Response of Wheat DREB Transcription Factor to Osmotic Stress Based on DNA Methylation
Abstract
:1. Introduction
2. Results
2.1. Amplification and Sequence Analysis of DREBs
2.2. The Expression Patterns of DREBs in Wheat
2.3. Promoter Analysis of Wheat DREB Genes
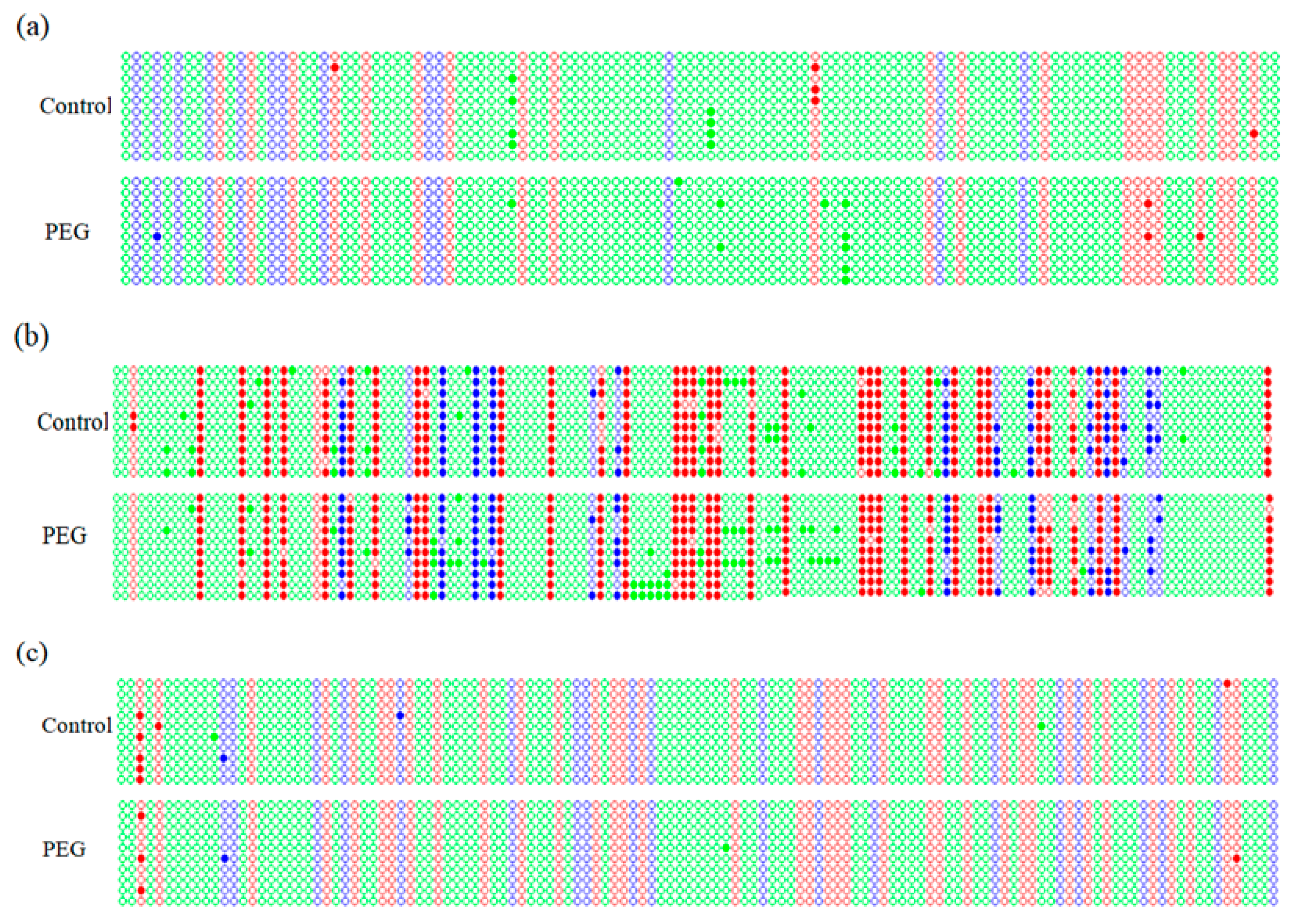
2.4. Promoter Methylation Analysis of DREB Genes
2.5. Methylation Level of DREB Promoters under Osmotic Stress
2.6. Methylation Status in DREB Promoters under Osmotic Stress
2.7. Correlation Analysis between Promoter Methylation and Expression of DREBs
3. Discussion
4. Materials and Methods
4.1. Cultivation and Treatment of Wheat Seedlings
4.2. Extraction of Genomic DNA
4.3. Isolation and Reverse Transcription of RNA
4.4. Amplification and Bioinformatic Analysis of DREB Genes
4.5. Fluorescence Quantitative Real-Time PCR
4.6. Isolation and Analysis of Promoter Sequence
4.7. Methylation Analysis of Promoter
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BSP | bisulfite sequencing PCR |
| CTAB | cetyltriethyl ammonium bromide |
| DRE/CRT | dehydration responsive element/C-repeat |
| DREB | dehydration responsive element binding protein |
| qRT-PCR | quantitative real-time PCR |
References
- Reis, R.R.; Cunha, B.A.D.; Martins, P.K.; Martins, M.T.B.; Alekcevetch, J.C.; Chalfun-Junior, A.; Andrade, A.C.; Ribeiro, A.P.; Qin, F.; Mizoi, J.; et al. Induced over-expression of AtDREB2A CA improves drought tolerance in sugarcane. Plant Sci. 2014, 221, 59–68. [Google Scholar] [CrossRef]
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of drought and heat stresses on plant growth and yield: A review. Int. Agrophys. 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Wang, P.; Du, Y.; Zhao, X.; Miao, Y.; Song, C.P. The MPK6-ERF6-ROS-responsive cis-acting element7/GCC box complex modulates oxidative gene transcription and the oxidative response in Arabidopsis. Plant Physiol. 2013, 161, 1392–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.C.; Chu, S.J.; Guo, Y.M.; Ji, Y.J.; Hu, D.Q.; Cheng, J.; Lu, G.H.; Yang, R.W.; Tang, C.Y.; Qi, J.L.; et al. Novel mechanisms for organic acid-mediated aluminium tolerance in roots and leaves of two contrasting soybean genotypes. AoB Plants 2017, 9, plx064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Liu, H.J.; Dong, Z.Y.; Xiao, J.W.; Su, B.D.; Fan, L.S.; Kmis, G.; Samaj, J.; Lin, J.X.; Li, R.L. The dynamics and endocytosis of Flot1 protein in response to flg22 in Arabidopsis. J. Plant Physiol. 2017, 215, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Sazegari, S.; Niazi, A.; Ahmadi, F.S. A study on the regulatory network with promoter analysis for Arabidopsis DREB genes. Bioinformation 2015, 11, 101–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Qin, F.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 2006, 18, 1292–1309. [Google Scholar] [CrossRef] [Green Version]
- Jaglo-Ottosen, K.R.; Gilmour, S.J.; Zarka, D.G.; Schabenberger, O.; Thomashow, M.F. ArabidopsisCBF1 overexpression induces cor genes and enhances freezing tolerance. Science 1998, 280, 104–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dossa, K.; Wei, X.; Li, D.; Fonceka, D.; Zhang, Y.X.; Wang, L.H.; Yu, J.Y.; Boshou, L.; Diouf, D.; Cisse, N.; et al. Insight into the AP2/ERF transcription factor superfamily in sesame and expression profiling of DREB subfamily under drought stress. BMC Plant Boil. 2016, 16, 171. [Google Scholar] [CrossRef] [Green Version]
- Gehring, M.; Henikoff, S. DNA methylation dynamics in plant genomes. Biochim. Biophys. Acta 2007, 1769, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.Y.; Liu, W.X.; Li, J.Y.; Ding, W.K.; Zhu, Y.Q.; Wang, H.N.; Jiang, L.N.; Zhou, Y.Q. Growth and DNA methylation level of Triticum aestivum seedlings treated with 5-azacytidine. Pak. J. Bot. 2016, 48, 1585–1591. [Google Scholar]
- Zheng, X.G.; Chen, L.; Lou, Q.J.; Li, M.S.; Luo, L.J. Changes in DNA methylation pattern in a water-saving and drought-resistance rice variety at three-leaf and four-leaf stages after drought domestication. Chin. J. Rice Sci. 2014, 28, 32–40. [Google Scholar]
- Fan, H.H.; Li, T.C.; Li, Z.P.; Lin, Y.; Cai, Y.P.; Jin, Q. MSAP analysis of epigenetic changes in Dendrobium huoshanense under PEG simulated drought stress. Acta Agric. Nucleatae Sin. 2011, 25, 363–368. [Google Scholar]
- Tang, X.M.; Tao, X.; Wang, Y.; Ma, D.W.; Li, D.; Yang, H.; Ma, X.R. Analysis of DNA methylation of perennial ryegrass under drought using the methylation-sensitive amplification polymorphism (MSAP) technique. Mol. Genet. Genom. 2014, 289, 1075–1084. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J. Epigenetic regulation of stress responses in plants. Curr. Opin. Plant Biol. 2009, 12, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Zeng, F.S. Drought resistance and DNA methylation of interspecific hybrids between Fraxinus mandshurica and Fraxinu samericana. Trees 2014, 28, 1679–1692. [Google Scholar] [CrossRef]
- Yang, M.N.; Yang, G.L.; Guo, T.; Liu, Y.Z.; Zhang, J.G.; Chen, Z.Q.; Wang, H. DNA methylation under stresses and its prospects in plant drought-resistant breeding. Chin. Agric. Sci. Bull. 2013, 6, 6–11. [Google Scholar]
- Zhang, C.Y. Study on the Genetic Epigenetic Variation and Related Physiological Metabolism Changes in Rice under Drought Stress; Northeast Normal University: Changchun, China, 2013. [Google Scholar]
- Ahmed, M.D.; Khan, A.S.; Kashif, M.; Khan, S. Genetic mechanism of leaf venation and stomatal traits for breeding drought tolerant lines in wheat. Bangladesh J. Bot. 2017, 46, 35–41. [Google Scholar]
- Gupta, P.; Balyan, H.S.; Gahlaut, V. QTL analysis for drought tolerance in wheat: Present status and future possibilities. Agronomy 2017, 7, 5. [Google Scholar] [CrossRef]
- Ni, Z.Y.; Xu, Z.S.; Liu, L.; Li, L.C.; Chai, Y.; Chen, M.; Ma, Y.Z. Isolation and characterization of a transcription factor TaDREB6 gene from Triticum aestivum L. J. Triticeae Crop. 2008, 28, 357–363. [Google Scholar]
- Pan, L. Excavation of Natural Allelic Variation of Wheat DREB2 Transcription Factor; University of Electronic Science and Technology of China: Chengdu, China, 2010. [Google Scholar]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice (Oryza sativa L.) encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003, 33, 751. [Google Scholar] [CrossRef] [PubMed]
- Li, L.B.; Zhao, H.; Liu, L.; He, C.F.; Bai, R.; Wang, T. Isolation and characterisation of a cold-induced DREB gene from Cymbidium insigne. J. Hortic. Sci. Biotechnol. 2011, 86, 43–49. [Google Scholar] [CrossRef]
- Lopato, S.; Bazanova, N.; Morran, S.; Milligan, A.S.; Shirley, N.; Langridge, P. Isolation of plant transcription factors using a modified yeast one-hybrid system. Plant Methods 2006, 2, 45–66. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.B. Cloning and expressing analysis of a DREB transcription factor in Sunflower. Ningxia J. Agric. For. Sci. Technol. 2015, 1, 31–34. [Google Scholar]
- Huang, Y.; Xu, Z.S.; Wang, F.; Song, X.; Tian, C.; Xiong, A.S. Isolation and expression profiles analysis of two Dc DREB-A1 group transcription factor genes from Carrot. J. Nucl. Agric. Sci. 2015, 29, 29–39. [Google Scholar]
- Fang, Z.W.; Xu, X.Y.; Gao, J.F.; Wang, P.K.; Liu, Z.X.; Feng, B.L. Characterization of FeDREB1 promoter involved in cold- and drought-inducible expression from common buckwheat (Fagopyrum esculentum). Genet. Mol. Res. 2015, 14, 7990–8000. [Google Scholar] [CrossRef]
- Sreenivasulu, G.; Senthilkumaran, B.; Sudhakumari, C.C.; Guan, G.; Oba, Y.; Kagawac, H.; Nagahama, Y. 20β-hydroxysteroid dehydrogenase gene promoter: Potential role for cyclic AMP and xenobiotic responsive elements. Gene 2012, 509, 68–76. [Google Scholar] [CrossRef]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Abid, G.; Mingeot, D.; Muhovski, Y.; Mergeai, G.; Aouida, M.; Abdelkarim, S.; Aroua, I.; Ayed, M.E.; Mhamdi, M.; Sassif, K. Analysis of DNA methylation patterns associated with drought stress response in faba bean (Vicia faba L.) using methylation-sensitive amplification polymorphism (MSAP). Environ. Exp. Bot. 2017, 142, 34–44. [Google Scholar] [CrossRef]
- Duan, H.Y.; Li, J.Y.; Zhu, Y.Q.; Jia, W.J.; Wang, H.H.; Jiang, L.N.; Zhou, Y.Q. Responsive changes of DNA methylation in wheat (Triticum aestivum) under water deficit. Sci. Rep. 2020, 10, 7938. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A. Epigenetic regulation of abiotic stress tolerance in plants. Adv. Plants Agric. Res. 2016, 5, 179. [Google Scholar] [CrossRef]
- Zilberman, D.; Gehring, M.; Tran, R.; Ballinger, T.; Henikoff, S. Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat. Genet. 2007, 39, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.G.; Ma, Z.Q.; Qiu, N.W.; Dong, W. DNA methylation modification status of CUC1 during in vitro organogenesis in Arabidopsis. Plant Physiol. J. 2016, 6, 926–932. [Google Scholar]
- Wang, R.X. Research of Transcriptional Regulation of Opaque2 Gene and DNA Methylation of 19-kDa α-zeins in Maize; Zhejiang University: Hangzhou, China, 2015. [Google Scholar]
- Zhang, X.; Yazaki, J.; Sundaresan, A.; Cokus, S.; Chan, A.; Chen, H.M.; Henderson, I.R.; Shin, P.; Pellegrini, M.; Jacobsen, S.E.; et al. Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell 2006, 126, 1189–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Pan, Y.; Su, C.G. Analysis of the DNA methylation of SlGLD1 regulated by JMJ524 in tomato. Biotechnol. Bull. 2016, 32, 79–85. [Google Scholar]
- Wang, H.N.; Li, B.Y.; Chen, F.F.; Zhou, Y.Q.; Li, J.J.; Zheng, X.; Duan, H.Y. Analysis on phenotype, catalpol accumulation and methylation of Rehmannia glutinosa. Pak. J. Bot. 2019, 51, 461–467. [Google Scholar] [CrossRef]
- Duan, H.Y.; Liu, W.X.; Zeng, Y.P.; Jia, W.J.; Wang, H.H.; Zhou, Y.Q. Expression analysis of key enzymes involved in the accumulation of iridoid in Rehmannia glutinosa. Plant Omics 2019, 12, 102–108. [Google Scholar] [CrossRef]


| Gene | Pattern | Pattern Frequency (%) | Methylation Rate (%) | Total Methylation Rate (%) |
|---|---|---|---|---|
| DREB2 | CG | 19.09 | 2.38 | 1.17 |
| CHG | 11.82 | 0.00 | ||
| CHH | 69.09 | 1.03 | ||
| DREB6 | CG | 25.93 | 88.08 | 31.89 |
| CHG | 11.11 | 51.36 | ||
| CHH | 62.96 | 4.93 | ||
| Wdreb2 | CG | 29.60 | 1.89 | 0.88 |
| CHG | 16.00 | 1.00 | ||
| CHH | 54.40 | 0.29 |
| Gene | Cytosine Type | No. of Cytosine | No. of Methylation Site | |
|---|---|---|---|---|
| Hypermethylation Site | Demethylation Site | |||
| DREB2 | CG | 21 | 2 | 3 |
| CHG | 13 | 1 | 0 | |
| CHH | 76 | 3 | 1 | |
| DREB6 | CG | 34 | 1 | 1 |
| CHG | 15 | 1 | 1 | |
| CHH | 84 | 10 | 8 | |
| Wdreb2 | CG | 37 | 1 | 2 |
| CHG | 20 | 0 | 1 | |
| CHH | 68 | 1 | 2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhu, Y.; Yuan, P.; Song, S.; Dong, T.; Chen, P.; Duan, Z.; Jiang, L.; Lu, L.; Duan, H. Response of Wheat DREB Transcription Factor to Osmotic Stress Based on DNA Methylation. Int. J. Mol. Sci. 2021, 22, 7670. https://doi.org/10.3390/ijms22147670
Wang H, Zhu Y, Yuan P, Song S, Dong T, Chen P, Duan Z, Jiang L, Lu L, Duan H. Response of Wheat DREB Transcription Factor to Osmotic Stress Based on DNA Methylation. International Journal of Molecular Sciences. 2021; 22(14):7670. https://doi.org/10.3390/ijms22147670
Chicago/Turabian StyleWang, Huihui, Yanqiu Zhu, Ping Yuan, Shanglin Song, Tianyu Dong, Peilei Chen, Zhikun Duan, Lina Jiang, Longdou Lu, and Hongying Duan. 2021. "Response of Wheat DREB Transcription Factor to Osmotic Stress Based on DNA Methylation" International Journal of Molecular Sciences 22, no. 14: 7670. https://doi.org/10.3390/ijms22147670





