MCAM/MUC18/CD146 as a Multifaceted Warning Marker of Melanoma Progression in Liquid Biopsy
Abstract
:1. Introduction
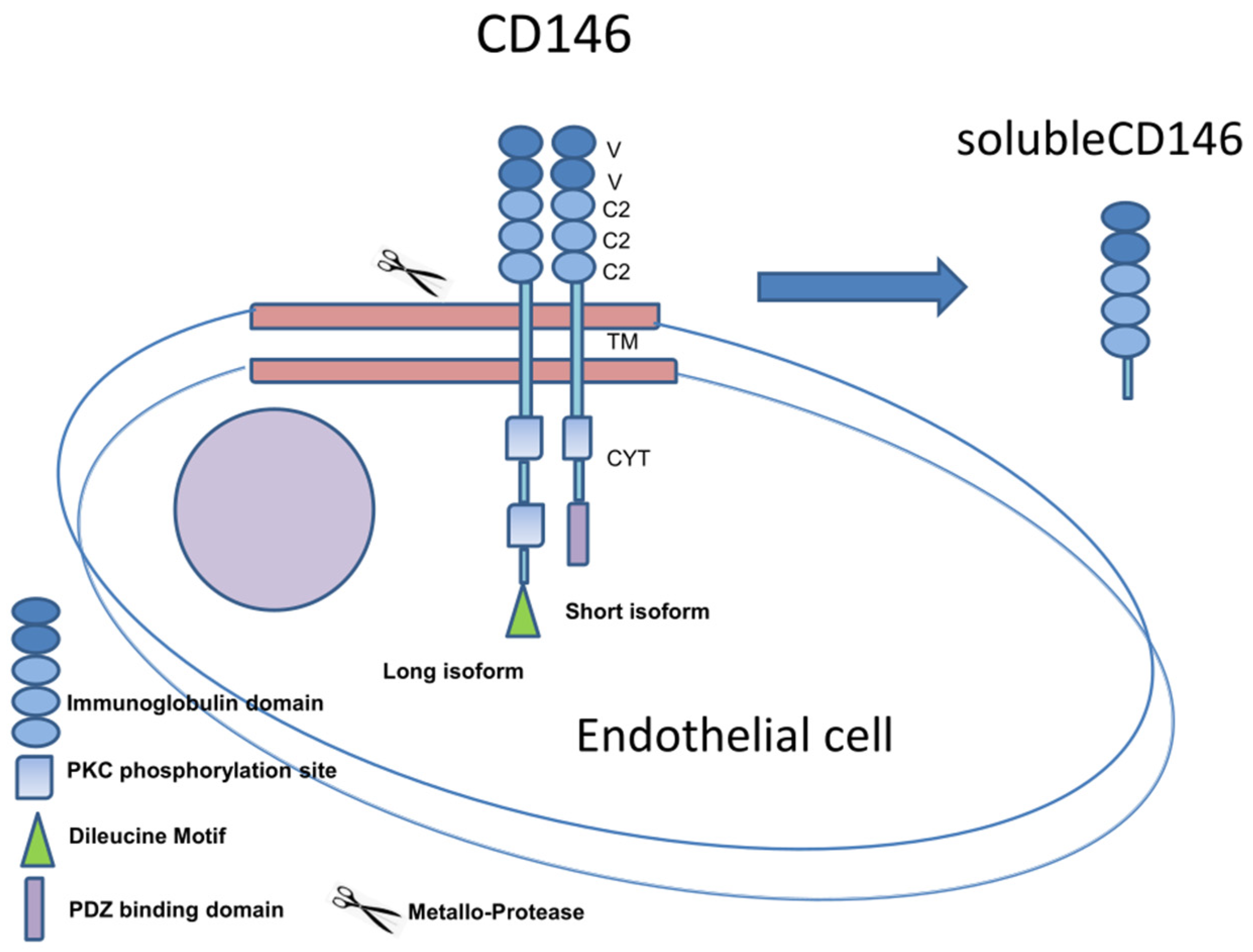
2. CD146 Molecular Expression as a Melanoma-Associated Marker in Peripheral Blood
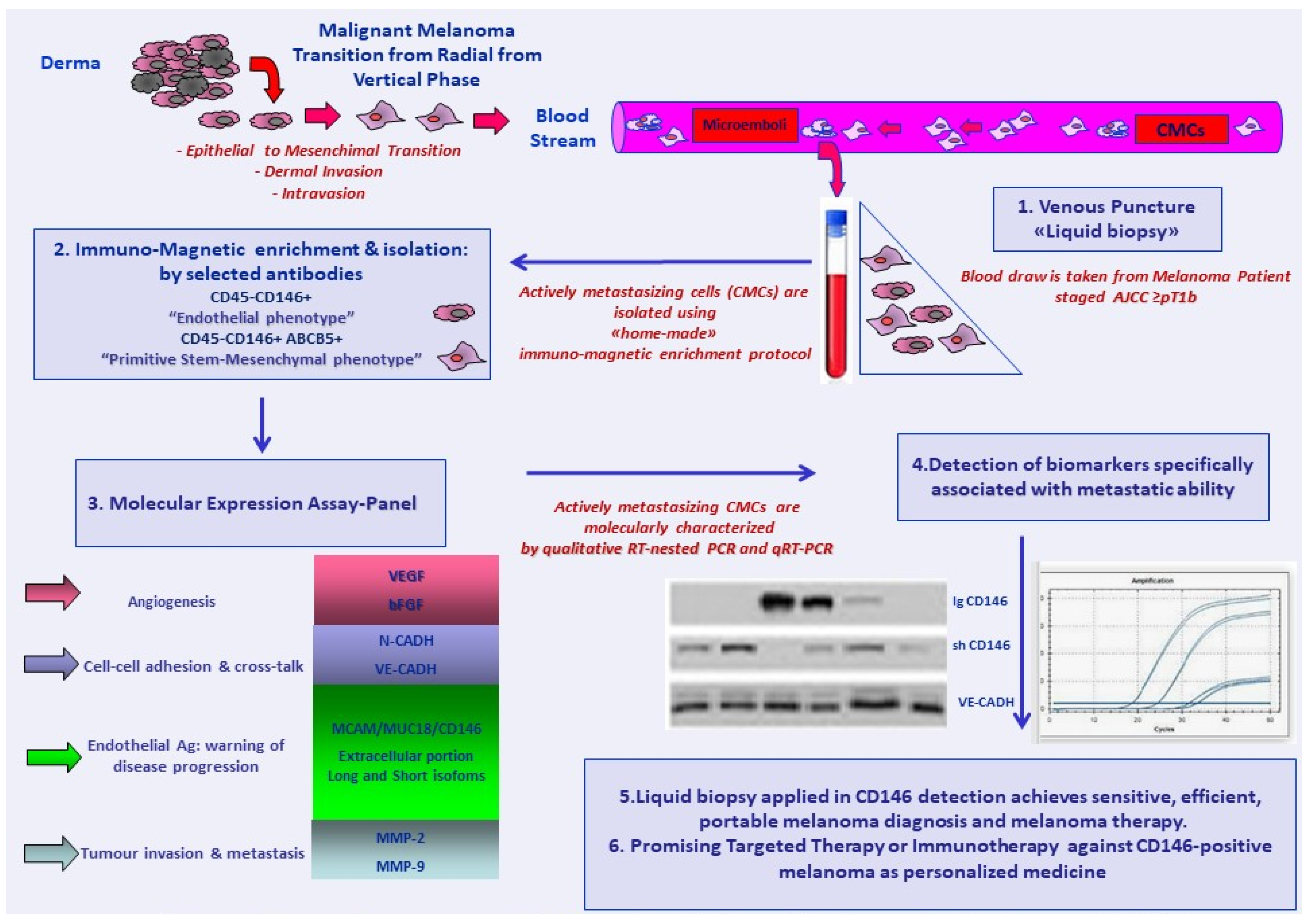
3. CD146 as an Enrichment and Capture Marker for Circulating Melanoma Cells
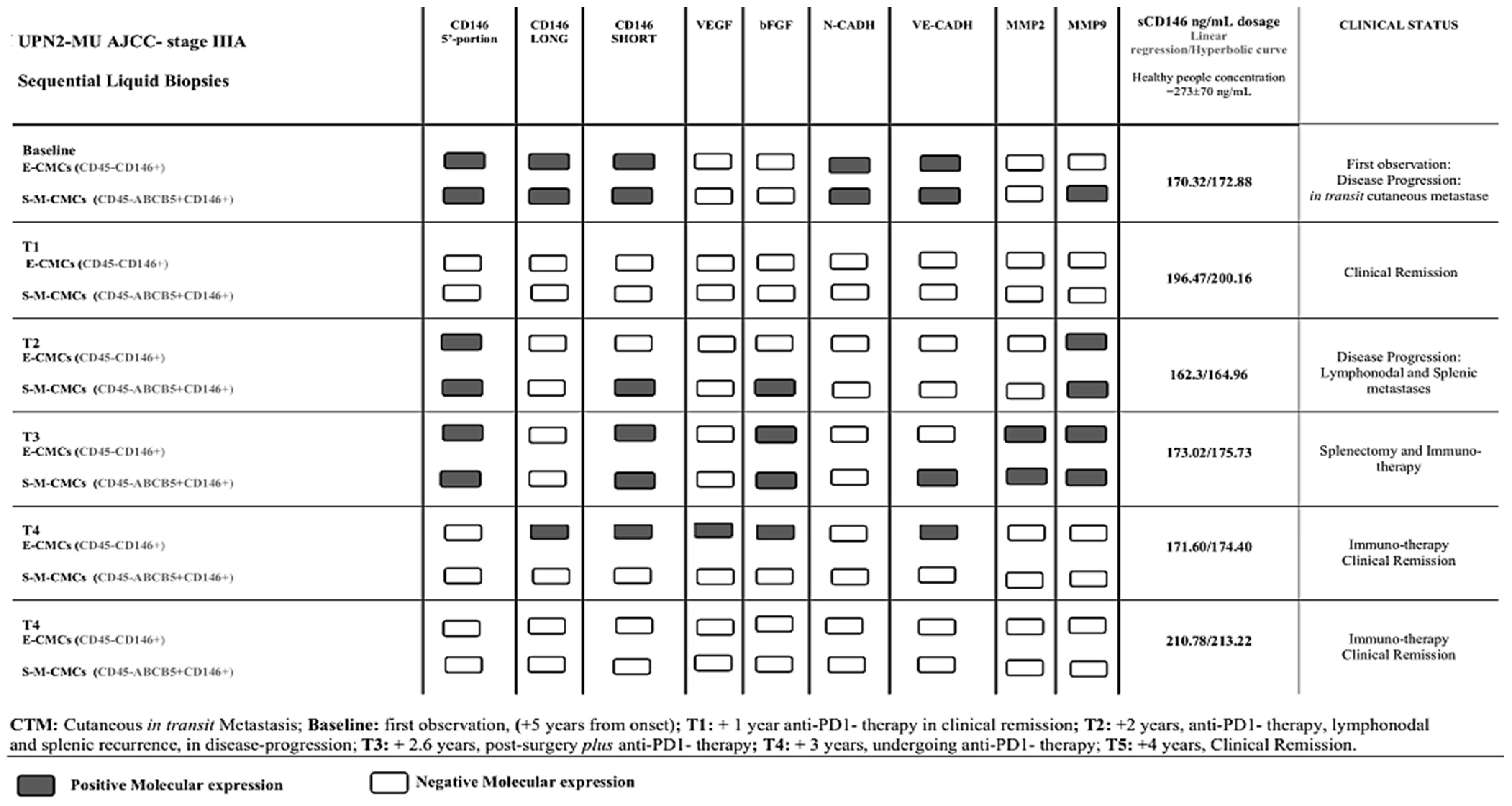
4. Current Findings: CD146 as an Enrichment and Capture Marker at Melanoma Onset or Disease Recurrence
5. Current Findings: CD146 as an Enrichment and Capture Antigen and Molecular Expression Marker in Magnetically Immune CMC Fractions during Melanoma Follow-Up Time Course
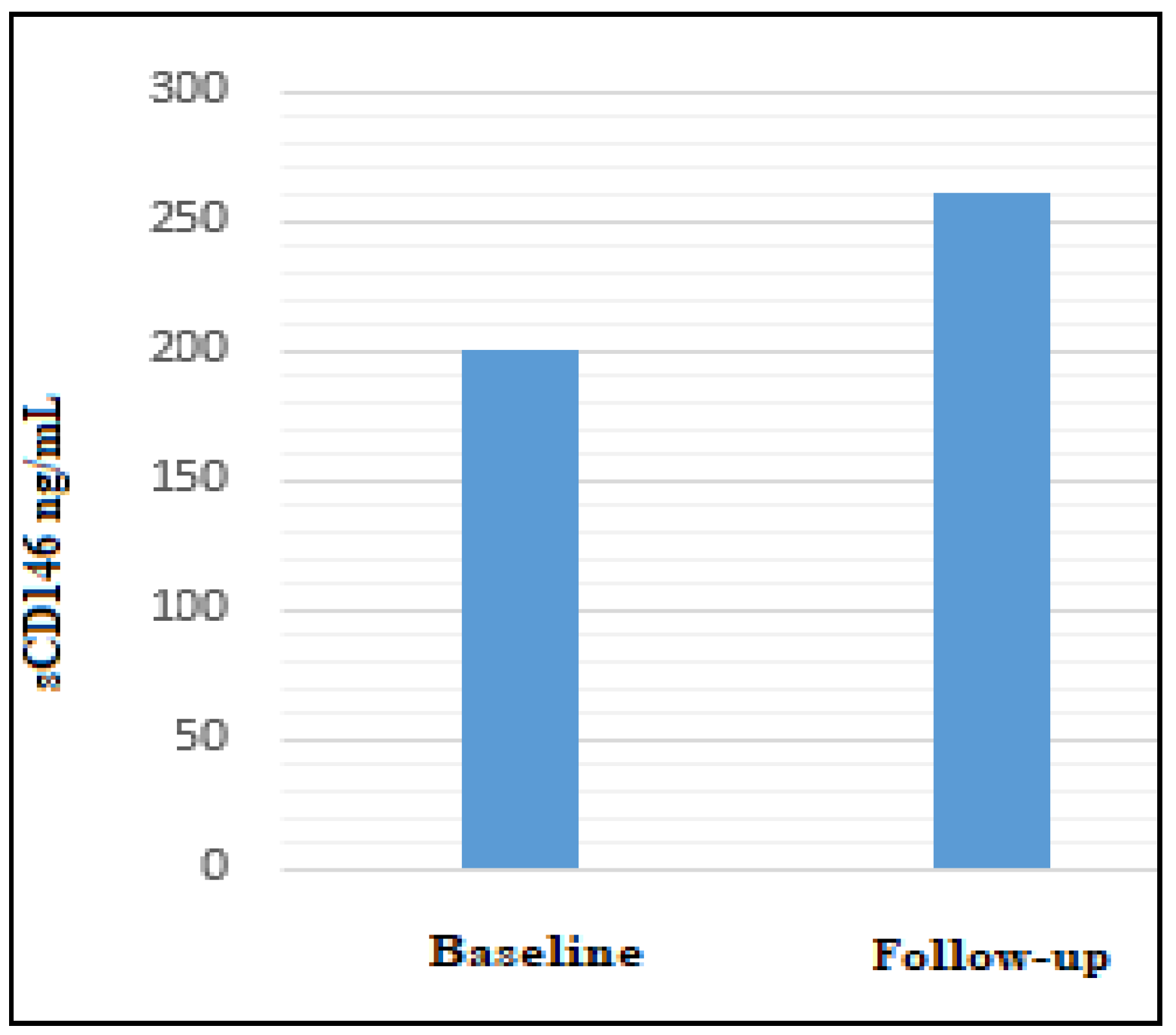
6. Soluble CD146 Form in Melanoma Patients
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Braeuer, R.R.; Watson, I.R.; Wu, C.-J.; Mobley, A.K.; Kamiya, T.; Shoshan, E.; Bar-Eli, M. Why is melanoma so metastatic? Pigment Cell Melanoma Res. 2014, 27, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.M.; Holzmann, B.; Breitbart, E.; Schmiegelow, P.; Riethmuller, G.; Johnson, J.P. Discrimination between benign and malignant cells of melanocytic lineage by two novel antigens, a glycoprotein with a molecular weight of 113,000 and a protein with a molecular weight of 76,000. Cancer Res. 1987, 47, 841–845. [Google Scholar] [PubMed]
- Shih, I.M. The role of CD146 (Mel-CAM) in biology and pathology. J. Pathol. 1999, 189, 4–11. [Google Scholar] [CrossRef]
- Mobley, A.K.; Braeuer, R.R.; Kamiya, T.; Shoshan, E.; Bar-Eli, M. Driving transcriptional regulators in melanoma metastasis. Cancer Metastasis Rev. 2012, 31, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Shih, L.M.; Hsu, M.Y.; Palazzo, J.P.; Herlyn, M. The cell-cell adhesion receptor Mel-CAM acts as a tumor suppressor in breast carcinoma. Am. J. Pathol. 1997, 151, 745–751. [Google Scholar] [PubMed]
- Holzmann, B.; Bröcker, E.-B.; Lehmann, J.-M.; Ruiter, D.-J.; Sorg, C.; Riethmüller, G.; Johnson, J.P. Tumor progression in human malignant melanoma: Five stages defined by their antigenic phenotypes. Int. J. Cancer 1987, 39, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, V.O.; Bar-Eli, M. Bioimmunotherapy for melanoma using fully human antibodies targeting MCAM/MUC18 and IL-8. Pigment Cell Res. 2006, 19, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Hoon, D.; Ambrosi, A.; Nitti, D.; Rossi, C.R. The Prognostic Value of Circulating Tumor Cells in Patients with Melanoma: A Systematic Review and Meta-analysis. Clin. Cancer Res. 2006, 12, 4605–4613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, J.-M.; Riethmuller, G.; Johnson, J.P. Muc18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc. Natl. Acad. Sci. USA 1989, 86, 9891–9895. [Google Scholar] [CrossRef] [Green Version]
- Luca, M.; Hunt, B.; Bucana, C.-D.; Johnson, J.-P.; Fidler, I.-J.; Bar-Eli, M. Direct correlation between muc18 expression and metastatic potential of human melanoma cells. Melanoma Res. 1993, 3, 35–41. [Google Scholar] [CrossRef]
- Xie, S.; Luca, M.; Huang, S.; Gutman, M.; Reich, R.; Johnson, J.-P.; Bar-Eli, M. Expression of mcam/muc18 by human melanoma cells leads to increased tumor growth and metastasis. Cancer Res. 1997, 57, 2295–2303. [Google Scholar] [PubMed]
- Satyamoorthy, K.; Muyrers, J.; Meier, F.; Patel, D.; Herlyn, M. Mel-CAM-specific genetic suppressor elements inhibit melanoma growth and invasion through loss of gap junctional communication. Oncogene 2001, 20, 4676–4684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haass, N.K.; Smalley, K.S.; Li, L.; Herlyn, M. Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res. 2005, 18, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Wondimu, Z.; Oikawa, Y.; Gentilcore, G.; Kiessling, R.; Egyhazi Brage, S.; Hansson, J.; Patarroyo, M. Laminins 411 and 421 differentially promote tumor cell migration via alpha6beta1 integrin and MCAM (CD146). Matrix Biol. 2014, 38, 69–83. [Google Scholar] [CrossRef]
- Ishikawa, T.; Wondimu, Z.; Oikawa, Y.; Ingerpuu, S.; Virtanen, I.; Patarroyo, M. Monoclonal antibodies to human laminin alpha4 chain globular domain inhibit tumor cell adhesion and migration on laminins 411 and 421, and binding of alpha6beta1 integrin and MCAM to alpha4-laminins. Matrix Biol. 2014, 36, 5–14. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Q.; Zhang, N.; Du, X.; Xu, G.; Yan, X. CD146, from a melanoma cell adhesion molecule to a signalling receptor. Signal Trasd. Targeted Ther. 2020, 148, 1–15. [Google Scholar]
- Sumardika, W.; Tomonobu, N.; Kinoshita, R.; Inoue, Y.; Iioka, R.; Mitsui, Y.; Saito, K.; Winarsa Ruma, I.; Hiroki Sato, H.; Yamauchi, A.; et al. Critical role of the MCAM-ETV4 axis triggered by extracellular S100A8/A9 in breast cancer aggressiveness. Neoplasia 2017, 21, 627–640. [Google Scholar]
- Sers, C.; Kirsch, K.; Rothbächer, U.; Riethmüller, G.; Johnson, J.P. Genomic organization of the melanoma-associted glycoprotein MUC18: Implications for the evolution of the immunoglobulin domains. Proc. Natl. Acad. Sci. USA 1993, 90, 8514–8518. [Google Scholar] [CrossRef] [Green Version]
- Joshkon, A.; Heim, X.; Dubrou, C.; Bachelier, R.; Traboulsi, W.; Stalin, J.; Fayyad-Kazan, H.; Badran, B.; Foucault-Bertaud, A.; Leroyer, A.S.; et al. Role of CD146 (MCAM) in Physiological and Pathological Angiogenesis—Contribution of New Antibodies for Therapy. Biomedicines 2020, 8, 633. [Google Scholar] [CrossRef]
- Bardin, N.; Francès, V.; Combes, V.; Sampol, J.; Dignat-George, F. CD146: Biosynthesis and production of a soluble form in human cultured endothelial cells. FEBS Lett. 1998, 421, 12–14. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.; Guan, C.-E.; Song, Y.; Wang, H. The multifaceted role of CD146/MCAM in the promotion of melanoma progression. Cancer Cell Intern. 2015, 15, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroyer, A.S.; Blin, M.G.; Bachelier, R.; Bardin, N.; Blot-Chabaud, M.; Dignat-George, F. CD146 (Cluster of Differentiation 146): An Adhesion Molecule Involved in Vessel Homeostasis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Kebir, A.; Harhouri, K.; Guillet, B.; Liu, J.-W.; Foucault-Bertaud, A.; Lamy, E.; Kaspi, E.; Elganfoud, N.; Vely, F.; Sabatier, F.; et al. CD146 Short Isoform Increases the Proangiogenic Potential of Endothelial Progenitor Cells In Vitro and In Vivo. Circ. Res. 2010, 107, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maru, G.B.; Gandhi, K.; Ramchandani, A.; Kumar, G. The role of inflammation in skin cancer. Adv. Exp. Med. Biol. 2014, 816, 437–469. [Google Scholar] [CrossRef]
- Coffelt, S.B.; de Visser, K.E. Cancer: Inflammation lights the way to metastasis. Nature 2014, 507, 48–49. [Google Scholar] [CrossRef]
- Xing, S.; Luo, Y.; Liu, Z.; Bu, P.; Duan, H.; Li, D.; Wang, P.; Yang, J.; Song, L.; Feng, J.; et al. Targeting Endothelial CD146 Attenuates Colitis and Prevents Colitis-Associated Carcinogenesis. Am. J. Pathol. 2014, 5, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Yeremenko, N.; Noordenbos, T.; Cantaert, T.; van Tok, M.; van de Sande, M.; Cañete, J.D.; Baeten, D. Disease-specific and inflammation-independent stromal alterations in spondylarthritis synovitis. Arthritis Rheum. 2013, 65, 174–185. [Google Scholar] [CrossRef]
- Wu, Q.; Case, S.R.; Minor, M.N.; Di, J.; Martin, R.J.; Bowler, R.P.; Wang, J.; Hartney, J.; Karimpour-Fard, A.; Chu, H.W. A novel function of MUC18: Amplification of lung inflammation during bacterial infection. Am. J. Pathol. 2013, 182, 819–827. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Duan, H.; Qian, Y.; Feng, L.; Wu, Z.; Wang, F.; Feng, J.; Yang, D.; Qin, Z.; Yan, X. Macrophagic CD146 promotes foam cell formation and retention during atherosclerosis. Cell Res. 2017, 27, 352–372. [Google Scholar] [CrossRef] [Green Version]
- Kaspi, E.; Heim, X.; Granel, B.; Guillet, B.; Stalin, J.; Nollet, M.; Bertaud-Foucault, A.; Robaglia-Schlupp, A.; Roll, P.; Cau, P.; et al. Identification of CD146 as a novel molecular actor involved in systemic sclerosis. J. Allergy Clin. Immunol. 2017, 140, 1448–1451.e6. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Fei, Y.; Zheng, L.; Wang, J.; Xiao, W.; Wen, J.; Xu, Y.; Wang, Y.; He, L.; Guan, J.; et al. Expression of Endothelial Cell Injury Marker Cd146 Correlates with Disease Severity and Predicts the Renal Outcomes in Patients with Diabetic Nephropathy. Cell Physiol. Biochem. 2018, 48, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Tamura, N.; Okuda, S.; Tada, K.; Matsushita, M.; Yamaji, K.; Kato, K.; Takasaki, Y. Elevated serum levels of soluble CD146 in patients with systemic sclerosis. Clin. Rheumatol. 2017, 36, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Neidhart, M.; Wehrli, R.; Brühlmann, P.; Michel, B.A.; Gay, R.E.; Gay, S. Synovial fluid CD146 (MUC18), a marker for synovial membrane angiogenesis in rheumatoid arthritis. Arthritis Rheum. 1999, 42, 622–630. [Google Scholar] [CrossRef]
- Duan, H.; Luo, Y.; Hao, H.; Feng, L.; Zhang, Y.; Lu, D.; Xing, S.; Feng, J.; Yang, D.; Song, L.; et al. Soluble CD146 in cerebrospinal fluid of active multiple sclerosis. Neuroscience 2013, 235, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Bardin, N.; Blot-Chabaud, M.; Despoix, N.; Kebir, A.; Harhouri, K.; Arsanto, J.P.; Espinosa, L.; Perrin, P.; Robert, S.; Vely, F.; et al. CD146 and its soluble form regulate monocyte transendothelial migration. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 746–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.; Lin, Y.; Yang, D.; Shen, Y.; Yuan, M.; Zhang, Z.; Li, P.; Xia, H.; Li, L.; Luo, D.; et al. A novel anti-CD146 monoclonal antibody, AA98, inhibits angiogenesis and tumor growth. Blood 2003, 102, 184–191. [Google Scholar] [CrossRef]
- Mills, L.; Tellez, C.; Huang, S.; Baker, C.; McCarty, M.; Green, L.; Gudas, J.M.; Feng, X.; Bar-Eli, M. Fully human antibodies to MCAM/MUC18 inhibit tumor growth and metastasis of human melanoma. Cancer Res. 2002, 62, 5106–5114. [Google Scholar]
- Nollet, M.; Stalin, J.; Moyon, A.; Traboulsi, W.; Essaadi, A.; Robert, S.; Malissen, N.; Bachelier, R.; Daniel, L.; Foucault-Bertaud, A.; et al. A novel anti-CD146 antibody specifically targets cancer cells by internalizing the molecule. Oncotarget 2017, 8, 112283–112296. [Google Scholar] [CrossRef]
- Stalin, J.; Nollet, M.; Garigue, P.; Fernandez, S.; Vivancos, L.; Essaadi, A.; Muller, A.; Bachelier, R.; Foucault-Bertaud, A.; Fugazza, L.; et al. Targeting soluble CD146 with a neutralizing antibody inhibits vascularization, growth and survival of CD146-positive tumors. Oncogene 2016, 35, 5489–5500. [Google Scholar] [CrossRef] [PubMed]
- Stalin, J.; Nollet, M.; Dignat-George, F.; Bardin, N.; Blot-Chabaud, M. Therapeutic and Diagnostic Antibodies to CD146: Thirty Years of Research on Its Potential for Detection and Treatment of Tumors. Antibodies 2017, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Mel, Z. Circulating melanoma cells. Breaktroughs. In Melanoma Research; Yohei Tanaka—INTECH Open Science-Open Minds: London, UK, 2011; Chapter 3. [Google Scholar]
- Rose, T.M.; Plowman, G.D.; Teplow, D.B.; Dreyer, W.J.; Hellström, K.E.; Brown, J.P. Primary structure of the human melanoma-associated antigen p97 (melanotransferrin) deduced from the mRNA sequence. Proc. Natl. Acad. Sci. USA 1986, 83, 1261–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keilholz, U.; Goldin-Lang, P.; Bechrakis, N.E.; Max, N.; Letsch, A.; Schmittel, A.; Scheibenbogen, C.; Heufelder, K.; Eggermont, A.; Thiel, E. Quantitative detection of circulating tumor cells in cutaneous and ocular melanoma and quality assessment by real-time reverse transcriptase-polymerase chain reaction. Clin Cancer Res. 2004, 10, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaugler, B.; Eynde, B.V.D.; Van Der Bruggen, P.; Romero, P.; Gaforio, J.J.; De Plaen, E.; Lethe, B.; Brasseur, F.; Boon, T. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J. Exp. Med. 1994, 179, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Rapanotti, M.C.; Bianchi, L.; Ricozzi, I.; Campione, E.; Pierantozzi, A.; Orlandi, A.; Chimenti, S.; Federici, G.; Bernardini, S. Melanoma-associated markers expression in blood: MUC-18 is associated with advanced stages in melanoma patients. Br. J. Dermatol. 2009, 160, 338–344. [Google Scholar] [CrossRef]
- Hoon, D.; Wang, Y.; Dale, P.S.; Conrad, A.J.; Schmid, P.; Garrison, D.; Kuo, C.; Foshag, L.J.; Nizze, A.J.; Morton, D.L. Detection of occult melanoma cells in blood with a multiple-marker polymerase chain reaction assay. J. Clin. Oncol. 1995, 13, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Scolyer, R.A.; Thomson, J.F.; Stretch, J.R.; Sharma, R.; McCarthy, S.W. Pathology of melanocytic: New, controversial and clinically important issues. J. Surg. Oncol. 2004, 86, 200–211. [Google Scholar] [CrossRef]
- Curry, B.J.; Myers, K.; Hersey, P. MART-1 is expressed less frequently on circulating melanoma cells in patients who develop distant compared with locoregional metastase. J. Clin. Oncol. 1999, 17, 2562–7251. [Google Scholar] [CrossRef] [PubMed]
- Mellado, B.; Gutierrez, L.; Castel, T.; Colomer, D.; Fontanillas, M.; Castro, J.; Estapé, J. Prognostic significance of the detection of circulating malignant cells by reverse transcriptase- polymerase chain reaction in long-term clinically disease-free melanoma patients. Clin. Cancer Res. 1999, 5, 1843–1848. [Google Scholar]
- Palmieri, G.; Strazzullo, M.; Ascierto, P.A.; Satriano, S.M.; Daponte, A. Castello for the Melanoma Cooperative Group, G. Polymerase chain reaction-based detection circulating melanoma cells as an effective marker of tumor progression. J. Clin. Oncol. 1999, 17, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Schittek, B.; Bodingbauer, Y.; Ellwanger, U.; Blaheta, H.J.; Garbe, C. Amplification of Melan A messenger RNA in addition to tyrosinase increases sensitivity of melanoma cell detection in peripheral blood and is associated with the clinical stage and prognosis of malignant melanoma. Br. J. Dermatol. 1999, 141, 30–36. [Google Scholar] [CrossRef]
- Aubin, F.; Chtourou, M.; Teyssier, J.R.; Laubriet, A.; Mougin, C.H.; Blanc, D.; Humbert, P. The detection of tyrosinase mRNA in the peripheral blood of stage I melanoma patients is not of clinical relevance in predicting metastasis risk and survival. Melanoma Res. 2000, 10, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, G.; Ascierto, P.A.; Perrone, F.; Satriano, S.M.; Ottaiano, A.; Daponte, A.; Napolitano, M.; Caracò, C.; Mozzillo, N.; Melucci, M.T.; et al. Prognostic value of circulating melanoma cells detected by reverse transcriptase-polymerase chain reaction. J. Clin. Oncol. 2003, 21, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Wascher, R.A.; Morton, D.L.; Kuo, C.; Elashoff, R.M.; Wang, H.-J.; Gerami, M.; Hoon, D.S. Molecular tumor markers in the blood: Early prediction of disease outcome in melanoma patients treated with a melanoma vaccine. J. Clin. Oncol. 2003, 21, 2558–2563. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.R.; Hoon, D.S.B. Molecular markers in malignant cutaneous melanoma: Gift horse or one-trick pony? J. Cell BioChem. 2005, 96, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.; Nadiminti, U.; Sober, A.J.; Bigby, M. A meta-analysis of reverse transcriptase-polymerase chain reaction for tyrosinase mRNA as a marker for circulating tumor cells in cutaneous melanoma. Arch. Dermatol. 2001, 137, 325–330. [Google Scholar] [PubMed]
- Brownbridge, G.G.; Gold, J.; Edward, M.; Mackie, R.M. Evaluation of the use of tyrosinase-specific and MelanA/MART-1-specific reverse transcriptase-coupled-polymerase chain reaction to detect melanoma cells in peripheral blood samples from 299 patients with malignant melanoma. Br. J. Dermatol. 2001, 144, 279–287. [Google Scholar] [CrossRef]
- Rapanotti, M.C.; Ricozzi, I.; Campione, E.; Orlandi, A.; Bianchi, L. Blood MUC-18/MCAM expression in patients with melanoma: A suitable marker of poor outcome. Br. J. Dermatol. 2013, 169, 221–222. [Google Scholar] [CrossRef]
- Rapanotti, M.C.; Suarez Viguria, T.M.; Costanza, G.; Ricozzi, I.; Pierantozzi, A.; Di Stefani, A.; Campione, E.; Bernardini, S.; Chimenti, S.; Orlandi, A.; et al. Sequential molecular analysis of circulating MCAM/MUC18 expression: A promising disease biomarker related to clinical outcome in melanoma. Arch Dermatol. Res. 2014, 306, 527–537. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.Y.; Oskarsson, T.; Acharyya, S.; Nguyen, D.X.; Zhang, X.H.; Norton, L.; Massagué, J. Tumor self-seeding by circulating cancer cells. Cell 2009, 139, 1315–1326. [Google Scholar] [CrossRef] [Green Version]
- Pantel, K.; Alix-Panabières, C. Real-time liquid biopsy in cancer patients: Fact or fiction? Cancer Res. 2013, 73, 6384–6388. [Google Scholar] [CrossRef] [Green Version]
- Ghossein, R.A.; Rosai, J. Polymerase chain reaction in the detection of micrometastases and circulating tumor cells. Cancer 1996, 78, 10–16. [Google Scholar] [CrossRef]
- Klinac, D.; Gray, E.S.; Freeman, J.B.; Reid, A.; Bowyer, S.; Millward, M.; Ziman, M. Monitoring changes in circulating tumour cells as a prognostic indicator of overall survival and treatment response in patients with metastatic melanoma. BMC Cancer 2014, 14, 423. [Google Scholar] [CrossRef] [Green Version]
- Rao, C.; Bui, T.; Connelly, M.; Doyle, G.; Karydis, I.; Middleton, M.R.; Clack, G.; Malone, M.; Coumans, F.A.W.; Terstappen, L.W.M.M. Circulating melanoma cells and survival in metastatic melanoma. Int. J. Oncol. 2011, 38, 755–760. [Google Scholar] [PubMed] [Green Version]
- Khoja, L.; Lorigan, P.; Zhou, C.; Lancashire, M.; Booth, J.; Cummings, J.; Califano, R.; Clack, G.; Hughes, A.; Dive, C. Biomarker utility of circulating tumor cells in metastatic cutaneous melanoma. J. Invest. Dermatol. 2013, 133, 1582–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boiko, A.D.; Razorenova, O.V.; van de Rijn, M.; Swetter, S.M.; Johnson, D.L.; Ly, D.P.; Butler, P.D.; Yang, G.P.; Joshua, B.; Kaplan, M.J.; et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature 2010, 466, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Mitra, D.; Sullivan, R.J.; Wittner, B.S.; Kimura, A.M.; Pan, S.; Hoang, M.P.; Brannigan, B.W.; Lawrence, D.P.; Flaherty, K.T.; et al. Isolation and molecular characterization of circulating melanoma cells. Cell Rep. 2014, 7, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapanotti, M.C.; Campione, E.; Suarez Viguria, T.M.; Spallone, G.; Costanza, G.; Rossi, P.; Orlandi, A.; Valenti, P.; Bernardini, S.; Bianchi, L. Stem-Mesenchymal Signature Cell Genes Detected in Heterogeneous Circulating Melanoma Cells Correlate with Disease Stage in Melanoma Patients. Front. Mol. Biosci. 2020, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Rapanotti, M.C. Molecular Expression of Bone Marrow Angiogenic Factors, Cell-Cell Adhesion Molecules and Matrix-Metallo-Proteinases in Plasmacellular Disorders: A Molecular Panel to İnvestigate Disease Progression. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018059. [Google Scholar] [CrossRef]
- Rapanotti, M.C.; Viguria, T.M.S.; Spallone, G.; Terrinoni, A.; Rossi, P.; Costanza, G.; Campione, E.; Lombardo, P.; Pathirannehalage, C.D.; Orlandi, A.; et al. Minimal Residual Disease in Melanoma:molecular characterization of in transit cutaneous metastases and Circulating Melanoma Cells recognizes an expression panel potentially related to disease progression. Cancer Treat Res. Commun. 2020, 25, 100262. [Google Scholar] [CrossRef]
- Frank, N.Y.; Margaryan, A.; Huang, Y.; Schatton, T.; Waaga-Gasser, A.M.; Gasser, M.; Sayegh, M.H.; Sadee, W.; Frank, M. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005, 65, 4320–4333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harhouri, K.; Kebir, A.; Guillet, B.; Foucault-Bertaud, A.; Voytenko, S.; Piercecchi-Marti, M.D.; Berenguer, C.; Lamy, E.; Vely, F.; Pisano, P.; et al. Soluble CD146 displays angiogenic properties and promotes neovascularization in experimental hind-limb ischemia. Blood 2010, 115, 3843–3851. [Google Scholar] [CrossRef]
- Moal, V.; Anfosso, F.; Daniel, L.; Brunet, P.; Sampol, J.; George, F.D.; Bardin, N. Soluble CD146, a novel endothelial marker, is increased in physiopathological settings linked to endothelial junctional alteration. Thromb. Haemost. 2003, 90, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Bardin, N.; Reumaux, D.; Geboes, K.; Colombel, J.F.; Blot-Chabaud, M.; Sampol, J.; Duthilleul, P.; Dignat-George, F. Increased expression of CD146, a new marker of the endothelial junction in active inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Ilié, M.; Long, E.; Hofman, V.; Selva, E.; Bonnetaud, C.; Boyer, J.; Vénissac, N.; Sanfiorenzo, C.; Ferrua, B.; Marquette, C.H.; et al. Clinical value of circulating endothelial cells and of soluble CD146 levels in patients undergoing surgery for non-small cell lung cancer. Br. J. Cancer 2014, 110, 1236–1243. [Google Scholar] [CrossRef] [Green Version]
- Tsiolakidou, G.; Koutroubakis, I.E.; Tzardi, M.; Kouroumalis, E.A. Increased expression of VEGF and CD146 in patients with inflammatory bowel disease. Dig. Liver Dis. 2008, 40, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Saito, O.; Kawano, T.; Tamemoto, H.; Kusano, E.; Kawakami, M.; Ishikawa, S.E. Elevation of serum adiponectin and CD146 levels in diabetic nephropathy. Diabetes Res. Clin. Pract. 2007, 78, 85–92. [Google Scholar] [CrossRef]
- Hohenstein, B.; Hausknecht, B.; Boehmer, K.; Riess, R.; Brekken, R.A.; Hugo, C.P. Local VEGF activity but not VEGF expression is tightly regulated during diabetic nephropathy in man. Kidney Int. 2006, 69, 1654–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Distler, O.; Del Rosso, A.; Giacomelli, R.; Cipriani, P.; Conforti, M.L.; Guiducci, S.; Gay, R.E.; Michel, B.A.; Brühlmann, P.; Müller-Ladner, U.; et al. Angiogenic and angiostatic factors in systemic sclerosis: Increased levels of vascular endothelial growth factor are a feature of the earliest disease stages and are associated with the absence of fingertip ulcers. Arthritis Res. 2002, 4, R11. [Google Scholar] [CrossRef] [Green Version]
- Kowal-Bielecka, O.; Fransen, J.; Avouac, J.; Becker, M.; Kulak, A.; Allanore, Y.; Distler, O.; Clements, P.; Cutolo, M.; Czirjak, L.; et al. EUSTAR Coauthors. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 1327–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larochelle, C.; Lécuyer, M.A.; Alvarez, J.I.; Charabati, M.; Saint-Laurent, O.; Ghannam, S.; Kebir, H.; Flanagan, K.; Yednock, T.; Duquette, P.; et al. Melanoma cell adhesion molecule-positive CD8 T lymphocytes mediate central nervous system inflammation. Ann. Neurol. 2015, 78, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.R.; Ammitzbøll, C.; Søndergaard, H.B.; Oturai, A.B.; Sørensen, P.S.; Nilsson, A.C.; Börnsen, L.; von Essen, M.; Sellebjerg, F. Expression of melanoma cell adhesion molecule-1 (MCAM-1) in natalizumab-treated multiple sclerosis. J. Neuroimmunol. 2019, 337, 577085. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Funovits, J.; Smolen, J.S. Physical disability in rheumatoid arthritis is associated with cartilage damage rather than bone destruction. Ann. Rheum. Dis. 2011, 70, 733–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagur, P.K.; McCoy, J.P., Jr. Endothelial-binding, proinflammatory T cells identified by MCAM (CD146) expression: Characterization and role in human autoimmune diseases. Autoimmun. Rev. 2015, 14, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Queirolo, P.; Boutros, A.; Tanda, E.; Spagnolo, F.; Quaglino, P. Immune-checkpoint inhibitors for the treatment of metastatic melanoma: A model of cancer immunotherapy. Semin. Cancer Biol. 2019, 59, 290–297. [Google Scholar] [CrossRef]
- Tanda, E.T.; Vanni, I.; Boutros, A.; Andreotti, V.; Bruno, W.; Ghiorzo, P.; Spagnolo, F. Current State of Target Treatment in BRAF Mutated Melanoma. Front. Mol. Biosci. 2020, 7, 154. [Google Scholar] [CrossRef]
- Chiou, V.L.; Burotto, M. Pseudoprogression and Immune-Related Response in Solid Tumors. J. Clin. Oncol. 2015, 33, 3541–3543. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Long, G.V.; Menzies, A.M.; Lo, S.; Guminski, A.; Whitbourne, K.; Peranec, M.; Scolyer, R.; Kefford, R.F.; Rizos, H.; et al. Association Between Circulating Tumor DNA and Pseudoprogression in Patients with Metastatic Melanoma Treated with Anti-Programmed Cell Death 1 Antibodies. JAMA Oncol. 2018, 4, 717–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggermont, A.M.; Maio, M.; Robert, C. Immune checkpoint inhibitors in melanoma provide the cornerstones for curative therapies. Semin. Oncol. 2015, 42, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Mauri, D.; Tsiouris, S.; Gkoura, S.; Gazouli, I.; Ntellas, P.; Amylidis, A.; Kampletsas, L.; Fotopoulos, A. Is there a role for Gallium-67 SPECT in distinguishing progression and pseudoprogresion in oncologic patients receiving immunotherapy? Cancer Treat Res. Commun. 2021, 28, 100441. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Alix-Panabières, C.; Riethdorf, S. Cancer micrometastases. Nat. Rev. Clin. Oncol. 2009, 6, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Lianidou, E.S.; Markou, A. Circulating tumor cells in breast cancer: Detection systems, molecular characterization, and future challenges. Clin. Chem. 2011, 57, 1242–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecot, C.V.; Bischoff, F.Z.; Mayer, J.A.; Wong, K.L.; Pham, T.; Bottsford-Miller, J.; Stone, R.L.; Lin, Y.G.; Jaladurgam, P.; Roh, J.W.; et al. A novel platform for detection of CK+ and CK- CTCs. Cancer Discov. 2011, 1, 580–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.X.; Hyun, K.A.; Moon, H.S.; Sim, T.S.; Lee, J.G.; Park, J.C.; Lee, S.S.; Jung, H.I. Continuous labeling of circulating tumor cells with microbeads using a vortex micromixer for highly selective isolation. Biosens. Bioelectron. 2013, 40, 63–67. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okabe, H.; Ishimoto, T.; Mima, K.; Nakagawa, S.; Hayashi, H.; Kuroki, H.; Imai, K.; Nitta, H.; Saito, S.; Hashimoto, D.; et al. CD44s signals the acquisition of the mesenchymal phenotype required for anchorage-independent cell survival in hepatocellular carcinoma. Br. J. Cancer 2014, 110, 958–966. [Google Scholar] [CrossRef]
- Yeung, K.T.; Yang, J. Epithelial-mesenchymal transition in tumor metastasis. Mol. Oncol. 2017, 11, 28–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Weinberg, R.A. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front. Med. 2018, 12, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Banyard, J.; Bielenberg, D.R. The role of EMT and MET in cancer dissemination. Connect Tissue Res. 2015, 56, 403–413. [Google Scholar] [CrossRef] [Green Version]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer. 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Scheel, C.; Weinberg, R.A. Cancer stem cells and epithelial-mesenchymal transition: Concepts and molecular links. Semin Cancer Biol. 2012, 22, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Donaher, J.L.; Murphy, D.A.; Chau, S.; Yang, J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012, 22, 725–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolly, M.K.; Boareto, M.; Huang, B.; Jia, D.; Lu, M.; Ben-Jacob, E.; Onuchic, J.N.; Levine, H. Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis. Front. Oncol. 2015, 5, 155. [Google Scholar] [CrossRef] [Green Version]
- Ashworth, T.R. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Australian Med. J. 1869, 14, 146–147. [Google Scholar]
- Galvis, M.M.; Romero, C.S.; Bueno, T.O.; Teng, Y. Toward a New Era for the Management of Circulating Tumor Cells. Adv. Exp. Med. Biol. 2021, 1286, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy: Potential and challenges. Mol Oncol. 2016, 10, 371–373. [Google Scholar] [CrossRef] [Green Version]
- Gray, E.S.; Reid, A.L.; Bowyer, S.; Calapre, L.; Siew, K.; Pearce, R.; Cowell, L.; Frank, M.H.; Millward, M.; Ziman, M. Circulating Melanoma Cell Subpopulations: Their Heterogeneity and Differential Responses to Treatment. J. Invest. Dermatol. 2015, 135, 2040–2048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aya-Bonilla, C.; Gray, E.S.; Manikandan, J.; Freeman, J.B.; Zaenker, P.; Reid, A.; Khattak, M.A.; Frank, M.H.; Millward, M.; Ziman, M. Immunomagnetic-enriched subpopulations of melanoma circulating tumour cells (CTCs) exhibit distinct transcriptome profiles. Cancers 2019, 11, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aya-Bonilla, C.A.; Morici, M.; Hong, X.; McEvoy, A.C.; Sullivan, R.J.; Freeman, J.; Calapre, L.; Khattak, M.A.; Meniawy, T.; Millward, M.; et al. Detection and prognostic role of heterogeneous populations of melanoma circulating tumour cells. Br. J. Cancer. 2020, 122, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Kiniwa, Y.; Nakamura, K.; Mikoshiba, A.; Akiyama, Y.; Morimoto, A.; Okuyama, R. Diversity of circulating tumor cells in peripheral blood: Detection of heterogeneous BRAF mutations in a patient with advanced melanoma by single-cell analysis. J. Dermatol. Sci. 2018, 90, 211–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, A.; Mogami, T.; Watanabe, M.; Iijima, K.; Akiyama, Y.; Katayama, K.; Futami, T.; Yamamoto, N.; Sawada, T.; Koizumi, F.; et al. High-Density Dielectrophoretic Microwell Array for Detection, Capture, and Single-Cell Analysis of Rare Tumor Cells in Peripheral Blood. PLoS ONE 2015, 10, e0130418. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, H.; Nawa, T.; Yamamoto, Y.; Shimizu, K.; Kobayashi, K.; Kitazawa, S.; Kanbara, H.; Odagiri, T.; Endo, K.; Matsunaga, T.; et al. Detection of circulating tumor cells in patients with lung cancer using metallic micro-cavity array filter: A pilot study. Mol. Clin. Oncol. 2020, 12, 278–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raimondi, C.; Nicolazzo, C.; Gradilone, A. Circulating tumor cells isolation: The “post-EpCAM era”. Chin. J. Cancer Res. 2015, 27, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Laga, M.D.; George, F.; Murphy, M.D. Cellular Heterogeneity in Vertical Growth Phase Melanoma. Arch. Pathol. Lab. Med. 2010, 134, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Schatton, T.; Murphy, G.F.; Frank, N.Y.; Yamaura, K.; Waaga-Gasser, A.M.; Gasser, M.; Zhan, Q.; Jordan, S.; Duncan, L.M.; Weishaupt, C.; et al. Identification of cells initiating human melanomas. Nature 2008, 451, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Klein, W.M.; Wu, B.P.; Zhao, S.; Wu, H.; Klein-Szanto, A.J.P.; Tahan, S.R. Increased expression of stem cell markers in malignant melanoma. Mod. Pathol. 2007, 20, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Bardin, N.; Anfosso, F.; Massé, J.-M.; Cramer, E.; Sabatier, F.; Le Bivic, A.; Sampol, J.; Dignat-George, F. Identification of CD146 as a component of the endothelial junction involved in the control of cell-cell cohesion. Blood 2001, 98, 3677–3684. [Google Scholar] [CrossRef] [Green Version]
- Guezguez, B.; Vigneron, P.; Lamerant, N.; Kieda, C.; Jaffredo, T.; Dunon, D. Dual role of melanoma cell adhesion molecule (MCAM)/CD146 in lymphocyte endothelium interaction: MCAM/CD146 promotes rolling via microvilli induction in lymphocyte and is an endothelial adhesion receptor. J. Immunol. 2007, 179, 6673–6685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocken, M. Early tumor dissemination, but late metastasis: Insights into tumor dormancy. J. Clin. Invest. 2010, 120, 1800–1803. [Google Scholar] [CrossRef]
- Friberg, S.; Nyström, A. Cancer Metastases: Early Dissemination and Late Recurrences. Cancer Growth Metastasis 2015, 8, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Kittler, O.; Ragg, T.; Daskalakis, A.; Granzow, M.; Ahr, A.; Blankenstein, T.J.F.; Kaufmann, M.; Diebold, J.; Arnholdt, H.; Müller, P.; et al. From latent disseminated cells to overt metastasis: Genetic analysis of systemic breast cancer progression. Proc. Natl. Acad. Sci. USA 2003, 100, 7737–7742. [Google Scholar] [CrossRef] [Green Version]
- Naik, M.U.; Naik, U.P. Junctional adhesion molecule-A-induced endothelial cell migration on vitronectin is integrin alpha v beta 3 specific. J. Cell Sci. 2006, 119 Pt 3, 490–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.J.; Yang, Y.; Yang, G.K.; Wan, J.; Cui, D.L.; Ma, Z.H.; Du, L.J.; Zhang, G.M. Slit2 suppresses endothelial cell proliferation and migration by inhibiting the VEGF-Notch signaling pathway. Mol. Med. Rep. 2017, 15, 1981–1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, H.; Yan, L.; Du, W.; Zhang, M.; Chen, H.; Zhang, L.; Li, G.; Li, J.; Dong, Y.; et al. MMP-2 and MMP-9 contribute to the angiogenic effect produced by hypoxia/15-HETE in pulmonary endothelial cells. J. Mol. Cell Cardiol. 2018, 121, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Barillari, G.; Iovane, A.; Bacigalupo, I.; Palladino, C.; Bellino, S.; Leone, P.; Monini, P.; Ensoli, B. Ritonavir or saquinavir impairs the invasion of cervical intraepithelial neoplasia cells via a reduction of MMP expression and activity. AIDS 2012, 26, 909–919. [Google Scholar] [CrossRef] [Green Version]
- Veidal, S.S.; Larsen, D.V.; Chen, X.; Sun, S.; Zheng, Q.; Bay-Jensen, A.C.; Leeming, D.J.; Nawrocki, A.; Larsen, M.R.; Schett, G.; et al. MMP mediated type V collagen degradation (C5M) is elevated in ankylosing spondylitis. Clin. Biochem. 2012, 45, 541–546. [Google Scholar] [CrossRef]
- Li, W.; Liu, Z.; Zhao, C.; Zhai, L. Binding of MMP-9-degraded fibronectin to β6 integrin promotes invasion via the FAK-Src-related Erk1/2 and PI3K/Akt/Smad-1/5/8 pathways in breast cancer. Oncol. Rep. 2015, 34, 1345–1352. [Google Scholar] [CrossRef]
- Barillari, G. The Impact of Matrix Metalloproteinase-9 on the Sequential Steps of the Metastatic Process. Int. J. Mol. Sci. 2020, 21, 4526. [Google Scholar] [CrossRef] [PubMed]
- Roudier, E.; Chapados, N.; Decary, S.; Gineste, C.; Le Bel, C.; Lavoie, J.M.; Bergeron, R.; Birot, O. Angiomotin p80/p130 ratio: A new indicator of exercise-induced angiogenic activity in skeletal muscles from obese and non-obese rats? J. Physiol. 2009, 587, 4105–4119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Watkins, G.; Douglas-Jones, A.; Holmgren, L.; Mansel, R.E. Angiomotin and angiomotin like proteins, their expression and correlation with angiogenesis and clinical outcome in human breast cancer. BMC Cancer 2006, 6, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Stalin, J.; Harhouri, K.; Hubert, L.; Subrini, C.; Lafitte, D.; Lissitzky, J.-C.; Elganfoud, N.; Robert, S.; Foucault-Bertaud, A.; Kaspi, E.; et al. Melanoma cell adhesion molecule (smcam/scd146) promotes angiogenic effects on endothelial progenitor cells through angiomotin. J. Biol. Chem. 2013, 288, 8991–9000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satchi-Fainaro, R.; Ferber, S.; Segal, E.; Ma, L.; Dixit, N.; Ijaz, A.; Hlatky, L.; Abdollahi, A.; Almog, N. Prospective identification of glioblastoma cells generating dormant tumors. PLoS ONE 2012, 7, e44395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, L.; Lu, Q.; Wang, L.H.; Liu, C.-Y.; Lei, Q.-Y.; Guan, K.-L. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011, 25, 51–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Chan, H.L.; Chen, P. Immune Checkpoint Inhibitors: Basics and Challenges. Curr. Med. Chem. 2019, 26, 3009–3025. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Maleki Vareki, S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J. Immunother. Cancer. 2018, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- De Guillebon, E.; Dardenne, A.; Saldmann, A.; Séguier, S.; Tran, T.; Paolini, L.; Lebbe, C.; Tartour, E. Beyond the concept of cold and hot tumors for the development of novel predictive biomarkers and the rational design of immunotherapy combination. Int. J. Cancer. 2020, 147, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Rapanotti, M.C.; Campione, E.; Spallone, G.; Orlandi, A.; Bernardini, S.; Bianchi, L. Minimal residual disease in melanoma: Circulating melanoma cells and predictive role of MCAM/MUC18/MelCAM/CD146. Cell Death Discov. 2017, 3, 17005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Sex | N° | % |
| Female | 13 | 43.33 |
| Male | 17 | 56.66 |
| Age (Years) | 44 (mean) | 23–84 (range) |
| Primary Tumour Site | N° | % |
| Head and Neck * | 2 | 6.67 |
| Trunk | 17 | 56.67 |
| Extremity | 7 | 23.33 |
| Unknown | 4 | 13.33 |
| AJCC** Stage | N° | % |
| >IB | 5 | 16.67 |
| II: IIA (3); IIB (2); | 5 | 16.67 |
| III ***: IIIA (2); IIIB (3); IIIC (2); | 7 | 23.33 |
| IV | 13 | 43.33 |
| Time from Diagnosis | Years | Pts/AJCC Stage |
| Onset | 0–11 | 18: IB (5); IIA (3); IIB (2); IIIA (1); IIIB (1); IIIC (2); IV (4) |
| First Observation–Baseline | 12: IIIA (1); IIIB (2); IV (9) | |
| Clinical Status | % | |
| Clinically Disease-Free | 12 | 40 |
| Clinically Evident Disease | 18 | 60 |
| Clinically Remission Expression Panel Patients Baseline Samples (#12) | CD146 5′-Portion | CD146 Long Isoform | CD146 Short Isoform | VE-Cadh | N-Cadh | VEGF | b-FGF | MMP-2 | MMP-9 |
|---|---|---|---|---|---|---|---|---|---|
| Endothelial CMCs (E-CMCs) (CD45-MCAM/CD16 +) | 16.6% 2/12 | 33.3% 4/12 | 33.3% 4/12 | 25% 3/12 | 33.3% 4/12 | 8.3% 1/12 | 33.3% 4/12 | 41.6% 5/12 | 41.6% 5/12 |
| Stem-Mesenchimal CMCs (S-M-CMCs) (CD45-MCAM + ABCB5 +) | 8.3% 1/12 | 25% 3/12 | 16.6% 2/12 | 33.3% 4/12 | 25.0% 3/12 | 16.6% 2/12 | 16.6% 2/12 | 50.0% 6/12 | 33.3% 4/12 |
| |||||||||
| Clinically Evident Disease Patients Expression Panel Baseline Samples (#18) | |||||||||
| Endothelial CMCs (E-CMCs) (CD45-MCAM/CD16 +) | 44.4% 8/18 | 72.2% 13/18 | 61.1% 11/18 | 55.5% 10/18 | 33.3% 6/18 | 16.6% 3/18 | 11.1% 2/18 | 66.6% 12/18 | 55.5% 12/18 |
| Stem-Mesenchimal CMCs (S-M-CMCs) (CD45-MCAM + ABCB5 +) | 50.0% 9/18 | 77.7% 14/18 | 72.2% 13/18 | 55.5% 10/18 | 27.7% 5/18 | 5.5% 1/18 | 22.2% 4/18 | 66.6% 12/18 | 72.2% 13/18 |
| UPN | Sex | Age at Baseline | Primary Tumor Site | Histology | Breslow Grade (mm) | AJCC Status at Baseline | Incurrence of Progression from Diagnosis | Therapy after Diagnosis or Lymphnodal/Cutaneous in Transit/Metastases Incurrence | Follow-Up and Clinical Status |
|---|---|---|---|---|---|---|---|---|---|
| UPN1- AV | f | 80 | Unknown | / | / | IV | +1 year | Checkpoint inhibitors (anti-PD1-PD1L) | At +2 years: disease progression Actually in therapy |
| UPN2- MU | m | 47 | Trunk | SSM | 1.8 | IIIA | +5 years | Targeted therapy (anti-BRAF and anti-MEK) | At +4 years: disease progression Actually clinical remission |
| UPN3- FM | m | 40 | Trunk | SSM | 1.25 | IIB | +3 years | Pretargeted therapy (anti-BRAF and anti-MEK) | At +1 year: stable disease Actually in therapy |
| UPN4- VM | m | 60 | Trunk | NM | 4.5 | IIB | +1 year | Checkpoint inhibitors (anti-PD1-PD1L) | At +2 years: continuous clinical remission |
| UPN5- CAD | f | 82 | Acral | NM | 2.4 | IV | +13 years | Checkpoint inhibitors(anti-PD1-PD1L) | At +1 year: disease progression and death |
| UPN6- PN | m | 64 | Trunk | NM | 4.0 | IV | +1 year | Targeted therapy (anti-BRAF and anti-MEK) | At +1 year: disease progression and death |
| UPN7- ZF | m | 42 | Head | NM | 2.2 | IB | / | Checkpoint inhibitors (anti-PD1-PD1L) | At +2 years: continuous clinical remission |
| UPN8- GD | m | 35 | Acral | NM | 2.2 | IIA | +7 years | Targeted therapy (anti-BRAF and anti-MEK) | At +1 year: continuous clinical remission |
| UPN9- RETL | f | 53 | Acral | ALM | / | IIIC | / | Targeted therapy (anti-BRAF and anti-MEK) | At +2 years: clinical remission Actually stopped therapy |
| UPN10- PME | f | 75 | Nasal cavity | Mucous MM | / | IIB | / | Checkpoint inhibitors (anti-PD1-PD1L) | At +6 months: disease progression Actually in therapy |
| UPN11- DMM | f | 34 | Trunk | NM | 1.5 | IIIA | +2 years | IFN | At +6 years: continuous clinical remission Actually stop-therapy |
| UPN12- SD | f | 41 | Trunk | NM | 7 | IIB | +2 years | Checkpoint inhibitors (anti-PD1-PD1L) | At +4 years: disease progression Actually in therapy |
| UPN13- BC | m | 86 | Trunk | Trunk | 5 | IIB | +4 years | Refusal of any therapy | At +1 year: stable disease |
| (A) | |||||||||
| * Clinically Remission Patients | CD146 5′-Portion | CD146 Long Isoform | CD146 Short Isoform | VEGF | bFGF | N-Cadh | VE-Cadh | MMP-2 | MMP-9 |
| Baseline Expression Panel (#7) | |||||||||
| Endothelial CMCs (E-CMCs) (CD45-MCAM +) | 42.8% 3/7 | 85.7% 6/7 | 100% 7/7 | 57.1% 4/7 | 28.6% 2/7 | 71.4% 5/7 | 85.7% 6/7 | 85.7% 6/7 | 71.4% 5/7 |
| Stem-Mesenchimal CMCs (S-M-CMCs) (CD45-MCAM + ABCB5+) | 42.8% 3/7 | 85.7% 6/7 | 100% 7/7 | 28.6% 2/7 | 57.1% 4/7 | 57.1% 4/7 | 71.4% 5/7 | 85.7% 6/7 | 71.4% 5/7 |
| Follow-Up Expression Panel (#7) | |||||||||
| Endothelial CMCs (E-CMCs) (CD45-MCAM +) | 0% 0/7 | 0% 0/7 | 0% 0/7 | 14.3% 1/7 | 14.3% 1/7 | 42.8% 3/7 | 0% 0/7 | 28.6% 2/7 | 28.6% 2/7 |
| Stem-Mesenchimal CMCs (S-M-CMCs) (CD45-MCAM + ABCB5+) | 0% 0/7 | 0% 0/7 | 14.3% 1/7 | 0% 0/7 | 14.3% 1/7 | 0% 0/7 | 0% 0/7 | 0% 0/7 | 0% 0/7 |
| (B) | |||||||||
| Baseline Expression Panel (#8) | |||||||||
| Endothelial CMCs (E-CMCs) (CD45-MCAM+) | 37.5% 3/8 | 62.5% 5/8 | 50.0% 4/8 | 12.5% 1/8 | 25.0% 2/8 | 25.0% 2/8 | 50.0% 4/8 | 62.5% 5/8 | 50.0% 4/8 |
| Stem-Mesenchimal CMCs (S-M-CMCs) (CD45-MCAM + ABCB5+) | 50.0% 4/8 | 75.00% 6/8 | 62.5% 5/8 | 25.0% 2/8 | 50.0% 4/8 | 37.5% 3/8 | 62.5% 5/8 | 62.5% 5/8 | 87.5% 7/8 |
| Follow-Up Expression Panel (#8) | |||||||||
| Endothelial CMCs (CD45-MCAM + E-CMCs) | 37.5% 3/8 | 62.5% 5/8 | 100% 8/8 | 25.0% 2/8 | 25.0% 2/8 | 50.0% 4/8 | 62.5% 5/8 | 62.5% 5/8 | 87.5% 7/8 |
| Stem-Mesenchimal CMCs (S-M-CMCs) (CD45-MCAM + ABCB5+) | 50.0% 4/8 | 87.5% 7/8 | 62.5% 5/8 | 37.5% 3/8 | 12.5% 1/8 | 25.0% 2/8 | 62.5% 5/8 | 62.5% 5/8 | 100% 8/8 |
| Comparison Between Clinical Serum Classes | sCD146 ng/mL Dosage Mean/Median Healthy People Concentration = 273 ± 70 ng/mL |
|---|---|
| (A) Melanoma Baseline (Onset/First Observation) Sera (#30) | 199.55/186.78 Linear regression 201.01/191.40 Hyperbolic curve |
| Melanoma Follow Up Sera (#21) | 258,28/230.60 Linear regression 260.26/232.85 Hyperbolic curve |
| (B) Clinically Remission Patient Sera (#13) | 212.651/197.48 Linear regression 215.72/203.255 Hyperbolic curve |
| Clinically Evident Disease Patient Sera (#22) | 262.13/259.16 Linear regression 263.88/261.22 Hyperbolic curve |
| (C) Treatment-Naïve Patient Sera (#12) | 217.58/218.68 Linear regression 198.5/206.35 Hyperbolic curve |
| Treated-Patient Sera (#22) (Checkpoint Inhibitor Therapy–Targeted Therapy–Other Therapies) | 253.85/235.33 Linear regression 255.87/237.82 Hyperbolic curve |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapanotti, M.C.; Cugini, E.; Nuccetelli, M.; Terrinoni, A.; Di Raimondo, C.; Lombardo, P.; Costanza, G.; Cosio, T.; Rossi, P.; Orlandi, A.; et al. MCAM/MUC18/CD146 as a Multifaceted Warning Marker of Melanoma Progression in Liquid Biopsy. Int. J. Mol. Sci. 2021, 22, 12416. https://doi.org/10.3390/ijms222212416
Rapanotti MC, Cugini E, Nuccetelli M, Terrinoni A, Di Raimondo C, Lombardo P, Costanza G, Cosio T, Rossi P, Orlandi A, et al. MCAM/MUC18/CD146 as a Multifaceted Warning Marker of Melanoma Progression in Liquid Biopsy. International Journal of Molecular Sciences. 2021; 22(22):12416. https://doi.org/10.3390/ijms222212416
Chicago/Turabian StyleRapanotti, Maria Cristina, Elisa Cugini, Marzia Nuccetelli, Alessandro Terrinoni, Cosimo Di Raimondo, Paolo Lombardo, Gaetana Costanza, Terenzio Cosio, Piero Rossi, Augusto Orlandi, and et al. 2021. "MCAM/MUC18/CD146 as a Multifaceted Warning Marker of Melanoma Progression in Liquid Biopsy" International Journal of Molecular Sciences 22, no. 22: 12416. https://doi.org/10.3390/ijms222212416








