Immunomodulation and Regenerative Capacity of MSCs for Long-COVID
Abstract
:1. Introduction
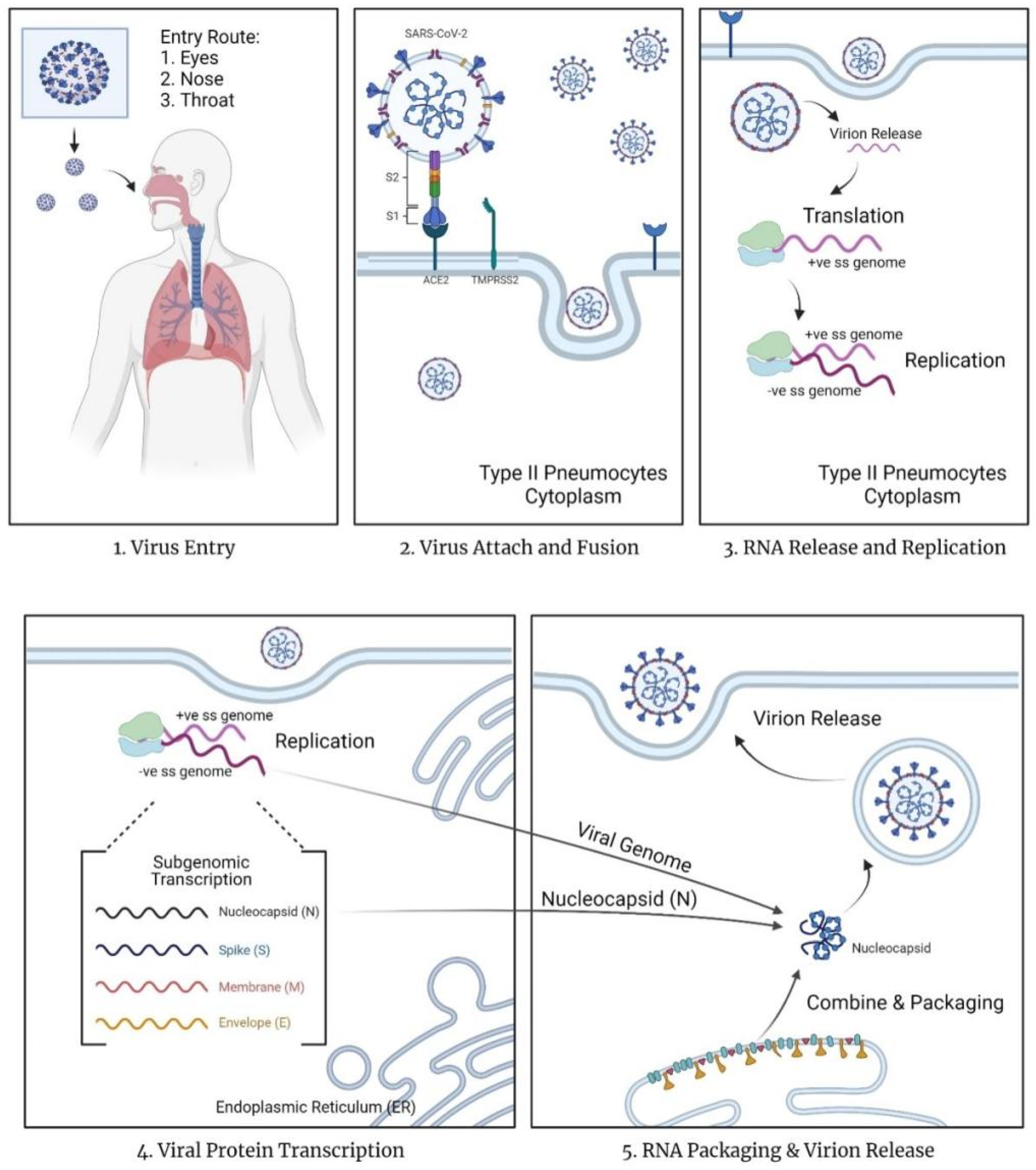
1.1. Mechanism of COVID-19
1.2. Severity of the COVID-19
1.3. Current Problems with COVID-19 Treatment and Vaccines
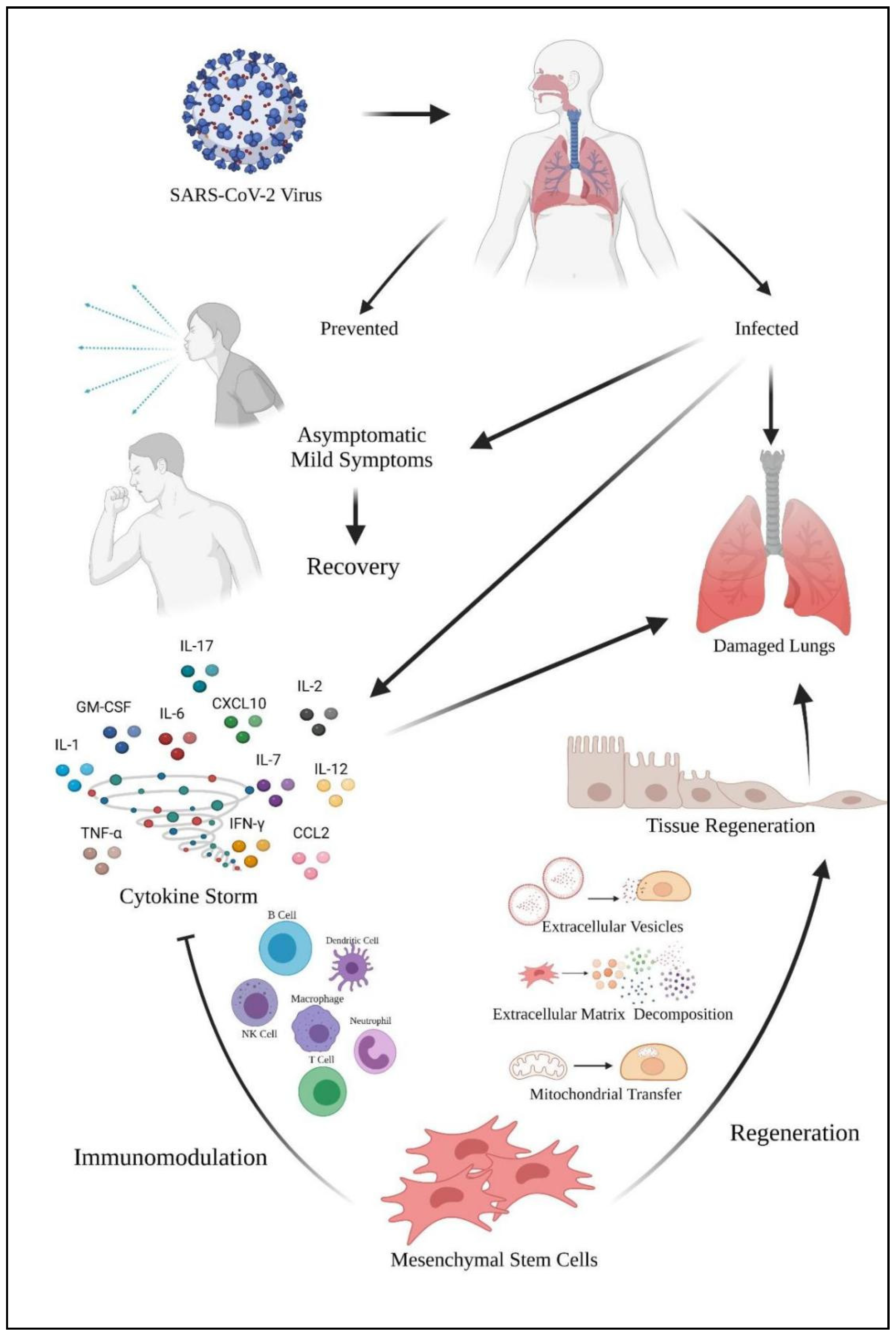
1.4. Cytokine Storm and Immunomodulatory Mechanism
2. Immunomodulatory Effects of MSCs in COVID-19
2.1. Immunological Mechanism during COVID-19
2.2. Immunomodulation Mechanism of MSCs Involving Molecular Signaling
2.2.1. Immunomodulating Effects of MSCs on Macrophages and Neutrophils
2.2.2. Immunomodulating Effects of MSCs on DCs
2.2.3. Immunomodulating Effects of MSCs on T Cells and B Cells
2.2.4. Immunomodulating Effects of MSCs on NK Cells
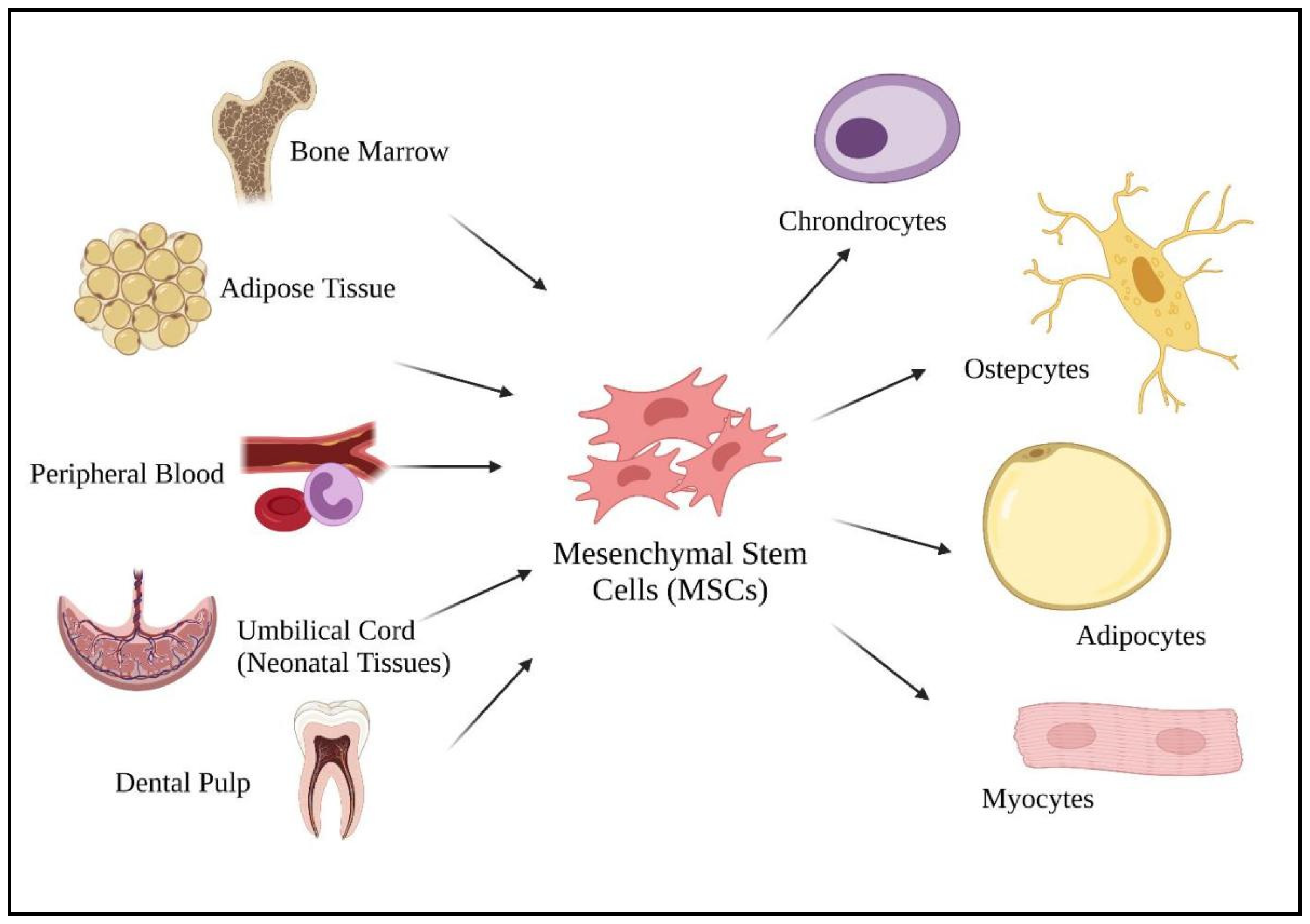
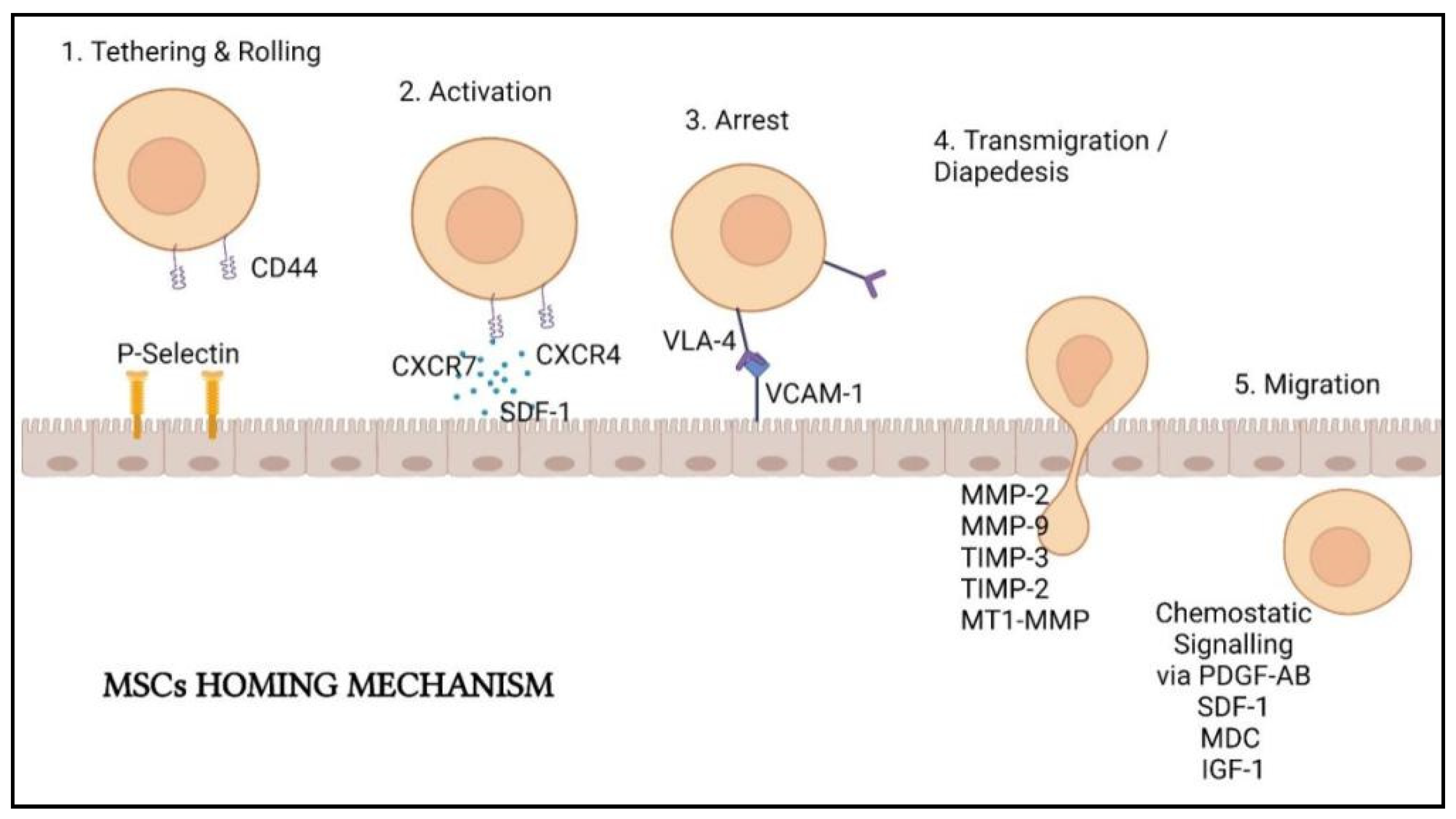
3. Regenerative Mechanisms of MSCs Post-COVID-19 Infection
3.1. Regenerative Mechanism on Damaged Organ Due to COVID-19
3.2. Mechanism of MSCs Tissue Regeneration in Lung Cell Therapy
3.2.1. Secretion at Local and Systemic Levels
3.2.2. Replenish the Cellular Composition of the Niche
4. Current Clinical Trials of MSCs in COVID-19 Treatment and Their Challenges
4.1. Clinical Trials of MSCs in COVID-19 Treatment
4.2. Regulatory Control on MSCs Used in COVID-19
4.3. Safety and Efficacy of MSCs for COVID-19 as a Therapeutic Approach
5. Conclusions
Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard with Vaccination Data. 2021. Available online: https://covid19.who.int/ (accessed on 2 September 2021).
- Elengoe, A. COVID-19 outbreak in Malaysia. Osong Public Health Res. Perspect. J. 2020, 11, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Chen, C.S.; Chan, Y.J. The outbreak of COVID-19: An overview. J. Chin. Med. Assoc. 2020, 83, 217–220. [Google Scholar] [CrossRef]
- Cucinotta, D.; Vanelli, M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar]
- COVID-19 Pandemic, Declared by the World Health Organization (WHO). 12 March 2020. Available online: https://www.pharmaceutical-technology.com/news/who-declares-covid-19-pandemic/ (accessed on 3 August 2021).
- Çetin, İ.; Topçul, M. Can mesenchymal stem cells be used to treat COVID-19-induced pneumonia? Biomed. Rep. 2020, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Centers of Disease Control and Prevention. How to Protect Yourself & Others. 13 August 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html (accessed on 3 August 2021).
- How Ventilators Help with Severe COVID-19 Symptoms. 8 June 2020. Available online: https://www.uchealth.com/en/media-room/covid-19/ventilators-and-covid-19 (accessed on 3 August 2021).
- Banerjee, S. COVID-19: Variants of Concern and Variants of Interest. 13 July 2021. Available online: https://www.thehindu.com/sci-tech/health/covid-19-variants-of-concern-and-variants-of-interest/article35301681.ece (accessed on 3 August 2021).
- World Health Organization. Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 3 August 2021).
- Boopathi, S.; Poma, A.B.; Kolandaivel, P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J. Biomol. Struct. Dyn. 2021, 39, 3409–3418. [Google Scholar] [CrossRef] [Green Version]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and t cellreplication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Kumar, V.; Doshi, K.U.; Khan, W.H.; Rathore, A.S. COVID-19 pandemic: Mechanism, diagnosis, and treatment. J. Chem. Technol. Biotechnol. 2021, 96, 299–308. [Google Scholar] [CrossRef]
- Lin, P.; Wang, M.; Wei, Y.; Kim, T.; Wei, X. Coronavirus in human diseases, mechanisms and advances in clinical treatment. MedComm 2020, 1, 270–301. [Google Scholar] [CrossRef]
- Simmons, G.; Gosalia, D.N.; Rennekamp, A.J.; Reeves, J.D.; Diamond, S.L.; Bates, P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA 2005, 102, 11876–11881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, A.; Alvites, R.D.; Branquinho, M.V.; Guerreiro, S.G.; Maurício, A.C. Mesenchymal stem cells (MSCs) as a potential therapeutic strategy in COVID-19 patients: Literature research. Front. Cell Dev. Biol. 2020, 8, 602647. [Google Scholar] [CrossRef]
- Moll, G.; Drzeniek, N.; Kamhieh-Milz, J.; Geissler, S.; Volk, H.D.; Reinke, P. MSC therapies for COVID-19: Importance of patient coagulopathy, thromboprophylaxis, cell product quality and mode of delivery for treatment safety and efficacy. Front. Immunol. 2020, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
- Reardon, S. The Most Worrying Mutations in Five Emerging Coronavirus Variants—Scientific American. 29 January 2021. Available online: https://www.scientificamerican.com/article/the-most-worrying-mutations-in-five-emerging-coronavirus-variants/ (accessed on 5 August 2021).
- Haider, N.; Rothman-Ostrow, P.; Osman, A.Y.; Arruda, L.B.; Macfarlane-Berry, L.; Elton, L.; Thomason, M.J.; Yeboah-Manu, D.; Ansumana, R.; Kapata, N.; et al. COVID-19—zoonosis or emerging infectious disease? Front. Public Health 2020, 8, 596944. [Google Scholar] [CrossRef] [PubMed]
- Kupferschmidt, K. New mutations raise specter of ‘immune escape’. Science 2021, 371, 329–330. [Google Scholar] [CrossRef]
- Ayres, J.S. Surviving COVID-19: A disease tolerance perspective. Sci. Adv. 2020, 6, 4–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelzman, F. COVID-19 and Disease Severity: Why Is There Such a Wide Range? Available online: https://healthmatters.nyp.org/covid-19-and-disease-severity/ (accessed on 7 August 2021).
- Centers for Disease Control and Prevention. Older Adults Risks and Vaccine Information. Available online: https://www.cdc.gov/aging/covid19/covid19-older-adults.html (accessed on 8 August 2021).
- Chen, P.L.; Lee, N.Y.; Cia, C.T.; Ko, W.C.; Hsueh, P.R. A review of treatment of coronavirus disease 2019 (COVID-19): Therapeutic repurposing and unmet clinical needs. Front. Pharmacol. 2020, 11, 584956. [Google Scholar] [CrossRef]
- Saha, R.P.; Sharma, A.R.; Singh, M.K.; Samanta, S.; Bhakta, S.; Mandal, S.; Bhattacharya, M.; Lee, S.S.; Chakraborty, C. Repurposing drugs, ongoing vaccine, and new therapeutic development initiatives against COVID-19. Front. Pharmacol. 2020, 11, 1258. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. Comirnaty and Pfizer-BioNTech Storm COVID-19 Vaccine. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine (accessed on 10 August 2021).
- World Health Organization. Vaccine Efficacy, Effectiveness and Protection. Available online: https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection (accessed on 10 August 2021).
- Cuffari, B. What Is a Cytokine Storm? Available online: https://www.news-medical.net/health/What-is-Cytokine-Storm.aspx (accessed on 10 August 2021).
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M. Cytokine storm and immunomodulatory therapy in COVID-19: Role of chloroquine and anti-IL-6 monoclonal antibodies. Int. J. Antimicrob. Agents 2020, 55, 105982. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Chen, X.; Wang, F.; Huang, Z.; Wu, Y.; Geng, J.; Wang, Y. Clinical applications of mesenchymal stromal cell-based therapies for pulmonary diseases: An update and concise review. Int. J. Med. Sci. 2021, 18, 2849–2870. [Google Scholar] [CrossRef] [PubMed]
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Van De Veerdonk, F.L.; Netea, M.G. Blocking IL-1 to prevent respiratory failure in COVID-19. Crit. Care 2020, 24, 445. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Cronstein, B.N. Interleukin-6—A key mediator of systemic and local symptoms in rheumatoid arthritis. Bull. NYU Hosp. Jt. Dis. 2007, 65 (Suppl. 1), S11–S15. [Google Scholar] [PubMed]
- Darif, D.; Hammi, I.; Kihel, A.; El Idrissi Saik, I.; Guessous, F.; Akarid, K. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microb. Pathog. 2021, 153, 104799. [Google Scholar] [CrossRef]
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta 2020, 509, 280–287. [Google Scholar] [CrossRef]
- Lawson, B.R.; Gonzalez-Quintial, R.; Eleftheriadis, T.; Farrar, M.A.; Miller, S.D.; Sauer, K.; McGavern, D.B.; Kono, D.H.; Baccala, R.; Theofilopoulos, A.N. Interleukin-7 is required for CD4 + T cell activation and autoimmune neuroinflammation. Clin. Immunol. 2015, 161, 260–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azmi, N.U.; Puteri, M.U.; Lukmanto, D. Cytokine storm in COVID-19: An overview, mechanism, treatment strategies, and stem cell therapy perspective. Pharm. Sci. Res. 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Gorelik, L.; Constant, S.; Flavell, R.A. Mechanism of transforming growth factor β-induced inhibition of T helper type 1 differentiation. J. Exp. Med. 2002, 195, 1499–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burleson, S.C.M.; Fick, R.B.; Mannie, M.D.; Olmstead, S.G.; Van Scott, M.R. Chapter 35—the immune basis of allergic lung disease. In Comparative Biology of the Normal Lung, 2nd ed.; Parent, R., Ed.; Academic Press: Cambridge, MA, USA, 2005; pp. 683–719. ISBN 978-0-12-404577-4. [Google Scholar]
- Shiomi, A.; Usui, T. Pivotal roles of GM-CSF in autoimmunity and inflammation. Mediat. Inflamm. 2015, 2015, 568543. [Google Scholar] [CrossRef] [Green Version]
- Kudlak, K.; Demuro, J.P.; Hanna, A.F.; Brem, H. Acute lung injury following the use of granulocyte-macrophage colony-stimulating factor. Int. J. Crit. Illn. Inj. Sci. 2013, 3, 279–281. [Google Scholar]
- Huang, K.J.; Su, I.J.; Theron, M.; Wu, Y.C.; Lai, S.K.; Liu, C.C.; Lei, H.Y. An interferon-γ-related cytokine storm in SARS patients. J. Med. Virol. 2005, 75, 185–194. [Google Scholar] [CrossRef]
- Meyerowitz, E.A.; Sen, P.; Schoenfeld, S.R.; Neilan, T.G.; Frigault, M.J.; Stone, J.H.; Kim, A.Y.; Mansour, M.K. Immunomodulation as treatment for severe coronavirus disease 2019: A systematic review of current modalities and future directions. Clin. Infect. Dis. 2021, 72, E1130–E1143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Meng, Z. Immunomodulation for severe COVID-19 pneumonia: The state of the art. Front. Immunol. 2020, 11, 2782. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘cytokine storm’’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Wakimoto, H.; Rossignoli, F.; Bhere, D.; Ciccocioppo, R.; Chen, K.S.; Khalsa, J.K.; Mastrolia, I.; Samarelli, A.V.; Dominici, M.; et al. Mesenchymal stem cell immunomodulation: In pursuit of controlling COVID-19 related cytokine storm. Stem Cells 2021, 39, 707–722. [Google Scholar] [PubMed]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by mesenchymal stem cells (MSCs): Mechanisms of action of living, apoptotic, and dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, M.A.; Hossain, N.; Kashem, M.A.; Shahid, M.A.; Alam, A. Immune response in COVID-19: A review. J. Infect. Public Health 2020, 13, 1619–1629. [Google Scholar] [CrossRef]
- Kavianpour, M.; Saleh, M.; Verdi, J. The role of mesenchymal stromal cells in immune modulation of COVID-19: Focus on cytokine storm. Stem Cell Res. Ther. 2020, 11, 404. [Google Scholar] [CrossRef]
- World Health Organization. What We Know About the COVID-19 Immune Response: The Latest in COVID-19 Immunity & the Current Global Situation. 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/risk-comms-updates/update-34-immunity-2nd.pdf?sfvrsn=8a488cb6_2 (accessed on 15 August 2021).
- Verma, Y.K.; Verma, R.; Tyagi, N.; Behl, A.; Kumar, S.; Gangenahalli, G.U. COVID-19 and its therapeutics: Special emphasis on mesenchymal stem cells based therapy. Stem Cell Rev. Rep. 2021, 17, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Huppert, L.A.; Matthay, M.A. Alveolar fluid clearance in pathologically relevant conditions: In vitro and in vivo models of acute respiratory distress syndrome. Front. Immunol. 2017, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Soudi, S.; Shafiee, A.; Hashemi, S.M. Mesenchymal stem cell therapies for COVID-19: Current status and mechanism of action. Life Sci. 2020, 262, 118493. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; John, A.; Koshy, S.; Ranjan, R.; Anudeep, T.C.; Jain, R.; Swati, K.; Jha, N.K.; Sharma, A.; Kesari, K.K.; et al. Fostering mesenchymal stem cell therapy to halt cytokine storm in COVID-19. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166014. [Google Scholar] [CrossRef] [PubMed]
- Mallis, P.; Michalopoulos, E.; Chatzistamatiou, T.; Stavropoulos-Giokas, C. Mesenchymal stromal cells as potential immunomodulatory players in severe acute respiratory distress syndrome induced by SARS-CoV-2 infection. World J. Stem Cells 2020, 12, 731–751. [Google Scholar] [CrossRef] [PubMed]
- Gur-Wahnon, D.; Borovsky, Z.; Beyth, S.; Liebergall, M.; Rachmilewitz, J. Contact-dependent induction of regulatory antigen-presenting cells by human mesenchymal stem cells is mediated via STAT3 signaling. Exp. Hematol. 2007, 35, 426–433. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, Z.; Zhang, R.; Yan, K.; Chen, L.; Chen, F.; Huang, W.; Lv, B.; Sun, C.; Jiang, X. MSCs inhibit bone marrow-derived DC maturation and function through the release of TSG-6. Biochem. Biophys. Res. Commun. 2014, 450, 1409–1415. [Google Scholar] [CrossRef]
- Yadav, P.; Vats, R.; Bano, A.; Bhardwaj, R. Mesenchymal stem cell immunomodulation and regeneration therapeutics as an ameliorative approach for COVID-19 pandemics. Life Sci. 2020, 263, 118588. [Google Scholar] [CrossRef]
- Proal, A.D.; Van Elzakker, M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): An overview of biological factors that may contribute to persistent symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Duncan, G. Long COVID: Scientists and Doctors Grapple with Unanswered Questions. 13 March 2021. Available online: https://www.thenationalnews.com/uae/health/long-covid-scientists-and-doctors-grapple-with-unanswered-questions-1.1182976 (accessed on 13 August 2021).
- Sang, L.; Guo, X.; Shi, J.; Hou, S.; Fan, H.; Lv, Q. Characteristics and developments in mesenchymal stem cell therapy for COVID-19: An update. Stem Cells Int. 2021, 2021, 5593584. [Google Scholar] [CrossRef]
- Kean, T.J.; Lin, P.; Caplan, A.I.; Dennis, J.E. MSCs: Delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013, 2013, 732742. [Google Scholar] [CrossRef] [Green Version]
- Rustad, K.C.; Gurtner, G.C. Mesenchymal stem cells home to sites of injury and inflammation. Adv. Wound Care 2012, 1, 152. [Google Scholar] [CrossRef] [Green Version]
- Ayala-Cuellar, A.P.; Kang, J.H.; Jeung, E.B.; Choi, K.C. Roles of mesenchymal stem cells in tissue regeneration and immunomodulation. Biomol. Ther. 2019, 27, 25–33. [Google Scholar] [CrossRef]
- García-Bernal, D.; García-Arranz, M.; Yáñez, R.M.; Hervás-Salcedo, R.; Cortés, A.; Fernández-García, M.; Hernando-Rodríguez, M.; Quintana-Bustamante, Ó.; Bueren, J.A.; García-Olmo, D.; et al. The current status of mesenchymal stromal cells: Controversies, unresolved issues and some promising solutions to improve their therapeutic efficacy. Front. Cell Dev. Biol. 2021, 9, 650664. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Soto-Gutierrez, A.; Parekkadan, B.; Kitagawa, Y.; Tompkins, R.G.; Kobayashi, N.; Yarmush, M.L. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transpl. 2010, 19, 667–679. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Zhao, F. Updates on clinical trials evaluating the regenerative potential of allogenic mesenchymal stem cells in COVID-19. NPJ Regen. Med. 2021, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jiang, J.; Gu, Z.; Zhang, J.; Chen, Y.; Liu, X. Mesenchymal stromal cell therapies: Immunomodulatory properties and clinical progress. Stem Cell Res. Ther. 2020, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Liu, D.D.; Thakor, A.S. mesenchymal stromal cell homing: Mechanisms and strategies for improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ip, J.E.; Wu, Y.; Huang, J.; Zhang, L.; Pratt, R.E.; Dzau, V.J. mesenchymal stem cells use integrin 1 not cxc chemokine receptor 4 for myocardial migration and engraftment. Mol. Biol. Cell 2007, 18, 2873–2882. [Google Scholar] [CrossRef] [Green Version]
- Son, B.-R.; Marquez-Curtis, L.A.; Kucia, M.; Wysoczynski, M.; Turner, A.R.; Ratajczak, J.; Ratajczak, M.Z.; Janowska-Wieczorek, A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-cxcr4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells 2006, 24, 1254–1264. [Google Scholar] [CrossRef]
- Sagaradze, G.D.; Basalova, N.A.; Efimenko, A.Y.; Tkachuk, V.A. Mesenchymal stromal cells as critical contributors to tissue regeneration. Front. Cell Dev. Biol. 2020, 8, 576176. [Google Scholar] [CrossRef]
- Purcaru, O.S.; Artene, S.A.; Barcan, E.; Silosi, C.A.; Stanciu, I.; Danoiu, S.; Tudorache, S.; Tataranu, L.G.; Dricu, A. The interference between SARS-CoV-2 and tyrosine kinase receptor signaling in cancer. Int. J. Mol. Sci. 2021, 22, 4830. [Google Scholar] [CrossRef]
- Behnke, J.; Kremer, S.; Shahzad, T.; Chao, C.M.; Böttcher-Friebertshäuser, E.; Morty, R.E.; Bellusci, S.; Ehrhardt, H. MSC based therapies—new perspectives for the injured lung. J. Clin. Med. 2020, 9, 682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal micrornas suppress myofibroblast differentiation by inhibiting the transforming growth factor-b/smad2 pathway during wound healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Mahrouf-Yorgov, M.; Augeul, L.; Da Silva, C.C.; Jourdan, M.; Rigolet, M.; Manin, S.; Ferrera, R.; Ovize, M.; Henry, A.; Guguin, A.; et al. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 2017, 24, 1224–1238. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Gai, H.; Mei, J.; Ding, F.; Bao, C.; Nguyen, D.M.; Zhong, H. Bone marrow mesenchymal stem cells can differentiate into type II alveolar epithelial cells in vitro. Cell Biol. Int. 2011, 35, 1261–1266. [Google Scholar] [CrossRef]
- Zhang, Y.; Ravikumar, M.; Ling, L.; Nurcombe, V.; Cool, S.M. Age-Related Changes in the Inflammatory Status of Human Mesenchymal Stem Cells: Implications for Cell Therapy. Stem Cell Rep. 2021, 16, 694–707. [Google Scholar] [CrossRef]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Henkle, S.L.; Betancourt, A.M. A New Mesenchymal Stem Cell (MSC) Paradigm: Polarization into a Pro-Inflammatory MSC1 or an Immunosuppressive MSC2 Phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef] [PubMed]
- Kurte, M.; Vega-Letter, A.M.; Luz-Crawford, P.; Djouad, F.; Noël, D.; Khoury, M.; Carrión, F. Time-Dependent LPS Exposure Commands MSC Immunoplasticity through TLR4 Activation Leading to Opposite Therapeutic Outcome in EAE. Stem Cell Res. Ther. 2020, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Shammaa, R.; El-Kadiry, A.E.H.; Abusarah, J.; Rafei, M. Mesenchymal Stem Cells Beyond Regenerative Medicine. Front. Cell Dev. Biol. 2020, 8, 72. [Google Scholar] [CrossRef] [Green Version]
- Betancourt, A.M.; Waterman, R.S. The Role of Mesenchymal Stem Cells in the Tumor Microenvironment. In Tumor Microenvironment and Myelomonocytic Cells; Biswas, S., Ed.; InTech: London, UK, 2012; pp. 255–286. ISBN 978-953-51-0439-1. [Google Scholar]
- Li, L.; Li, J.; Gao, M.; Fan, H.; Wang, Y.; Xu, X.; Chen, C.; Liu, J.; Kim, J.; Aliyari, R.; et al. Interleukin-8 as a Biomarker for Disease Prognosis of Coronavirus Disease-2019 Patients. Front. Immunol. 2020, 11, 602395. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, Cultivation, and Characterization of Human Mesenchymal Stem Cells. Cytom. Part A 2017, 93, 19–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukui, E.; Funaki, S.; Kimura, K.; Momozane, T.; Kimura, A.; Chijimatsu, R.; Kanzaki, R.; Kanou, T.; Ose, N.; Minami, M.; et al. Adipose Tissue-Derived Stem Cells Have the Ability to Differentiate into Alveolar Epithelial Cells and Ameliorate Lung Injury Caused by Elastase-Induced Emphysema in Mice. Stem Cells Int. 2019, 2019, 5179172. [Google Scholar] [CrossRef]
- Shi, L.; Huang, H.; Lu, X.; Yan, X.; Jiang, X.; Xu, R.; Wang, S.; Zhang, C.; Yuan, X.; Xu, Z.; et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: A randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Target. Ther. 2021, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, N.; Nor, S.N.M.; Mohamed, M.S. Regulation of stem cell technology in malaysia: Current status and recommendations. Sci. Eng. Ethics 2020, 26, 1–25. [Google Scholar] [CrossRef]
- Ministry of Health Malaysia. National Standards for Stem Cell Transplantation: Collection, Processing, Storage and Infusion of Hematopoietic Stem Cells and Therapeutic Cells. Available online: https://www.moh.gov.my/moh/resources/Arkib/National_Standards_For_Stem_cell_Transplatation_new.pdf (accessed on 27 August 2021).
- Ministry of Health Malaysia. Malaysian Guidelines for Stem Cell Research and Therapy. 2009. Available online: https://www.crc.gov.my/wp-content/uploads/documents/MALAYSIAN%20GUIDELINES%20FOR%20STEM%20CELL%20RESEARCH%20AND%20THERAPY%202009%20.pdf (accessed on 23 August 2021).
- International Society for Stem Cell Research. ISSCR Guidelines for Stem Cell Research and Clinical Translation. May 2021. Available online: https://www.isscr.org/docs/default-source/all-isscr-guidelines/2021-guidelines/isscr-guidelines-for-stem-cell-research-and-clinical-translation-2021.pdf?sfvrsn=979d58b1_4 (accessed on 28 August 2021).
- Elstner, A.; Damaschun, A.; Kurtz, A.; Stacey, G.; Arán, B.; Veiga, A.; Borstlap, J. The Changing Landscape of European and International Regulation on Embryonic Stem Cell Research. Stem Cell Res. 2009, 2, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, Z.; Zhu, R.; Hou, W.; Feng, Y.; Yang, Y.; Han, Q.; Shan, G.; Meng, F.; Du, D.; Wang, S.; et al. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020, 11, 216–228. [Google Scholar] [CrossRef] [Green Version]
- Merck &, Co. Merck and Ridgeback Announce Initiation of a Rolling Review by the European Medicines Agency for Molnupiravir, an Investigational Oral Antiviral Medicine, for the Treatment of COVID-19 in Adults. Available online: https://www.merck.com/news/merck-and-ridgeback-announce-initiation-of-a-rolling-review-by-the-european-medicines-agency-for-molnupiravir-an-investigational-oral-antiviral-medicine-for-the-treatment-of-covid-19-in-adults/ (accessed on 28 October 2021).
- Merck &, Co. Merck and Ridgeback’s Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study. Available online: https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat/ (accessed on 28 October 2021).
- Nowakowski, A.; Walczak, P.; Lukomska, B.; Janowski, M. Genetic engineering of mesenchymal stem cells to induce their migration and survival. Stem Cells Int. 2016, 2016, 4956063. [Google Scholar] [CrossRef] [Green Version]

| WHO Label | Pango Lineage | First Documented Date | First Documented Location |
|---|---|---|---|
| Variants of Concern (VOC) | |||
| α (Alpha) | B·1·1·7 | September 2020 | United Kingdom |
| β (Beta) | B·1·351 B·1·351·2 B·1·351·3 | May 2020 | South Africa |
| γ (Gamma) | P·1 P·1·1 P·1·2 P·1·4 P·1·6 P·1·7 | November 2020 | Brazil |
| δ (Delta) | B·1·617·2 AY·1 AY·2 AY·3 AY·3·1 | October 2020 | India |
| Variants of Interest (VOI) | |||
| ε (Epsilon *) | B·1·427 B·1·429 | March 2020 | United States of America |
| ζ (Zeta *) | P·2 | April 2020 | Brazil |
| η (Eta) | B·1·525 | December 2020 | Multiple Countries |
| θ (Theta *) | P·3 | January 2021 | Philippines |
| ι (Iota) | B·1·526 | November 2020 | United States of America |
| κ (Kappa) | B·1·617·1 | October 2020 | India |
| λ (Lambda) | C·37 | August 2020 | Peru |
| μ (Mu) | B·1·621 | January 2021 | Columbia |
| Sources | Actions | Results in COVID-19 Patients | Reference (s) | |
|---|---|---|---|---|
| Interleukins (IL) | ||||
| IL-1 | Macrophages Activated monocytes Dendritic cells B lymphocytes Neutrophils Synovial fibroblasts | Activates the secretion of other proinflammatory cytokines (IL-6 & TNF-α). Activate T helper (Th) 1 cell function. Recruit more neutrophils and monocytes to the site of infection. Induced secretion of IL-1β in monocytes and macrophages. | Destruction of lung cells and loss of pulmonary functions by increasing the viral load. Lung damage and increasing mortality risks (IL-1α). Lung cell pyroptosis, tissue damage in airway inflammation, results in fever, pain, vasodilation, and hypotension in patients (IL-1β). Secretion of pulmonary inflammation molecules resulting in extensive lung involvement (Th1 cells). | [29,34,35] |
| IL-2 | T cells | Promotes the proliferation and activation of T, B, and NK cells. Generate effector and memory T cells. | Prevent pulmonary edema and acute lung microvascular injuries (NK cells). Prevents scarring of the lungs, leading to interstitial pneumonia, severe respiratory insufficiency of patients, and vascular leak syndrome (T cells). | [34,36] |
| IL-6 | T and B lymphocytes Monocytes Macrophages Dendritic cells Endothelial cells | Involved in inflammation, immune response, and hematopoiesis. Stimulates the growth and differentiation of B lymphocytes and increases the generation of platelets. Stimulate T cells dysfunctionality. Activate the coagulation system and increase vascular permeability. | Formation of rheumatoid factor and other autoantibodies resulting in scarring within the lungs. T cells capacity in relation to dendritic cells is damaged. Coagulation system provides a condition for the rapid spread of inflammation. | [29,34,37,38,39] |
| IL-7 | Epithelial cells | Activates T cells. Increase the secretion of other proinflammatory cytokines. Negatively regulates TGF-β. | T cells activated will induce anti-apoptotic BCL-2, promoting longer T cell proliferation. Prevent induced bronchial asthma (downregulation of TGF- β). | [34,40] |
| IL-10 | Regulatory T cells Th9 cells | Inhibiting the production of proinflammatory cytokines (IFN-γ, TNF-α, IL-1β, IL-6). Prevents dendritic cell maturation by blocking IL-12. Stimulate IFN-γ production via CD8+ T cells. | Prevent further lung tissues damage by producing immunostimulatory molecules Increasing lung cell viral resistance. Ameliorates lung tissue injury by inducing collagen production and fibrocyte recruitment into the lung. | [34,36,41] |
| IL-12 | Dendritic cells Macrophages Monocytes B cells | Develop Th1 and Th2 cells. Induced secretion of IFN-γ by T cells and NK cells via positive feedback mechanism. Acting in synergy with IL-18. | NK cells increase the binding to vascular endothelial cells. Increase in viral load in the lung’s microenvironment. Th2 regulates the immune system of the host against infection and promotes lung tissue repair. | [18,34,36] |
| IL-13 | Th2 cells | Induce TGF-β secretion. | TGF-β inhibits naive T cells differentiation into effector cells. TGF-β inhibits Th1 cell differentiation. | [34,42] |
| IL-17 | Th17 cells | Elevate inflammatory process and autoimmune diseases. Induced production of antimicrobial peptides. | Tissue damage of the lungs, physiological stress, and infection. Increase in viral load and disease severity, eventually causing multiorgan failure. Aggravation of lung lesions and acute respiratory distress syndrome occur when Th17 cells increase and are high in CD8 + T cells. | [34,38] |
| Colony-Stimulating Factors (CSFs) | ||||
| Granulocyte-macrophage CSF (GM-CSF) | Fibroblasts cells Endothelial cells Epithelial cells Hematopoietic cells T cells | Stimulate the proliferation and activation of macrophages, neutrophils, and dendritic cells. Increase proinflammatory cytokine production. | Activation of macrophages allows easy antigenic presentation and phagocytosis of pathogens. Increase the number of activated and life span of neutrophils to lung tissues, leading to substantial lung tissue injury and the development of ALI. GM-CSF upregulates the expression of TLR2, TLR4, and CD14 to increase cytokine production, and induce Th17-induced inflammation response. | [34,43,44,45] |
| Chemokines | ||||
| CXCL10 (IP10) | Neutrophils Endothelial cells Keratinocytes Fibroblasts Dendritic cells Hepatocytes | Induced by IFN-γ. Regulates immune system responses by activating and recruiting leukocytes (T cells, monocytes, and NK cells) Activate Th1 cell function. | Active recruitment of leukocytes causes lung tissue damage, leading to worse disease progression. IFN-γ stimulate higher viral load in the lung microenvironment, increase disease severity, and eventually to mortality. Th1 cells lead to pulmonary inflammation and extensive lung involvement in patients by initiation of lung injuries. | [29,34,38] |
| CCL2 (MCP-1) | Endothelial cells Epithelial cells Monocytes Microglial cells | Associated with antiviral responses in tissue. Regulates the migration and infiltration of monocytes, T cells, and NK cells. Activate Th1 cell function. | NK cells prevent pulmonary edema and acute lung microvascular injuries. Th1 cells lead to pulmonary inflammation and extensive lung involvement in patients by initiation of lung injuries. | [29,34] |
| Interferon (IFN) | ||||
| IFN-γ | T cells NK cells Monocytes Macrophages | Participates in numerous immune and adaptive immunological functions. Elevates after the activation of Th1 cells. Promotes macrophage activation and antigen presentation. | IFN-γ upregulates the viral loads in the lung microenvironment and causes tissue damage. Enhancement of major histocompatibility complex expression, activate macrophage function, stimulate chemokine production, induce apoptosis, arrest cell cycle, and enhance Fas expression. | [29,34,46] |
| Tumor Necrosis Factors (TNF) | ||||
| TNF-α | Monocytes Macrophages T cells Epithelial cells | Mediated by IL-1β and IL-6. Decrease the T cells count. Causes poor prognosis of COVID-19. Induces macrophage activation syndrome. | Elevated TNF-α leads to apoptotic death of lung epithelial and endothelial cells, resulting in tissue damage of the lungs. Macrophage activation syndrome triggers the endothelial cells, macrophages, and neutrophils to express TF within the lungs to initiate and further augment pulmonary coagulopathy and microvascular thrombosis. | [34,38] |
| Immune Cells | Mechanism of Actions | Implicated Biomolecules | Action Pathway | Results |
|---|---|---|---|---|
| Macrophages and Neutrophils | Soluble Factors | PGE 2 | Activation of signal transducer activators of transcription-3 (STAT 3) | M2 macrophage phenotype switch |
| DCs | Soluble Factors | PGE 2 | Lower the expression of CD38, CD80, CD86, IL-12, and IL-6 | Inhibit DCs maturation |
| CCR7–CCL21 interaction | Lowering the migratory ability of DCs | |||
| TNF-α-stimulating gene 6 | Inactivation of mitogen activated protein kinase (MAPK) and nuclear factor-kappa B (NF-κB) | Suppress DCs maturation | ||
| HLA-G | Blocking the secretion of cytokines such as TNF-α, ΙL1-α, β, IL-6, IL-7, IL-8, IL-9, GM-CSF, and IFN-γ | Prevent the differentiation of monocytes to DCs | ||
| EVs | miR-21-5p, miR-142-3p, miR-223-3p, and miR-126-3p | Interaction with JAG1, PDCD4, IL-12p35, downregulation of IL-6 expression | Inhibition of DCs maturation | |
| T Cells and B Cells | Soluble Factors | PGE2 | CAMP production in T cells | Downregulate the IL-2 and IL-2R expression |
| Negatively regulating the phosphatidylinositol hydrolysis and the diacylglycerol and inositol phosphate (IP) production | T cells inactivation | |||
| Orchestrating regulatory T (Treg) responses | Promote Th2 immune response | |||
| IDO | Blocks the metabolism from tryptophan to kynurenine in combination with TGF-β1 and HGF | Suppress T cells proliferation | ||
| NO | Activating the transcription 5 phosphorylation | Inhibition of TCR-mediated T cells proliferation and inflammatory cytokine production | ||
| Galectin 1 and 3 | Preventing the clustering of TCR via crosslink interaction mechanism | Suppress the proliferation of T cells proliferation | ||
| HLA-G with IDO and IL-10 | Suppress proliferation of T cells | Indirectly blocked the secretion of cytokine (TNF-α, ΙL1-α, ΙL1-β, IL-6, IL-7, IL-8, IL-9, GM-CSF, and IFN-γ) | ||
| Cell–Cell Interactions | Fas/Fas ligand death signaling pathway | Downstream activation of the Fas-associated death domain and caspases | T cells apoptosis | |
| TNF-related apoptosis-inducing ligand (TRAIL)/death receptor (DR) signaling pathway | High production of TRAIL and binds to DR on T cells | T cells apoptosis | ||
| Programmed death ligand-1 (PD-L1)/programmed death-1 (PD-1) | Inhibition of MAPK followed by Src-homology 2 domain containing protein tyrosine phosphatases (SHP)-1 and SHP-2 phosphorylation | Reduces T cells proliferation | ||
| NK Cells | Cell-cell Interaction | HLA class I | Upregulate the expression of HLA class I molecules to interact with killer cell immunoglobulin-like receptors (KIRs) | Inhibit cytolytic activity of NK cells |
| Interacting with KIR2DL4 | Inhibit NK cells and cytokine production | |||
| Toll-like receptors (TLRs) | Activation of TLR3 | Increases the immunosuppression against NK cells | ||
| Soluble Factors | IDO and PGE2 with aid from TGF-β1 and HGF molecules | Inhibit IL-2 induced NK response | Immunosuppression of NK cells |
| Clinical Trials Identifier | Study | ||||
|---|---|---|---|---|---|
| MSCs Source | Title | Outcome Measured | Trial Duration | Location | |
| NCT04573270 | UC-MSCs | Mesenchymal Stem Cells for The Treatment of COVID-19 | Safety and Efficacy of Stem Cell Therapy for The Treatment of Patients Admitted to Hospital Suffering Complications from COVID-19 | April 2020–September 2020 | United States |
| NCT04288102 | UC-MSCs | Treatment with Human Umbilical Cord-Derived Mesenchymal Stem Cells for Severe Coronavirus Disease 2019 (COVID-19) | Safety and Efficacy of Human Umbilical Cord-Derived MSCs (UC-MSCs) for Severe COVID-19 Patients with Lung Damage | March 2020–July 2020 | China |
| NCT04355728 | UC-MSCs | Use of UC-MSCs for COVID-19 Patients | Safety and Efficacy of Human Umbilical Cord Derived Mesenchymal Stem Cells (UC-MSCs) for Treatment of COVID-19 Patients with Severe Complications of ALI/ARDS | April 2020–October 2020 | United States |
| NCT04492501 | BM-MSCs | Investigational Treatments for COVID-19 In Tertiary Care Hospital of Pakistan | Mortality and Morbidity Benefit of Different Investigational Treatment | April 2020–July 2020 | Pakistan |
| NCT04491240 | MSCs-Derived Exosomes | Evaluation of Safety and Efficiency of Method of Exosome Inhalation in SARS-CoV-2 Associated Pneumonia. | Safety and Efficiency of Aerosol Inhalation of The Exosomes in The Treatment of Severe Patients Hospitalized with Novel Coronavirus Pneumonia (NCP) | July 2020–October 2020 | Russian Federation |
| NCT04288102 | UC-MSCs | Treatment with Human Umbilical Cord-Derived Mesenchymal Stem Cells for Severe Coronavirus Disease 2019 (COVID-19) | Safe and Effective MSCs Therapeutic Approach to COVID-19 | February 2020–August 2020 | China |
| NCT04535856 | DW-MSCs | Therapeutic Study to Evaluate the Safety and Efficacy of DW-MSCs in COVID-19 Patients | Safety and Efficacy of DW-MSCs in COVID-19 Patients | September 2020–January 27 2021 | Indonesia |
| NCT04269525 | UC-MSCs | Umbilical Cord (UC)-Derived Mesenchymal Stem Cells (MSCs) Treatment for the 2019-Novel Coronavirus(nCOV) Pneumonia | Availability and Safety of UC-MSCs Treatment for Serious Pneumonia and Critical Pneumonia Caused by the 2019-nCOV Infection | February 2020–December 2020 | China |
| NCT04252118 | UC-MSCs | Mesenchymal Stem Cell Treatment for Pneumonia Patients Infected with COVID-19 | Safety and Efficiency of Mesenchymal Stem Cells (MSCs) Therapy for Pneumonia Patients Infected with SARS-CoV-2 | February 2020–April 2020 | China |
| NCT04392778 | UC-MSCs | Clinical Use of Stem Cells for The Treatment of COVID-19 | Regenerative And Repair Abilities of Stem Cells to Fight Against the Harmful Effects of The Novel Coronavirus COVID-19 | May 2020–May 2021 | Turkey |
| NCT04898088 | N/A | A Proof-Of-Concept Study for The DNA Repair Driven by the Mesenchymal Stem Cells in Critical COVID-19 Patients (Repair) | Positive Effect of Stem Cell Therapy Applied on Critically Ill Patients with Coronavirus Infection on DNA Repair Genes | January 2020–September 2020 | Turkey |
| NCT04276987 | AdMSCs-Derived Exosomes | A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia | Safety And Efficiency of Aerosol Inhalation of The Exosomes Derived from Allogenic Adipose Mesenchymal Stem Cells (MSCs-Exo) in the Treatment of Severe Patients Hospitalized with Novel Coronavirus Pneumonia (NCP) | February 2020–July 2020 | China |
| ChiCTR2000029990 | N/A | Clinical Trials of Mesenchymal Stem Cells for The Treatment of Pneumonitis Caused by Novel Coronavirus Pneumonia (COVID-19) | Safety And Efficacy of Human Mesenchymal Stem Cells in The Treatment of New Coronavirus (2019-nCOV) Pneumonia. | January 2020–March 2020 | China |
| ChiCTR2000029569 | UC-MSCs | Safety And Efficacy of Umbilical Cord Blood Mononuclear Cells Conditioned Medium in the Treatment of Severe and Critically Novel Coronavirus Pneumonia (COVID-19): A Randomized Controlled Trial | Effectiveness of Conventional Treatment Group and Conventional Treatment Combined with Umbilical Cord Mesenchymal Stem Cell Conditioned Medium Group in Treating Patients with Severe and Critical 2019-nCOV Coronavirus Pneumonia | February 2020–April 2020 | China |
| ChiCTR2000031139 | hESC- Derived M Cells | Safety and Effectiveness of Human Embryonic Stem Cell-Derived M Cells (CAStem) for Pulmonary Fibrosis Correlated with Novel Coronavirus Pneumonia (COVID-19) | Safety and Tolerability of CAStem Cells for COVID-19-Related Pulmonary Fibrosis | March 2020–March 2021 | China |
| ChiCTR2000031319 | DP-MSCs | Safety And Efficacy Study of Allogeneic Human Dental Pulp Mesenchymal Stem Cells to Treat Severe Novel Coronavirus Pneumonia (COVID-19) Patients | Safety and Efficacy of Allogeneic Human Dental Pulp Mesenchymal Stem Cells in The Treatment of Severe Pneumonia Caused By COVID-19, Reducing Mortality and Improving Clinical Prognosis | April 2020–July 2020 | China |
| NCT04400032 | N/A | Cellular Immuno-Therapy for COVID-19 acute respiratory distress syndrome (CIRCA-19) | Tolerability and Potential Signs of Efficacy of using MSC Therapy for Patients with Severe Infections (Sepsis) Associated with ARDS | May 2020–April 2021 | Canada |
| NCT04382547 | Mucosa-Derived MSCs | Treatment of COVID-19 Associated Pneumonia with Allogenic Pooled Olfactory Mucosa-derived Mesenchymal Stem Cells | Ability of Treatment of Severe COVID-19 Associated Interstitial Pneumonia using Allogeneic Mesenchymal Stem Cells | May 2020–June 2021 | Belarus |
| Study | ||||||
|---|---|---|---|---|---|---|
| Clinical Trials Identifier | MSCs Source | Title | Outcome Measured | Phase | Estimated Trial Duration | Location |
| NCT04371393 | MSCs Drug (Remestemcel-L) | MSCs in COVID-19 ARDS | Number of Days Alive Off Mechanical Ventilatory Support, Total Number of Events Over 30 Days, Number of Participants Alive and/or Improvement of ARDS, Severity of ARDS, Hospital Length of Stay, Number of Readmission and Stay in Intensive Care Unit, Change in Plasma Hs-CRP Concentration and Serum Hs-CRP Concentration, Change In IL-6, IL-8, TNF-Alpha, Inflammatory Marker Level, Pulmonary Symptoms. | 3 | April 2020–February 2022 | United States |
| NCT04361942 | N/A | Treatment of Severe COVID-19 Pneumonia with Allogeneic Mesenchymal Stromal Cells (COVID_MSV) | Proportion of Patients Achieved Withdrawal of Invasive Mechanical Ventilation, Rate of Mortality, Proportion of Patients Who Have Achieved Clinical Response and Radiological Responses, Blood White Cell Counts, Cellular Markers of Inflammation, Cytokines and Chemokines in Blood. | 2 | May 2020–December 2021 | Spain |
| NCT04615429 | N/A | Clinical Trial to Assess the Efficacy of MSCs in Patients with ARDS Due to COVID-19 | Change in the PaO2/FiO2 Ratio, All-Cause Mortality, SOFA Score, Oxygen Therapy-Free Days, Duration of Hospitalization and ICU Admission, Incidence of Non-Invasive Ventilation, Survival Rate. | 2 | September 2020–January 2022 | Spain |
| NCT04625738 | Ex-vivo expanded WJ-MSCs | Efficacy of Infusions of MSCs From Wharton Jelly in the SARS-CoV-2 (COVID-19) Related Acute Respiratory Distress Syndrome | Efficacy of WJ-MSCs on Respiratory Function Evolution, The Duration of Invasive Mechanical Ventilation During the Hospital Stay, The Evolution of Organ Failures, The Duration of Stay in Intensive Care Unit, The Mortality During Intensive Care Unit and Hospitalization, The Evolution of Viral Load, The Immediate or Delayed Tolerance Following the WJ-MSCs Injection. | 2 | November 2020–August 2022 | France |
| NCT04905836 | COVI-MSCs | Study of Allogeneic Adipose-Derived Mesenchymal Stem Cells for Treatment of COVID-19 Acute Respiratory Distress | Safety and Preliminary Efficacy of COVI-MSCs, All-Cause Mortality Rate, Incidence of All Adverse Events (AEs), Number of Ventilator-Free Days, Number of ICU days, Change in Oxygenation PaO2:FiO2 Ratio. | 2 | October 2021–March 2022 | United States |
| NCT05017298 | Autologous AD-MSCs | Clinical Study for Subjects With COVID-19 Using Allogeneic Adipose Tissue-Derived Mesenchymal Stem Cells | Safety of AdMSCs Injection, The Mortality Rate, Immune Measurements, Organ Functional Tests, Duration Weaning from Mechanical Ventilation, ICU Monitoring, Vasoactive Agent’s Usage, Hospitalization, Mortality rate. | 2 | November 2021–November 2024 | United States |
| NCT04466098 | N/A | Multiple Dosing of Mesenchymal Stromal Cells in Patients with ARDS (COVID-19) | Adverse Events Related to the Infusion of MSCs, Incidence of Reduction in Biomarkers of Inflammation, Trend Changes in PaO2:FiO2 Ratio, Airway Pressure, Changes in Positive End-Expiratory Airway Pressure (PEEP), Mortality, Number of ICU-free Days, Change in Acute Lung Injury (ALI) score. | 2 | July 2020–December 2021 | United States |
| NCT04869397 | Allogeneic WJ-MSCs | Treatment of Respiratory Complications Associated With COVID-19 Using Umbilical Cord Mesenchymal Stromal Cells (ProTrans19+) | Rate of Use of Mechanical Ventilation, Clinical Status Evaluation Assessed, Survival, Time to Clinical Improvement, Duration of Hospitalization and ICU Stay. | 2 | June 2021–July 2022 | Canada |
| NCT04428801 | Autologous AD-MSCs | Autologous Adipose-derived Stem Cells (AD-MSCs) for COVID-19 | Tolerability and Acute Safety of AdMSCs Infusion, IgM/IgG Antibodies Development Against SARS-CoV-2, Lymphocyte Count in White Blood Cell Counts, PaO2 Arterial Blood Gases, Mortality Rates, Change in Blood Test Values for Cytokine Panels. | 2 | September 2021–September 2024 | United States |
| NCT04780685 | hMSCs | A Phase II Study in Patients with Moderate to Severe ARDS Due to COVID-19 | Survival, Number of Patients with Treatment-Related Adverse Events. | 2 | March 2021–December 2021 | United States |
| NCT04336254 | DP-MSCs | Safety and Efficacy Study of Allogeneic Human Dental Pulp Mesenchymal Stem Cells to Treat Severe COVID-19 Patients | Safety and Efficacy in The Treatment of Severe Pneumonia Caused By COVID-19, Effects in the Treatment of Severe Pneumonia of COVID-19. | 2 | May 2020–December 2021 | China |
| NCT04753476 | MSCs Secretome | Treatment of Severe COVID-19 Patients Using Secretome of Hypoxia-Mesenchymal Stem Cells in Indonesia | Duration of Using a Ventilator, Length of Stay from the First Treatment to Final Outcome, Recovery, Death, Routine Blood Profile, CRP, D-dimer, Blood Gas Analysis (BGA), Photo Thorax. | 2 | June 2020–March 2022 | Indonesia |
| NCT04390139 | WJ-MSCs | Efficacy and Safety Evaluation of Mesenchymal Stem Cells for the Treatment of Patients with Respiratory Distress Due to COVID-19 | All-cause mortality, Safety of WJ-MSCs, Need for Treatment with Rescue Medication, Duration of Mechanical Ventilation, Evolution of PaO2 / FiO2 Ratio, SOFA Index, Duration of Hospitalization, Evolution of Markers of Immune Response, Feasibility of WJ-MSCs Administration, Evolution of Disease Biomarker. | 2 | May 2020–December 2021 | Spain |
| NCT04445454 | BM-MSCs | Mesenchymal Stromal Cell Therapy for Severe COVID-19 Infection | Safety of Intravenous Infusion of MSCs in Patients with COVID-19 Pneumonia, Efficacy of Intravenous Infusion of MSC, Effect of MSC Administration. | 2 | June 2020–September 2022 | Belgium |
| NCT04390152 | WJ-MSCs | Safety and Efficacy of Intravenous Wharton’s Jelly Derived Mesenchymal Stem Cells in Acute Respiratory Distress Syndrome Due to COVID-19 | Mortality Difference with Treatment, Efficacy of WJ-MSCs, Safety Evaluation of WJ-MSCs, Severity of Adverse Events. | 2 | January 2020–April 2022 | Colombia |
| NCT04614025 | Mesenchymal-like Adherent stromal Cells (PLX-PAD) | Open-label Multicenter Study to Evaluate the Efficacy of PLX-PAD for the Treatment of COVID-19 | Number of Ventilator-Free Days, All-Cause Mortality, Duration of Mechanical Ventilation. | 2 | October 2020–September 2022 | Germany |
| NCT04452097 | UC-MSCS | Use of hUC-MSCs Product (BX-U001) for the Treatment of COVID-19 With ARDS | Incidence of Infusion-Related Adverse Events, All-Cause Mortality, Duration of ICU Stay, Duration of Hospital Stay, Changes in Blood Cytokine Levels. | 2 | July 2021–March 2022 | United States |
| NCT04602442 | MSCs Exosomes | Safety and Efficiency of Method of Exosome Inhalation in COVID-19 Associated Pneumonia | Safety Assessment, Time to Clinical Recovery (TTCR), SpO2 Concentration Changes, Blood Biochemistry (CRP). | 2 | October 2020–December 2021 | Russian Federation |
| Clinical Trial | Outcome Measured and Description | Time Frame | Results |
|---|---|---|---|
| NCT04491240: Evaluation of Safety and Efficiency of Method of Exosome Inhalation in SARS-CoV-2 Associated Pneumonia. (COVID-19EXO) [90] | Number of Participants with Non-serious and Serious Adverse Events During Trial. (Safety assessments such as adverse events will be registered and will be monitored during all trials) | 30 days after clinic discharge | The number of participants with non-serious and serious adverse events for three groups of participants during the trial is 0.00%. |
| Number of Participants with Non-serious and Serious Adverse During Inhalation Procedure. (Safety assessments such as adverse events during the inhalation procedures will be registered) | After each inhalation for 10 days | The number of participants that show symptoms * during the inhalation procedure for three groups of participants is 0.00%. | |
| Time to Clinical Recovery (TTCR). (Measure and compare time to clinical recovery compared to placebo) | From first inhalation until discharge from the clinic, up to 30 days | The mean of TTCR is measured in days. Groups given with EXO1 require 13.8 days (SD: 1.55) while EXO2 groups require 14.8 days (SD: 2.35). As compared to the placebo group, it only requires 14.1 days (SD: 1.37). | |
| SpO2 Concentration. (Peripheral capillary oxygen saturation before and after each inhalation with a total 4 measures per day) | 10 days during inhalation | Group EXO-1 shows an increase in the median of SpO2 concentration from day 1 to 10 from 93.8 to 97.8. Group EXO-2 also shows an increase in the median of SpO2 concentration from day 1 to 10 from 94.5 to 98.5. For the placebo group, the median of SpO2 Concentration is 94 and increases to 98.8 on the 10th day. | |
| C-reactive Protein. (Blood biochemistry C reactive protein level in serum) | At the beginning of inhalation (day 1) and on the next day of last inhalation (day 11) | Group EXO-1 shows a major decrease in the mean of C-reactive Protein level from 78.3 (Day 1) to 5.04 (Day 11). Group EXO-2 also shows decreasing mean from 75.4 (Day 1) to 5.83 (Day11). For the placebo group, the mean protein level drops less than both exosome inhalation groups, which is from 61.5 (Day 1) to 8.29 (Day 11). | |
| Lactic Acid Dehydrogenase (LDH). (Lactic Acid Dehydrogenase (LDH) level in serum) | At the beginning of inhalation (day 1) and on the next day of last inhalation (day 11) | Group EXO-1 shows a major decrease in the mean of LDH from 773 U/L (Day 1) to 441 U/L (Day 11). Group EXO-2 also shows decreasing mean from 732 U/L (Day 1) to 365 U/L (Day11). For the placebo group, the mean of LDH drops less than both exosome inhalation groups, which is from 669 U/L (Day 1) to 430 U/L (Day 11). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loke, X.Y.; Imran, S.A.M.; Tye, G.J.; Wan Kamarul Zaman, W.S.; Nordin, F. Immunomodulation and Regenerative Capacity of MSCs for Long-COVID. Int. J. Mol. Sci. 2021, 22, 12421. https://doi.org/10.3390/ijms222212421
Loke XY, Imran SAM, Tye GJ, Wan Kamarul Zaman WS, Nordin F. Immunomodulation and Regenerative Capacity of MSCs for Long-COVID. International Journal of Molecular Sciences. 2021; 22(22):12421. https://doi.org/10.3390/ijms222212421
Chicago/Turabian StyleLoke, Xin Ya, Siti A. M. Imran, Gee Jun Tye, Wan Safwani Wan Kamarul Zaman, and Fazlina Nordin. 2021. "Immunomodulation and Regenerative Capacity of MSCs for Long-COVID" International Journal of Molecular Sciences 22, no. 22: 12421. https://doi.org/10.3390/ijms222212421






