Kobori, M.; Masumoto, S.; Akimoto, Y.; Oike, H. Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a Western-style diet in C57/BL6J mice. Mol. Nutr. Food Res. 2011, 55, 530–540. [Google Scholar] [CrossRef]
- McMichael-Phillips, D.F.; Harding, C.; Morton, M.; Roberts, S.A.; Howell, A.; Potten, C.S.; Bundred, N.J. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am. J. Clin. Nutr. 1998, 68, 1431S–1435S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alibin, C.P.; Kopilas, M.A.; Anderson, H.D. Suppression of Cardiac Myocyte Hypertrophy by Conjugated Linoleic Acid Role of Peroxisome Proliferator-Activated Receptors α and γ. J. Biol. Chem. 2008, 283, 10707–10715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodge, G.; Hodge, S.; Han, P. Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: Potential therapeutic use in the treatment of inflammatory bowel disease. Cytom. J. Int. Soc. Anal. Cytol. 2002, 48, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Kwiecień, I.; Włodek, L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ. Mol. Mutagenes. 2009, 50, 247–265. [Google Scholar] [CrossRef]
- Sato, N.; Moore, F.; Kone, B.; Zou, L.; Smith, M.; Childs, M.; Moore-Olufemi, S.; Schultz, S.; Kozar, R. Differential induction of PPAR-γ by luminal glutamine and iNOS by luminal arginine in the rodent postischemic small bowel. Am. J. Physiol.-Gastrointest. Liver Physiol. 2006, 290, G616–G623. [Google Scholar] [CrossRef]
- Von Lintig, J. Colors with functions: Elucidating the biochemical and molecular basis of carotenoid metabolism. Annu. Rev. Nutr. 2010, 30, 35–56. [Google Scholar] [CrossRef]
- Ortuño Sahagún, D.; Márquez-Aguirre, A.; Quintero-Fabián, S.; López-Roa, R.; Rojas-Mayorquín, A. Modulation of PPAR-γ by nutraceutics as complementary treatment for obesity-related disorders and inflammatory diseases. PPAR Res. 2012, 2012, 318613. [Google Scholar] [CrossRef] [Green Version]
- Ford, E.S.; Will, J.C.; Bowman, B.A.; Narayan, K.V. Diabetes mellitus and serum carotenoids: Findings from the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 1999, 149, 168–176. [Google Scholar] [CrossRef]
- Ylönen, K.; Alfthan, G.; Groop, L.; Saloranta, C.; Aro, A.; Virtanen, S.M.; Group, B.R. Dietary intakes and plasma concentrations of carotenoids and tocopherols in relation to glucose metabolism in subjects at high risk of type 2 diabetes: The Botnia Dietary Study. Am. J. Clin. Nutr. 2003, 77, 1434–1441. [Google Scholar] [CrossRef]
- Coyne, T.; Ibiebele, T.I.; Baade, P.D.; Dobson, A.; McClintock, C.; Dunn, S.; Leonard, D.; Shaw, J. Diabetes mellitus and serum carotenoids: Findings of a population-based study in Queensland, Australia. Am. J. Clin. Nutr. 2005, 82, 685–693. [Google Scholar] [CrossRef]
- Burrows, T.L.; Warren, J.M.; Colyvas, K.; Garg, M.L.; Collins, C.E. Validation of overweight children’s fruit and vegetable intake using plasma carotenoids. Obesity 2009, 17, 162–168. [Google Scholar] [CrossRef]
- Hessel, S.; Eichinger, A.; Isken, A.; Amengual, J.; Hunzelmann, S.; Hoeller, U.; Elste, V.; Hunziker, W.; Goralczyk, R.; Oberhauser, V. CMO1 deficiency abolishes vitamin A production from β-carotene and alters lipid metabolism in mice. J. Biol. Chem. 2007, 282, 33553–33561. [Google Scholar] [CrossRef] [Green Version]
- Boulanger, A.; McLemore, P.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Yu, S.S.; Gentleman, S.; Redmond, T.M. Identification of beta-carotene 15, 15′-monooxygenase as a peroxisome proliferator-activated receptor target gene. FASEB J. 2003, 17, 1304–1306. [Google Scholar] [CrossRef]
- Gong, X.; Tsai, S.-W.; Yan, B.; Rubin, L.P. Cooperation between MEF2 and PPARγ in human intestinal β, β-carotene 15, 15’-monooxygenase gene expression. BMC Mol. Biol. 2006, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Lobo, G.P.; Amengual, J.; Li, H.N.M.; Golczak, M.; Bonet, M.L.; Palczewski, K.; Von Lintig, J. β, β-carotene decreases peroxisome proliferator receptor γ activity and reduces lipid storage capacity of adipocytes in a β, β-carotene oxygenase 1-dependent manner. J. Biol. Chem. 2010, 285, 27891–27899. [Google Scholar] [CrossRef] [Green Version]
- Ziouzenkova, O.; Orasanu, G.; Sharlach, M.; Akiyama, T.E.; Berger, J.P.; Viereck, J.; Hamilton, J.A.; Tang, G.; Dolnikowski, G.G.; Vogel, S. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat. Med. 2007, 13, 695–702. [Google Scholar] [CrossRef]
- Eroglu, A.; Hruszkewycz, D.P.; Curley, R.W., Jr.; Harrison, E.H. The eccentric cleavage product of β-carotene, β-apo-13-carotenone, functions as an antagonist of RXRα. Arch. Biochem. Biophys. 2010, 504, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Amengual, J.; Gouranton, E.; van Helden, Y.G.; Hessel, S.; Ribot, J.; Kramer, E.; Kiec-Wilk, B.; Razny, U.; Lietz, G.; Wyss, A. Beta-carotene reduces body adiposity of mice via BCMO1. PLoS ONE 2011, 6, e20644. [Google Scholar] [CrossRef]
- Wang, X.; Quinn, P.J. Vitamin E and its function in membranes. Prog. Lipid Res. 1999, 38, 309–336. [Google Scholar] [CrossRef]
- Constantinou, C.; Papas, A.; Constantinou, A.I. Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs. Int. J. Cancer 2008, 123, 739–752. [Google Scholar] [CrossRef]
- Traber, M.G. Vitamin E regulatory mechanisms. Annu. Rev. Nutr. 2007, 27, 347–362. [Google Scholar] [CrossRef]
- Ross, A.C.; Caballero, B.; Cousins, R.J.; Tucker, K.L.; Ziegler, T.R. Modern Nutrition in Health and Disease, 11th ed.; Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Stone, W.L.; Krishnan, K.; Campbell, S.E.; Qui, M.; Whaley, S.G.; Yang, H. Tocopherols and the treatment of colon cancer. Ann. N. Y. Acad. Sci. 2004, 1031, 223–233. [Google Scholar] [CrossRef]
- Campbell, S.E.; Stone, W.L.; Whaley, S.G.; Qui, M.; Krishnan, K. Gamma (γ) tocopherol upregulates peroxisome proliferator activated receptor (PPAR) gamma (γ) expression in SW 480 human colon cancer cell lines. BMC Cancer 2003, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, A.A.; Tan, X.; Reis, J.C.; Badr, M.Z.; Papasian, C.J.; Morrison, D.C.; Qureshi, N. Inhibition of nitric oxide in LPS-stimulated macrophages of young and senescent mice by δ-tocotrienol and quercetin. Lipids Health Dis. 2011, 10, 239. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, A.A.; Reis, J.C.; Qureshi, N.; Papasian, C.J.; Morrison, D.C.; Schaefer, D.M. δ-Tocotrienol and quercetin reduce serum levels of nitric oxide and lipid parameters in female chickens. Lipids Health Dis. 2011, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Mosca, A.; Paleari, R.; Ivaldi, G.; Galanello, R.; Giordano, P. The role of haemoglobin A2 testing in the diagnosis of thalassaemias and related haemoglobinopathies. J. Clin. Pathol. 2009, 62, 13–17. [Google Scholar] [CrossRef]
- Uto-Kondo, H.; Ohmori, R.; Kiyose, C.; Kishimoto, Y.; Saito, H.; Igarashi, O.; Kondo, K. Tocotrienol suppresses adipocyte differentiation and Akt phosphorylation in 3T3-L1 preadipocytes. J. Nutr. 2009, 139, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Ribot, J.; Felipe, F.; Bonet, M.L.; Palou, A. Changes of adiposity in response to vitamin A status correlate with changes of PPARγ2 expression. Obesity Res. 2001, 9, 500–509. [Google Scholar] [CrossRef]
- Kuri-Harcuch, W. Differentiation of 3T3-F442A cells into adipocytes is inhibited by retinoic acid. Differentiation 1982, 23, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.J.; Reginato, M.J.; Shao, D.; Krakow, S.L.; Lazar, M.A. Retinoic acid blocks adipogenesis by inhibiting C/EBPbeta-mediated transcription. Mol. Cell. Biol. 1997, 17, 1552–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duque, G.; Macoritto, M.; Kremer, R. 1,25(OH)2D3 inhibits bone marrow adipogenesis in senescence accelerated mice (SAM-P/6) by decreasing the expression of peroxisome proliferator-activated receptor gamma 2 (PPARγ2). Exp. Gerontol. 2004, 39, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Hida, Y.; Kawada, T.; Kayahashi, S.; Ishihara, T.; Fushiki, T. Counteraction of retinoic acid and 1, 25-dihydroxyvitamin D3 on up-regulation of adipocyte differentiation with PPARγ ligand, an antidiabetic thiazolidinedione, in 3T3-L1 cells. Life Sci. 1998, 62, PL205–PL211. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Arnoldi, A.; Johnson, S.K. Phytoestrogens: End of a tale? Ann. Med. 2005, 37, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, M.; Hinge, A.; Limaye, L.S.; Gupta, R.K.; Surolia, A.; Kale, V.P. Mannose-binding dietary lectins induce adipogenic differentiation of the marrow-derived mesenchymal cells via an active insulin-like signaling mechanism. Glycobiology 2011, 21, 521–529. [Google Scholar] [CrossRef]
- Dang, Z.-C.; Audinot, V.; Papapoulos, S.E.; Boutin, J.A.; Löwik, C.W. Peroxisome proliferator-activated receptor γ (PPARγ) as a molecular target for the soy phytoestrogen genistein. J. Biol. Chem. 2003, 278, 962–967. [Google Scholar] [CrossRef] [Green Version]
- Sangeetha, T.; Quine, S.D. Protective effect of S-allyl cysteine sulphoxide (alliin) on glycoproteins and hematology in isoproterenol induced myocardial infarction in male Wistar rats. J. Appl. Toxicol. 2008, 28, 710–716. [Google Scholar] [CrossRef]
- Keophiphath, M.; Priem, F.; Jacquemond-Collet, I.; Clément, K.; Lacasa, D. 1,2-vinyldithiin from garlic inhibits differentiation and inflammation of human preadipocytes. J. Nutr. 2009, 139, 2055–2060. [Google Scholar] [CrossRef]
- Adapala, N.; Chan, M.M. Long-term use of an antiinflammatory, curcumin, suppressed type 1 immunity and exacerbated visceral leishmaniasis in a chronic experimental model. Lab. Investig. 2008, 88, 1329–1339. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos Costa, C.; Rohden, F.; Hammes, T.O.; Margis, R.; Bortolotto, J.W.; Padoin, A.V.; Mottin, C.C.; Guaragna, R.M. Resveratrol upregulated SIRT1, FOXO1, and adiponectin and downregulated PPARγ1–3 mRNA expression in human visceral adipocytes. Obesity Surg. 2011, 21, 356–361. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Zhong, Z.; Chen, L.; Wang, Y. Ganoderma lucidum polysaccharides: Immunomodulation and potential anti-tumor activities. Am. J. Chin. Med. 2011, 39, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Sawant, R.; Godghate, A. Qualitative phytochemical screening of rhizomes of Curcuma longa Linn. Int. J. Sci. Environ. 2013, 2, 634–641. [Google Scholar]
- Hinge, A.; Bajaj, M.; Limaye, L.; Surolia, A.; Kale, V. Oral administration of insulin receptor-interacting lectins leads to an enhancement in the hematopoietic stem and progenitor cell pool of mice. Stem Cells Development 2010, 19, 163–174. [Google Scholar] [CrossRef]
- Rayalam, S.; Della-Fera, M.A.; Yang, J.-Y.; Park, H.J.; Ambati, S.; Baile, C.A. Resveratrol potentiates genistein’s antiadipogenic and proapoptotic effects in 3T3-L1 adipocytes. J. Nutr. 2007, 137, 2668–2673. [Google Scholar] [CrossRef] [Green Version]
- Bionaz, M.; Hausman, G.J.; Loor, J.J.; Mandard, S. Physiological and nutritional roles of PPAR across species. PPAR Res. 2013, 2013, 807156. [Google Scholar] [CrossRef]
- Oliveira, A.C.P.; Bertollo, C.M.; Rocha, L.T.S.; Nascimento, E.B., Jr.; Costa, K.A.; Coelho, M.M. Antinociceptive and antiedematogenic activities of fenofibrate, an agonist of PPAR alpha, and pioglitazone, an agonist of PPAR gamma. Eur. J. Pharmacol. 2007, 561, 194–201. [Google Scholar] [CrossRef]
- Bionaz, M.; Loor, J.J. ACSL1, AGPAT6, FABP3, LPIN1, and SLC27A6 are the most abundant isoforms in bovine mammary tissue and their expression is affected by stage of lactation. J. Nutr. 2008, 138, 1019–1024. [Google Scholar] [CrossRef]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2011, 1812, 1007–1022. [Google Scholar] [CrossRef]
- Kushibiki, S.; Hodate, K.; Shingu, H.; Ueda, Y.; Shinoda, M.; Mori, Y.; Itoh, T.; Yokomizo, Y. Insulin resistance induced in dairy steers by tumor necrosis factor alpha is partially reversed by 2, 4–thiazolidinedione. Domestic Anim. Endocrinol. 2001, 21, 25–37. [Google Scholar] [CrossRef]
- Hauner, H. The mode of action of thiazolidinediones. Diabetes/Metabolism Res. Rev. 2002, 18, S10–S15. [Google Scholar] [CrossRef]
- Perdomo, M.C.; Santos, J.E.; Badinga, L. Trans-10, cis-12 conjugated linoleic acid and the PPAR-γ agonist rosiglitazone attenuate lipopolysaccharide-induced TNF-α production by bovine immune cells. Domest. Anim. Endocrinol. 2011, 41, 118–125. [Google Scholar] [CrossRef]
- He, X.; Gao, J.; Hou, H.; Qi, Z.; Chen, H.; Zhang, X.-X. Inhibition of mitochondrial fatty acid oxidation contributes to development of nonalcoholic fatty liver disease induced by environmental cadmium exposure. Environ. Sci. Technol. 2019, 53, 13992–14000. [Google Scholar] [CrossRef]
- Filip-Ciubotaru, F.; Foia, L.; Manciuc, C.; Grigore, C. PPARs: Structure, mechanisms of action and control. Note I. Revista Medico-Chirurgicala a Societatii de Medici si Naturalisti din Iasi 2011, 115, 477–484. [Google Scholar]
- Hasan, A.U.; Rahman, A.; Kobori, H. Interactions between host PPARs and gut microbiota in health and disease. Int. J. Mol. Sci. 2019, 20, 387. [Google Scholar] [CrossRef] [Green Version]
- Hasan, A.U.; Ohmori, K.; Hashimoto, T.; Kamitori, K.; Yamaguchi, F.; Rahman, A.; Tokuda, M.; Kobori, H. PPARγ activation mitigates glucocorticoid receptor-induced excessive lipolysis in adipocytes via homeostatic crosstalk. J. Cell. Biochem. 2018, 119, 4627–4635. [Google Scholar] [CrossRef]
- Kersten, S. Regulation of lipid metabolism via angiopoietin-like proteins. Biochem. Soc. Trans. 2005, 33, 1059–1062. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef] [Green Version]
- Loor, J.J.; Everts, R.E.; Bionaz, M.; Dann, H.M.; Morin, D.E.; Oliveira, R.; Rodriguez-Zas, S.L.; Drackley, J.K.; Lewin, H.A. Nutrition-induced ketosis alters metabolic and signaling gene networks in liver of periparturient dairy cows. Physiol. Genom. 2007, 32, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Schoenberg, K.M.; Giesy, S.L.; Harvatine, K.J.; Waldron, M.R.; Cheng, C.; Kharitonenkov, A.; Boisclair, Y.R. Plasma FGF21 is elevated by the intense lipid mobilization of lactation. Endocrinology 2011, 152, 4652–4661. [Google Scholar] [CrossRef] [Green Version]
- Riahi, Y.; Sin-Malia, Y.; Cohen, G.; Alpert, E.; Gruzman, A.; Eckel, J.; Staels, B.; Guichardant, M.; Sasson, S. The Natural Protective Mechanism Against Hyperglycemia in Vascular Endothelial Cells: Roles of the Lipid Peroxidation Product 4-Hydroxydodecadienal and Peroxisome Proliferator–Activated Receptor δ. Diabetes 2010, 59, 808–818. [Google Scholar] [CrossRef] [Green Version]
- Bionaz, M.; Trevisi, E.; Calamari, L.; Librandi, F.; Ferrari, A.; Bertoni, G. Plasma paraoxonase, health, inflammatory conditions, and liver function in transition dairy cows. J. Dairy Sci. 2007, 90, 1740–1750. [Google Scholar] [CrossRef] [Green Version]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine mammary protein synthesis during the lactation cycle. Bioinform. Biol. Insights 2011, 5, BBI.S7003. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Periasamy, K.; Rodriguez-Zas, S.L.; Everts, R.E.; Lewin, H.A.; Hurley, W.L.; Loor, J.J. Old and new stories: Revelations from functional analysis of the bovine mammary transcriptome during the lactation cycle. PLoS ONE 2012, 7, e33268. [Google Scholar] [CrossRef] [PubMed]
| Receptor Name | Abbreviation | Ligand |
|---|---|---|
| Progesterone receptor | PR | Progesterone |
| Estrogen receptor | ER | Estrogen |
| Liver X receptor | LXR | Oxysterols |
| Vitamin D3 receptor | VDR | Vitamin D3 |
| Androgen receptor | AR | Testosterone |
| Glucocorticoid receptor | GR | Cortisol |
| Thyroid hormone receptor | TR | Thyroid hormone |
| Retinoic acid-related receptor | RXR | Rexinoids |
| Mineralocorticoid receptor | MR | Aldosterone |
| Peroxisome proliferator activated receptor g | PPARγ | Fatty acid Metabolites |
| Retinoid orphan receptor | ROR | ? |
| Estrogen-related receptor | ERR | ? |
| Nutrients | PPARs Regulation | References |
|---|---|---|
| Polyunsaturated fatty acids (PUFA) | ||
| n-3 fatty acids | Activate both PPARα and PPARγ and lead to prevention of inflammation in adipocytes | [109,115] |
| n-6 fatty acid | Inhibitors of PPAR receptor signalling and regulate metabolic network | [109] |
| Oleoylethanolamide | Activate PPARα and induce lipolysis | [114] |
| Palmitoylethanolamide | Activate PPARα and provide anti-inflammatory activity | [114] |
| Conjugated Linoleic acid (CLA) Isomers | ||
| 9Z, 11E-CLA | Enhance PPAR-γ activation and exerts strong anti-cancer effects | [119,154] |
| 10E, 12Z-CLA | Inhibits the PPAR-γ activation causing inflammation, IR and adipocyte delipidation | [155] |
| 9Z, 11Z-CLA and 9Z, 11E-CLA | Enhanced activation of PPARβ/δ in preadipocytes | [122] |
| Dietary Amino acids | ||
| Glutamine | Increase the expression of PPARγ and prevent metabolic stress | [125] |
| Arginine | Decrease the jejunal TNFa and increase the expression of PPARγ and beneficial against gut injury | [81] |
| Vitamins | ||
| Vitamin-A [Beta Carotene (BC)] | BC supplementation can reduce the activity of PPAR-γ | [138] |
| Vitamin- E (Tocopherols) | α-tocopherol modulate PPAR-γ expression better than γ-tocopherol | [143,144] |
| 1,25-dihydroxy vitamin-D3 | Decrease the expression of PPAR-γ2 and regulate lipid metabolism | [152] |
| Phytochemicals | ||
| Isoflavone | Act as a ligand for PPAR to regulate lipid metabolism | [156] |
| Quercetin | Inhibits the activity of all isoform of PPARs except that of PPAR-γ and prevent accumulation of fat in the liver | [119] |
| Lectins | Up-regulate the PPAR-γ2 and provide an adipogenic effect on mesenchymal cells | [155] |
| Alliin | Activates the PPAR-γ and provides a cardioprotective effect | [157] |
| Allicin | Inhibit the PPAR-γ2 and therefore inhibits the differentiation and inflammation of the human preadipocytes | [158] |
| Curcumin | Activates the PPAR-γ and confer antioxidant and anti-inflammatory activity | [159] |
| Resveratrol | Down-regulate PPAR-γ1−3 mRNA expression in humans and provide anti-diabetic and anti-obesity effects | [160] |
| Triterpenes | Suppress PPAR-γ expression and prevent cancer development | [161] |
| Polysaccharides | Suppress PPAR-γ expression and exert anti-cancerous activity | [161] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, F.-u.; Nadeem, A.; Li, Z.; Javed, M.; Liu, Q.; Azhar, J.; Rehman, M.S.-u.; Cui, K.; Rehman, S.u. Role of Peroxisome Proliferator-Activated Receptors (PPARs) in Energy Homeostasis of Dairy Animals: Exploiting Their Modulation through Nutrigenomic Interventions. Int. J. Mol. Sci. 2021, 22, 12463. https://doi.org/10.3390/ijms222212463
Hassan F-u, Nadeem A, Li Z, Javed M, Liu Q, Azhar J, Rehman MS-u, Cui K, Rehman Su. Role of Peroxisome Proliferator-Activated Receptors (PPARs) in Energy Homeostasis of Dairy Animals: Exploiting Their Modulation through Nutrigenomic Interventions. International Journal of Molecular Sciences. 2021; 22(22):12463. https://doi.org/10.3390/ijms222212463
Chicago/Turabian StyleHassan, Faiz-ul, Asif Nadeem, Zhipeng Li, Maryam Javed, Qingyou Liu, Jahanzaib Azhar, Muhammad Saif-ur Rehman, Kuiqing Cui, and Saif ur Rehman. 2021. "Role of Peroxisome Proliferator-Activated Receptors (PPARs) in Energy Homeostasis of Dairy Animals: Exploiting Their Modulation through Nutrigenomic Interventions" International Journal of Molecular Sciences 22, no. 22: 12463. https://doi.org/10.3390/ijms222212463











