The Basal Pharmacology of Palmitoylethanolamide
Abstract
:1. Introduction
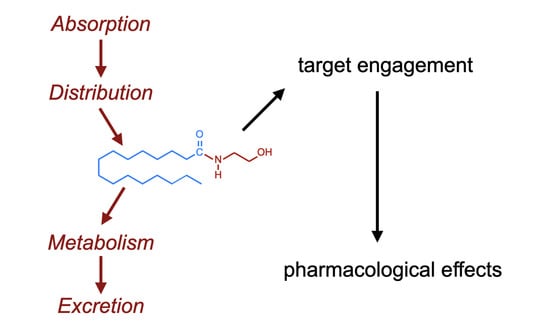
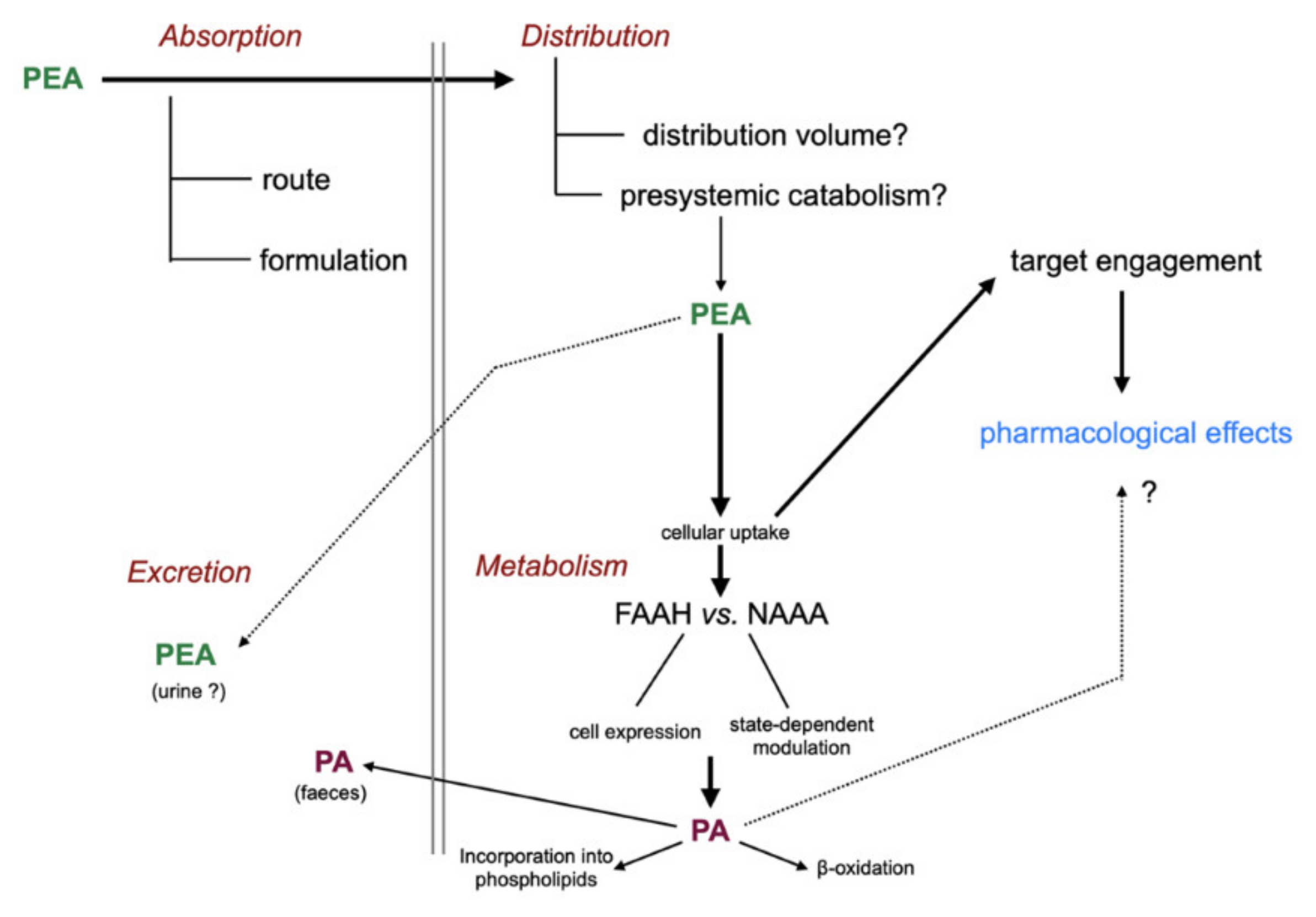
2. The ADME of PEA
2.1. Introduction
2.2. Absorption and Presystemic Metabolism of PEA
2.3. Distribution of PEA
2.4. Cellular Uptake of PEA
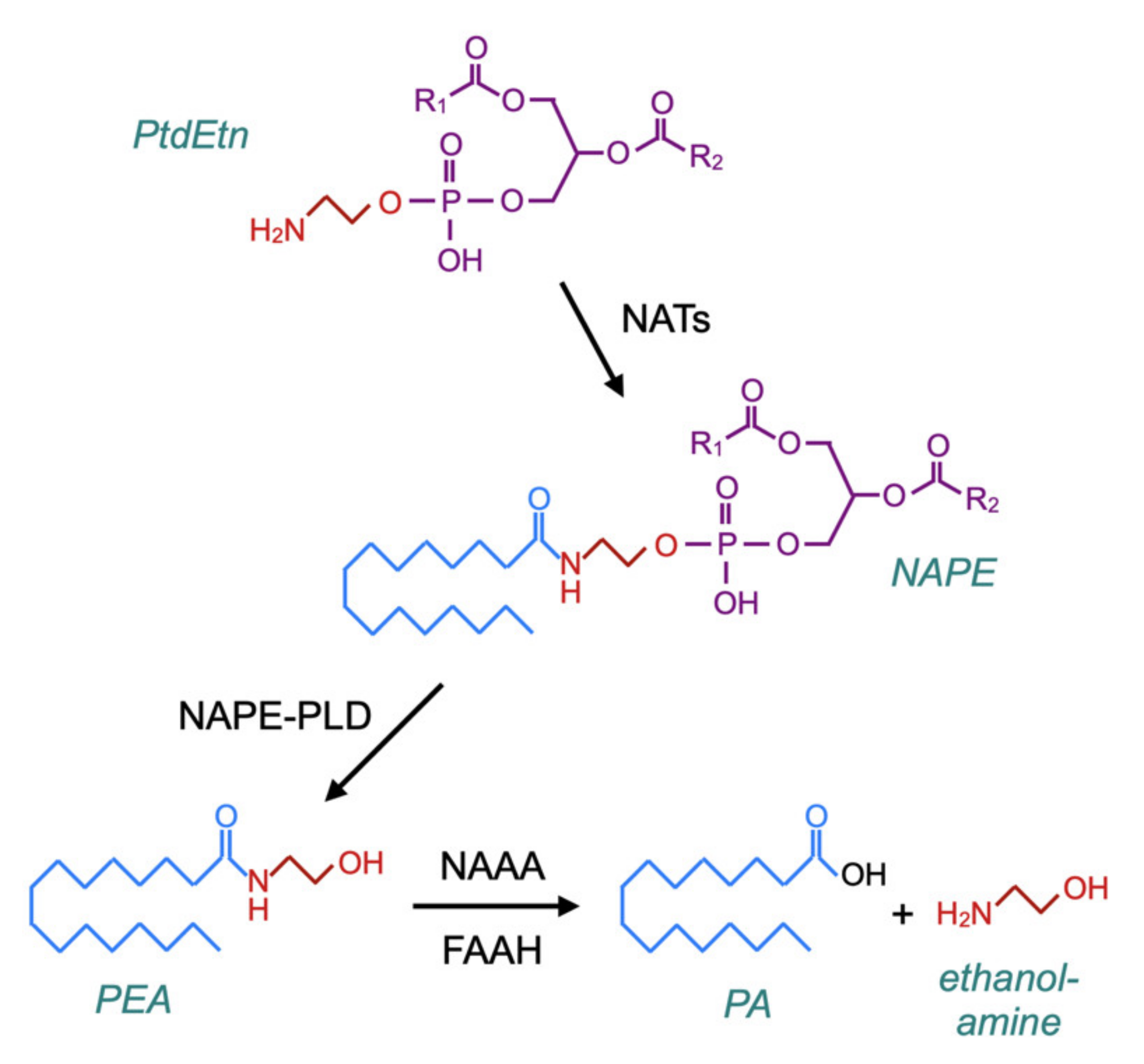
2.5. Metabolism of PEA
2.5.1. Hydrolysis of Endogenous PEA
2.5.2. Hydrolysis of Exogenous PEA
2.6. Excretion of PEA
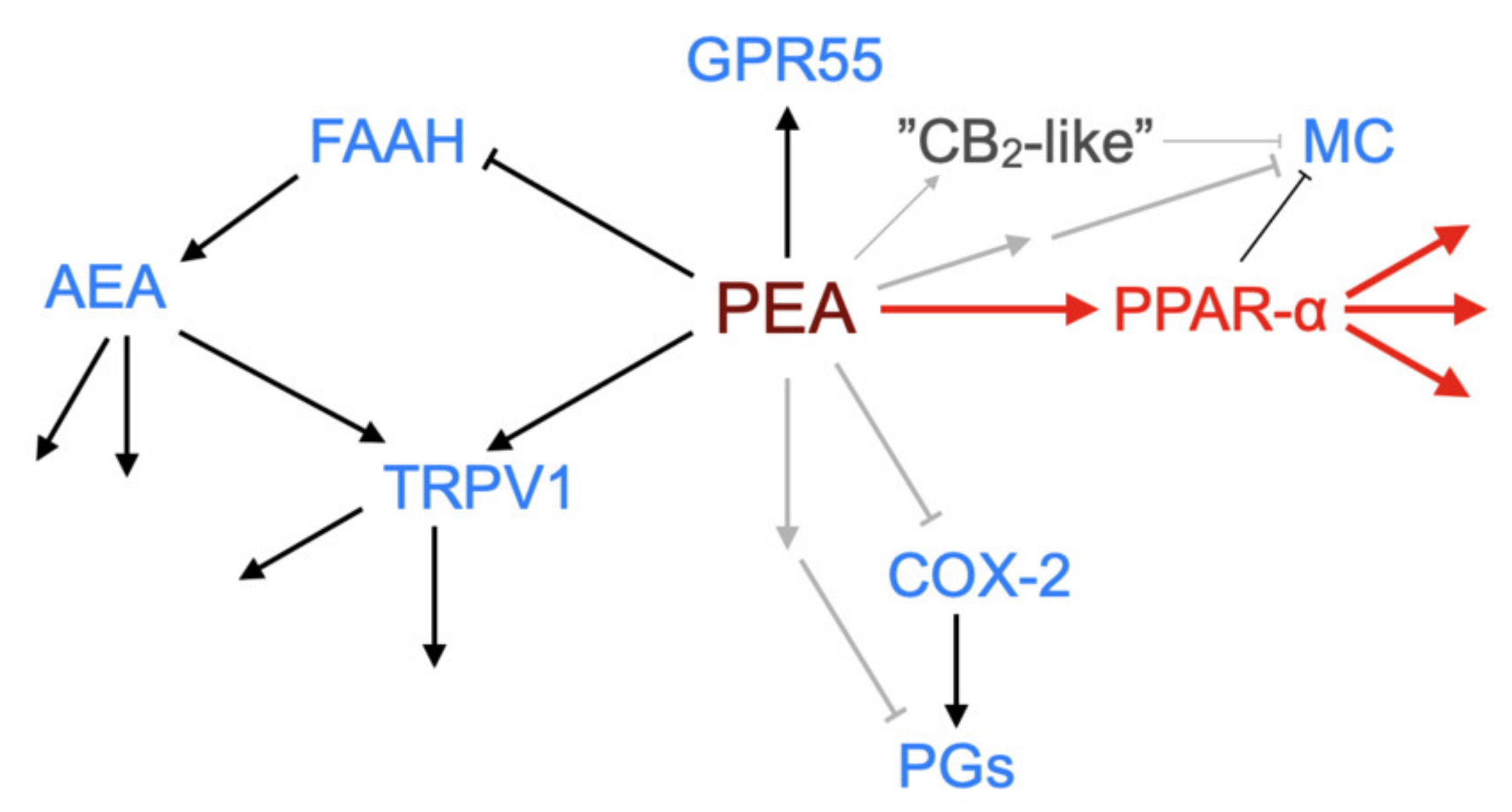
3. PEA Targets
3.1. Introduction
3.2. PPAR-α
3.3. NAE Turnover
3.4. Transient Receptor Potential Vanilloid 1 (TRPV1) Receptors
3.5. GPR55 and GPR119 Orphan Receptors
3.6. Downstream Effects of PEA
4. The Clinical Utility of PEA
5. Natural Sources of PEA
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ADME | absorption, distribution, metabolism and excretion |
| AEA | anandamide, arachidonoylethanolamide |
| COX-2 | cyclooxygenase-2 |
| FAAH | fatty acid amide hydrolase |
| MC | mast cell |
| NAAA | N-acylethanolamine acid amidase |
| NAE | N-acylethanolamine |
| NAPE | N-acylphosphatidylethanolamines |
| NAPE–PLD | NAP hydrolysing phospholipase D |
| NAT | N-acyltransferase |
| NNT | Number Needed to Treat |
| OEA | oleoylethanolamide |
| PA | palmitic acid |
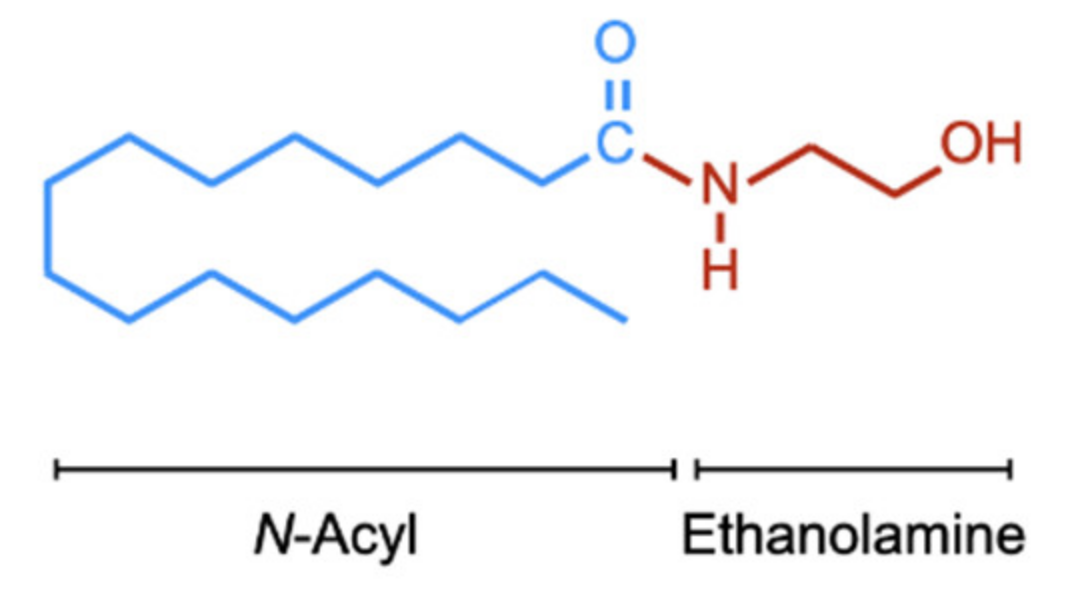
| PEA | palmitoylethanolamide, N-hexadecanoylethanolamide |
| PG | prostaglandin |
| PPAR-α | peroxisome proliferator-activated receptor-α |
| PtdEtn | phosphatidylethanolamine |
| TNF-α | tumour necrosis factor-α |
| TRPV1 | transient receptor potential vanilloid 1 |
| WOMAC | Western Ontario and McMaster Universities Osteoarthritis Index |
References
- Kuehl, F.; Jacob, T.; Ganley, O.; Ormond, R.; Meisinger, M. The identification of N-(2-hydroxyethyl)-palmitamide as a naturally occurring anti-inflammatory agent. J. Am. Chem. Soc. 1957, 79, 5577–5578. [Google Scholar] [CrossRef]
- Bachur, N.; Masek, K.; Melmon, K.; Udenfriend, S. Fatty acid amides of ethanolamine in mammalian tissues. J. Biol. Chem. 1965, 240, 1019–1024. [Google Scholar] [PubMed]
- Coburn, A.; Moore, L. Nutrition as a conditioning factor in the rheumatic state. Am. J. Dis. Child. 1943, 65, 744–756. [Google Scholar] [CrossRef]
- Coburn, A.; Graham, C.; Haninger, J. The effect of egg yolk in diets on anaphylactic arthritis (passive arthus phenomenon) in the guinea pig. J. Exp. Med. 1954, 100, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Guida, G.; De Martino, M.; De Fabiani, A.; Cantieri, L.A.; Alexandre, A.; Vassallo, G.M.; Rogai, M.; Lanaia, F.; Petrosino, S. La palmitoilethanolamida (Normast) en el dolor neuropático crónico por lumbociatalgia de tipo compresivo: Estudio clínico multicéntrico. Dolor 2010, 25, 35–42. [Google Scholar]
- Eberlein, B.; Eicke, C.; Reinhardt, H.W.; Ring, J. Adjuvant treatment of atopic eczema: Assessment of an emollient containing N-palmitoylethanolamine (ATOPA study). J. Eur. Acad. Dermatol. Venereol. 2008, 22, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Murru, E.; Bani, S.; Manca, C. Palmitic acid: Physiological role, metabolism and nutritional implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrosino, S.; Cordaro, M.; Verde, R.; Schiano Moriello, A.; Marcolongo, G.; Schievano, C.; Siracusa, R.; Piscitelli, F.; Peritore, A.F.; Crupi, R.; et al. Oral ultramicronized palmitoylethanolamide: Plasma and tissue levels and spinal anti-hyperalgesic effect. Front. Pharmacol. 2018, 9, 249. [Google Scholar] [CrossRef]
- Noli, C.; Della Valle, M.F.; Miolo, A.; Medori, C.; Schievano, C.; Skinalia Clinical Research Group. Efficacy of ultra-micronized palmitoylethanolamide in canine atopic dermatitis: An open-label multi-centre study. Vet. Dermatol. 2015, 26, 432–440. [Google Scholar] [CrossRef]
- Puglia, C.; Blasi, P.; Ostacolo, C.; Sommella, E.; Bucolo, C.; Platania, C.B.M.; Romano, G.L.; Geraci, F.; Drago, F.; Santonocito, D.; et al. Innovative nanoparticles enhance N-palmitoylethanolamide intraocular delivery. Front. Pharmacol. 2018, 9, 285. [Google Scholar] [CrossRef]
- Vacondio, F.; Bassi, M.; Silva, C.; Castelli, R.; Carmi, C.; Scalvini, L.; Lodola, A.; Vivo, V.; Flammini, L.; Barocelli, E.; et al. Amino acid derivatives as palmitoylethanolamide prodrugs: Synthesis, in vitro metabolism and in vivo plasma profile in rats. PLoS ONE 2015, 10, e0128699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, V.; Segerdahl, A.; Lambert, D.; Vandevoorde, S.; Blackbeard, J.; Pheby, T.; Hasnie, F.; Rice, A. The effect of the palmitoylethanolamide analogue, palmitoylallylamide (L-29) on pain behaviour in rodent models of neuropathy. Br. J. Pharmacol. 2007, 151, 1117–1128. [Google Scholar] [CrossRef] [Green Version]
- Petrosino, S.; Schiano Moriello, A.; Cerrato, S.; Fusco, M.; Puigdemont, A.; De Petrocellis, L.; Di Marzo, V. The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Br. J. Pharmacol. 2016, 173, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Gabrielsson, L.; Mattsson, S.; Fowler, C.J. Palmitoylethanolamide for the treatment of pain: Pharmacokinetics, safety and efficacy. Br. J. Clin. Pharmacol. 2016, 82, 932–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artamonov, M.; Zhukov, O.; Shuba, I.; Storozhuk, L.; Khmel, T.; Klimashevsky, V.; Mikosha, A.; Gula, N. Incorporation of labelled N-acylethanolamine (NAE) into rat brain regions in vivo and adaptive properties of saturated NAE under x-ray irradiation (1999). Ukr. Biokhimicheskii Zhurnal 2005, 77, 51–62. [Google Scholar]
- Gula, N.M.; Mel’nyk, O.O.; Vysots’kyĭ, M.V.; Balkov, D.I.; Volkov, G.L.; Govseieva, N.M. Distribution of [1-14C]N-palmitoylethanolamine and its metabolites in subcellular fractions of neuroblastoma C1300 N18 (1978). Ukr. Biokhimicheskii Zhurnal 1991, 63, 115–119. [Google Scholar]
- Nicolussi, S.; Gertsch, J. Endocannabinoid transport revisited. In Vitamins & Hormones; Academic Press: Waltham, MA, USA, 2015; Volume 98, pp. 441–485. [Google Scholar] [CrossRef]
- Fowler, C.J. Transport of endocannabinoids across the plasma membrane and within the cell. FEBS J. 2013, 280, 1895–1904. [Google Scholar] [CrossRef]
- Deutsch, D.; Glaser, S.; Howell, J.; Kunz, J.; Puffenbarger, R.; Hillard, C.; Abumrad, N. The cellular uptake of anandamide is coupled to its breakdown by fatty-acid amide hydrolase. J. Biol. Chem. 2001, 276, 6967–6973. [Google Scholar] [CrossRef] [Green Version]
- Bachur, N.; Udenfriend, S. Microsomal synthesis of fatty acid amides. J. Biol. Chem. 1966, 241, 1308–1313. [Google Scholar]
- Schmid, P.; Zuzarte-Augustin, M.; Schmid, H. Properties of rat liver N-acylethanolamine amidohydrolase. J. Biol. Chem. 1985, 260, 14145–14149. [Google Scholar]
- Boger, D.; Fecik, R.; Patterson, J.; Miyauchi, H.; Patricelli, M.; Cravatt, B. Fatty acid amide hydrolase substrate specificity. Bioorganic Med. Chem. Lett. 2000, 10, 2613–2616. [Google Scholar] [CrossRef]
- Bracey, M.; Hanson, M.; Masuda, K.; Stevens, R.; Cravatt, B. Structural adaptions in a membrane enzyme that terminates endocannabinoid signaling. Science 2002, 298, 1793–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, B.; Mikkelsen, T.; McKinney, M.; Lander, E.; Cravatt, B. A second fatty acid amide hydrolase with variable distribution among placental mammals. J. Biol. Chem. 2006, 281, 36569–36578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczocha, M.; Glaser, S.T.; Chae, J.; Brown, D.A.; Deutsch, D.G. Lipid droplets are novel sites of N-acylethanolamine inactivation by fatty acid amide hydrolase-2. J. Biol. Chem. 2010, 285, 2796–2806. [Google Scholar] [CrossRef] [Green Version]
- Ueda, N.; Yamanaka, K.; Yamamoto, S. Purification and characterization of an acid amidase selective for N-palmitoylethanolamine, a putative endogenous anti-inflammatory substance. J. Biol. Chem. 2001, 276, 35552–35557. [Google Scholar] [CrossRef] [Green Version]
- Tsuboi, K.; Sun, Y.-X.; Okamoto, Y.; Araki, N.; Tonai, T.; Ueda, N. Molecular characterization of N-acylethanolamine-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase. J. Biol. Chem. 2005, 280, 11082–11092. [Google Scholar] [CrossRef] [Green Version]
- Fegley, D.; Gaetani, S.; Duranti, A.; Tontini, A.; Mor, M.; Tarzia, G.; Piomelli, D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): Effects on anandamide and oleoylethanolamide deactivation. J. Pharmacol. Exp. Ther. 2005, 313, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Jhaveri, M.; Richardson, D.; Robinson, I.; Garle, M.; Patel, A.; Sun, Y.; Sagar, D.; Bennett, A.; Alexander, S.; Kendall, D.; et al. Inhibition of fatty acid amide hydrolase and cyclooxygenase-2 increases levels of endocannabinoid related molecules and produces analgesia via peroxisome proliferator-activated receptor-alpha in a model of inflammatory pain. Neuropharmacology 2008, 55, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.; Johnson, D.; Mileni, M.; Beidler, D.; Long, J.; McKinney, M.; Weerapana, E.; Sadagopan, N.; Liimatta, M.; Smith, S.; et al. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem. Biol. 2009, 16, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Alhouayek, M.; Bottemanne, P.; Subramanian, K.V.; Lambert, D.M.; Makriyannis, A.; Cani, P.D.; Muccioli, G.G. N-Acylethanolamine-hydrolyzing acid amidase inhibition increases colon N-palmitoylethanolamine levels and counteracts murine colitis. FASEB J. 2015, 29, 650–661. [Google Scholar] [CrossRef] [Green Version]
- Sasso, O.; Moreno-Sanz, G.; Martucci, C.; Realini, N.; Dionisi, M.; Mengatto, L.; Duranti, A.; Tarozzo, G.; Tarzia, G.; Mor, M.; et al. Antinociceptive effects of the N-acylethanolamine acid amidase inhibitor ARN077 in rodent pain models. Pain 2013, 154, 350–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonezzi, F.T.; Sasso, O.; Pontis, S.; Realini, N.; Romeo, E.; Ponzano, S.; Nuzzi, A.; Fiasella, A.; Bertozzi, F.; Piomelli, D. An important role for N-acylethanolamine acid amidase in the complete Freund’s adjuvant rat model of arthritis. J. Pharmacol. Exp. Ther. 2016, 356, 656–663. [Google Scholar] [CrossRef] [Green Version]
- Alhouayek, M.; Rankin, L.; Gouveia-Figueira, S.; Fowler, C.J. Interferon γ treatment increases endocannabinoid and related N-acylethanolamine levels in T84 human colon carcinoma cells. Br. J. Pharmacol. 2019, 176, 1470–1480. [Google Scholar] [CrossRef]
- Alhouayek, M.; Bottemanne, P.; Makriyannis, A.; Muccioli, G.G. N-acylethanolamine-hydrolyzing acid amidase and fatty acid amide hydrolase inhibition differentially affect N-acylethanolamine levels and macrophage activation. Biochim. Biophys. Acta 2017, 1862, 474–484. [Google Scholar] [CrossRef]
- Peng, X.; Studholme, K.; Kanjiya, M.P.; Luk, J.; Bogdan, D.; Elmes, M.W.; Carbonetti, G.; Tong, S.; Teng, Y.-H.G.; Rizzo, R.C.; et al. Fatty-acid-binding protein inhibition produces analgesic effects through peripheral and central mechanisms. Mol. Pain 2017, 13, 1744806917697007. [Google Scholar] [CrossRef] [Green Version]
- Gabrielsson, L.; Gouveia-Figueira, S.; Häggström, J.; Alhouayek, M.; Fowler, C.J. The anti-inflammatory compound palmitoylethanolamide inhibits prostaglandin and hydroxyeicosatetraenoic acid production by a macrophage cell line. Pharmacol. Res. Perspect. 2017, 5, e00300. [Google Scholar] [CrossRef] [PubMed]
- Raso, G.M.; Simeoli, R.; Russo, R.; Santoro, A.; Pirozzi, C.; Bianca, R.D.D.V.; Mitidieri, E.; Paciello, O.; Pagano, T.B.; Orefice, N.S.; et al. N-Palmitoylethanolamide protects the kidney from hypertensive injury in spontaneously hypertensive rats via inhibition of oxidative stress. Pharmacol. Res. 2013, 76, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Palmitoylethanolamide PEA Capsules. Available online: https://thelongevityspecialists.com/palmitoylethanolamide-pea-100-pure (accessed on 22 September 2020).
- Aloe, L.; Leon, A.; Levi-Montalcini, R. A proposed autacoid mechanism controlling mastocyte behaviour. Agents Actions 1993, 39, C145–C147. [Google Scholar] [CrossRef]
- Lerner, R.; Cuadrado, D.P.; Post, J.M.; Lutz, B.; Bindila, L. Broad lipidomic and transcriptional changes of prophylactic PEA administration in adult mice. Front. Neurosci. 2019, 13, 527. [Google Scholar] [CrossRef] [PubMed]
- Farquhar-Smith, W.; Jaggar, S.; Rice, A. Attenuation of nerve growth factor-induced visceral hyperalgesia via cannabinoid CB1 and CB2-like receptors. Pain 2002, 97, 11–21. [Google Scholar] [CrossRef]
- Costa, B.; Conti, S.; Giagnoni, G.; Colleoni, M. Therapeutic effect of the endogenous fatty acid amide, palmitoylethanolamide, in rat acute inflammation: Inhibition of nitric oxide and cyclo-oxygenase systems. Br. J. Pharmacol. 2002, 137, 413–420. [Google Scholar] [CrossRef]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular actions of PPARα in lipid metabolism and inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wójtowicz, S.; Strosznajder, A.K.; Jeżyna, M.; Strosznajder, J.B. The novel role of PPAR Alpha in the brain: Promising target in therapy of Alzheimer’s disease and other neurodegenerative disorders. Neurochem. Res. 2020, 45, 972–988. [Google Scholar] [CrossRef] [Green Version]
- Lo Verme, J.; Fu, J.; Astarita, G.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The nuclear receptor peroxisome proliferator-activated receptor-α mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005, 67, 15–19. [Google Scholar] [CrossRef]
- LoVerme, J.; Russo, R.; La Rana, G.; Fu, J.; Farthing, J.; Mattace-Raso, G.; Meli, R.; Hohmann, A.; Calignano, A.; Piomelli, D. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-α. J. Pharmacol. Exp. Ther. 2006, 319, 1051–1061. [Google Scholar] [CrossRef]
- Saliba, S.W.; Jauch, H.; Gargouri, B.; Keil, A.; Hurrle, T.; Volz, N.; Mohr, F.; van der Stelt, M.; Bräse, S.; Fiebich, B.L. Anti-neuroinflammatory effects of GPR55 antagonists in LPS-activated primary microglial cells. J. Neuroinflammation 2018, 15, 322. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, S.; Forstmann, B.U.; Wagenmakers, E.-J. Erroneous analyses of interactions in neuroscience: A problem of significance. Nat. Neurosci. 2011, 14, 1105–1107. [Google Scholar] [CrossRef]
- Ann, S.J.; Chung, J.H.; Park, B.H.; Kim, S.H.; Jang, J.; Park, S.; Kang, S.M.; Lee, S.H. PPARα agonists inhibit inflammatory activation of macrophages through upregulation of β-defensin 1. Atherosclerosis 2015, 240, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Wahba, M.G.; Messiha, B.A.; Abo-Saif, A.A. Protective effects of fenofibrate and resveratrol in an aggressive model of rheumatoid arthritis in rats. Pharm. Biol. 2016, 54, 1705–1715. [Google Scholar] [CrossRef]
- Usui-Ouchi, A.; Ouchi, Y.; Ebihara, N. The peroxisome proliferator-activated receptor pan-agonist bezafibrate suppresses microvascular inflammatory responses of retinal endothelial cells and vascular endothelial growth factor production in retinal pigmented epithelial cells. Int. Immunopharmacol. 2017, 52, 70–76. [Google Scholar] [CrossRef]
- Lipanthyl®. Available online: https://www.fass.se/LIF/product?userType=0&nplId=19991217000236 (accessed on 23 September 2020).
- Lopid®. Available online: https://www.fass.se/LIF/product?userType=0&nplId=19890609000116 (accessed on 23 September 2020).
- Okine, B.N.; Madasu, M.K.; McGowan, F.; Prendergast, C.; Gaspar, J.C.; Harhen, B.; Roche, M.; Finn, D.P. N-palmitoylethanolamide in the anterior cingulate cortex attenuates inflammatory pain behaviour indirectly via a CB1 receptor-mediated mechanism. Pain 2016, 157, 2687–2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaia, M.; Petrosino, S.; De Filippis, D.; Negro, L.; Guarino, A.; Carnuccio, R.; Di Marzo, V.; Iuvone, T. Palmitoylethanolamide reduces inflammation and itch in a mouse model of contact allergic dermatitis. Eur. J. Pharmacol. 2016, 791, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Cristino, L.; Karsak, M.; Gaffal, E.; Ueda, N.; Tuting, T.; Bisogno, T.; De Filippis, D.; D’Amico, A.; Saturnino, C.; et al. Protective role of palmitoylethanolamide in contact allergic dermatitis. Allergy 2010, 65, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Aldossary, S.A.; Alsalem, M.; Kalbouneh, H.; Haddad, M.; Azab, B.; Al-Shboul, O.; Mustafa, A.G.; Obiedat, S.; El-Salem, K. The role of transient receptor potential vanilloid receptor 1 and peroxisome proliferator-activated receptors-α in mediating the antinociceptive effects of palmitoylethanolamine in rats. Neuroreport 2019, 30, 32–37. [Google Scholar] [CrossRef]
- Alsalem, M.; Haddad, M.; Aldossary, S.A.; Kalbouneh, H.; Altarifi, A.; Jaffal, S.M.; Abbas, M.A.; Aldaoud, N.; El-Salem, K. Role of cannabinoid receptor 1 and the peroxisome proliferator-activated receptor α in mediating anti-nociceptive effects of synthetic cannabinoids and a cannabinoid-like compound. Inflammopharmacology 2019, 27, 1131–1142. [Google Scholar] [CrossRef]
- Borrelli, F.; Romano, B.; Petrosino, S.; Pagano, E.; Capasso, R.; Coppola, D.; Battista, G.; Orlando, P.; Di Marzo, V.; Izzo, A.A. Palmitoylethanolamide, a naturally occurring lipid, is an orally effective intestinal anti-inflammatory agent. Br. J. Pharmacol. 2015, 172, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Costa, B.; Comelli, F.; Bettoni, I.; Colleoni, M.; Giagnoni, G. The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: Involvement of CB1, TRPV1 and PPARγ receptors and neurotrophic factors. Pain 2008, 139, 541–550. [Google Scholar] [CrossRef]
- D’Agostino, G.; La Rana, G.; Russo, R.; Sasso, O.; Iacono, A.; Esposito, E.; Mattace Raso, G.; Cuzzocrea, S.; Lo Verme, J.; Piomelli, D.; et al. Acute intracerebroventricular administration of palmitoylethanolamide, an endogenous peroxisome proliferator-activated receptor-a agonist, modulates carrageenan-induced paw edema in mice. J. Pharmacol. Exp. Ther. 2007, 322, 1137–1143. [Google Scholar] [CrossRef] [Green Version]
- D’Agostino, G.; La Rana, G.; Russo, R.; Sasso, O.; Iacono, A.; Esposito, E.; Mattace Raso, G.; Cuzzocrea, S.; LoVerme, J.; Piomelli, D.; et al. Central administration of palmitoylethanolamide reduces hyperalgesia in mice via inhibition of NF-κB nuclear signalling in dorsal root ganglia. Eur. J. Pharmacol. 2009, 613, 54–59. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; D’Agostino, G.; Pacini, A.; Russo, R.; Zanardelli, M.; Ghelardini, C.; Calignano, A. Palmitoylethanolamide is a disease-modifying agent in peripheral neuropathy: Pain relief and neuroprotection share a PPAR-alpha-mediated mechanism. Mediat. Inflamm. 2013, 2013, 328797. [Google Scholar] [CrossRef]
- Di Paola, R.; Impellizzeri, D.; Mondello, P.; Velardi, E.; Aloisi, C.; Cappellani, A.; Esposito, E.; Cuzzocrea, S. Palmitoylethanolamide reduces early renal dysfunction and injury caused by experimental ischemia and reperfusion in mice. Shock 2012, 38, 356–366. [Google Scholar] [CrossRef] [Green Version]
- Di Paola, R.; Cordaro, M.; Crupi, R.; Siracusa, R.; Campolo, M.; Bruschetta, G.; Fusco, R.; Pugliatti, P.; Esposito, E.; Cuzzocrea, S. Protective Effects of ultramicronized palmitoylethanolamide (PEA-um) in myocardial ischaemia and reperfusion injury in vivo. Shock 2016, 46, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Donvito, G.; Wilkerson, J.L.; Damaj, M.I.; Lichtman, A.H. Palmitoylethanolamide reverses paclitaxel-induced allodynia in mice. J. Pharmacol. Exp. Ther. 2016, 359, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, E.; Impellizzeri, D.; Mazzon, E.; Paterniti, I.; Cuzzocrea, S. Neuroprotective activities of palmitoylethanolamide in an animal model of Parkinson’s disease. PLoS ONE 2012, 7, e41880. [Google Scholar] [CrossRef] [Green Version]
- Esposito, G.; Capoccia, E.; Turco, F.; Palumbo, I.; Lu, J.; Steardo, A.; Cuomo, R.; Sarnelli, G.; Steardo, L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut 2014, 63, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Crupi, R.; D’Amico, R.; Fusco, R.; Evangelista, M.; Cuzzocrea, S.; et al. The neuroprotective effects of micronized PEA (PEA-m) formulation on diabetic peripheral neuropathy in mice. FASEB J. 2019, 33, 11364–11380. [Google Scholar] [CrossRef]
- Paterniti, I.; Impellizzeri, D.; Crupi, R.; Morabito, R.; Campolo, M.; Esposito, E.; Cuzzocrea, S. Molecular evidence for the involvement of PPAR-δ and PPAR-γ in anti-inflammatory and neuroprotective activities of palmitoylethanolamide after spinal cord trauma. J. Neuroinflammation 2013, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Sarnelli, G.; D’Alessandro, A.; Iuvone, T.; Capoccia, E.; Gigli, S.; Pesce, M.; Seguella, L.; Nobile, N.; Aprea, G.; Maione, F.; et al. Palmitoylethanolamide modulates inflammation-associated vascular endothelial growth factor (VEGF) signaling via the Akt/mTOR pathway in a selective peroxisome proliferator-activated receptor alpha (PPAR-α)-dependent manner. PLoS ONE 2016, 11, e0156198. [Google Scholar] [CrossRef] [Green Version]
- Ye, S.; Chen, Q.; Jiang, N.; Liang, X.; Li, J.; Zong, R.; Huang, C.; Qiu, Y.; Ma, J.X.; Liu, Z. PPARα-dependent effects of palmitoylethanolamide against retinal neovascularization and fibrosis. Investig. Opthalmology Vis. Sci. 2020, 61, 15. [Google Scholar] [CrossRef] [Green Version]
- Holt, S.; Comelli, F.; Costa, B.; Fowler, C.J. Inhibitors of fatty acid amide hydrolase reduce carrageenan-induced hind paw inflammation in pentobarbital-treated mice: Comparison with indomethacin and possible involvement of cannabinoid receptors. Br. J. Pharmacol. 2005, 146, 467–476. [Google Scholar] [CrossRef] [Green Version]
- Di Marzo, V.; Melck, D.; Orlando, P.; Bisogno, T.; Zagoory, O.; Bifulco, M.; Vogel, Z.; De Petrocellis, L. Palmitoylethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti-proliferative effect of anandamide in human breast cancer cells. Biochem. J. 2001, 358, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Moriello, A.S.; Verde, R.; Allarà, M.; Imperatore, R.; Ligresti, A.; Mahmoud, A.M.; Peritore, A.F.; Iannotti, F.A.; Di Marzo, V. Palmitoylethanolamide counteracts substance P-induced mast cell activation in vitro by stimulating diacylglycerol lipase activity. J. Neuroinflammation 2019, 16, 274. [Google Scholar] [CrossRef] [Green Version]
- Huggins, J.P.; Smart, T.S.; Langman, S.; Taylor, L.; Young, T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain 2012, 153, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.M.E.; van Till, J.W.O.; Houbiers, J.G.A.; Martina, R.V.; Cerneus, D.P.; Melis, J.H.J.M.; Majek, A.; Vjaters, E.; Urban, M.; Ramonas, H.; et al. Fatty acid amide hydrolase inhibitor treatment in men with chronic prostatitis/chronic pelvic pain syndrome: An adaptive double-blind, randomized controlled trial. Urology 2017, 103, 191–197. [Google Scholar] [CrossRef]
- Natarajan, V.; Reddy, P.V.; Schmid, P.C.; Schmid, H.H.O. N-Acylation of ethanolamine phospholipids in canine myocardium. Biochim. Biophys. Acta 1982, 712, 342–355. [Google Scholar] [CrossRef]
- Natarajan, V.; Schmid, P.; Reddy, P.; Zuzarte-Augustin, M.; Schmid, H. Biosynthesis of N-acylethanolamine phospholipids by dog brain preparations. J. Neurochem. 1983, 41, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Reddy, P.; Natarajan, V.; Schmid, H. Metabolism of N-acylethanolamine phospholipids by a mammalian phosphodiesterase of the phospholipase D type. J. Biol. Chem. 1983, 258, 9302–9306. [Google Scholar]
- Leung, D.; Saghatelian, A.; Simon, G.; Cravatt, B. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry 2006, 45, 4720–4726. [Google Scholar] [CrossRef] [Green Version]
- Tsuboi, K.; Okamoto, Y.; Ikematsu, N.; Inoue, M.; Shimizu, Y.; Uyama, T.; Wang, J.; Deutsch, D.G.; Burns, M.P.; Ulloa, N.M.; et al. Enzymatic formation of N-acylethanolamines from N-acylethanolamine plasmalogen through N-acylphosphatidylethanolamine-hydrolyzing phospholipase D-dependent and -independent pathways. Biochim. Biophys. Acta 2011, 1811, 565–577. [Google Scholar] [CrossRef]
- Ueda, N.; Tsuboi, K.; Uyama, T. Metabolism of endocannabinoids and related N-acylethanolamines: Canonical and alternative pathways. FEBS J. 2013, 280, 1874–1894. [Google Scholar] [CrossRef]
- Fowler, C.J.; Doherty, P.; Alexander, S.P.H. Endocannabinoid turnover. Adv. Pharmacol. 2017, 80, 31–66. [Google Scholar] [CrossRef] [Green Version]
- Petrosino, S.; Di Marzo, V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef]
- Rankin, L. Chronic Pain. From the Study of Student Attitudes and Preferences to the In Vitro Investigation of a Novel Treatment Strategy. Ph.D. Thesis, Umeå University, Umeå, Sweden, 2020. Available online: https://umu.diva-portal.org/smash/get/diva2:1456215/FULLTEXT01.pdf (accessed on 23 September 2020).
- Korbecki, J.; Bajdak-Rusinek, K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popeijus, H.E.; van Otterdijk, S.D.; van der Krieken, S.E.; Konings, M.; Serbonij, K.; Plat, J.; Mensink, R.P. Fatty acid chain length and saturation influences PPARα transcriptional activation and repression in HepG2 cells. Mol. Nutr. Food Res. 2014, 58, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Davis, J.; Di Marzo, V. Palmitoylethanolamide enhances anandamide stimulation of human vanilloid VR1 receptors. FEBS Lett. 2001, 506, 253–256. [Google Scholar] [CrossRef]
- Smart, D.; Jonsson, K.-O.; Vandevoorde, S.; Lambert, D.M.; Fowler, C.J. ’Entourage’ effects of N-acyl ethanolamines at human vanilloid receptors. Comparison of effects upon anandamide-induced vanilloid receptor activation and upon anandamide metabolism. Br. J. Pharmacol. 2002, 136, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Ambrosino, P.; Soldovieri, M.V.; Russo, C.; Taglialatela, M. Activation and desensitization of TRPV1 channels in sensory neurons by the PPARα agonist palmitoylethanolamide. Br. J. Pharmacol. 2013, 168, 1430–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capasso, R.; Orlando, P.; Pagano, E.; Aveta, T.; Buono, L.; Borrelli, F.; Di Marzo, V.; Izzo, A.A. Palmitoylethanolamide normalizes intestinal motility in a model of post-inflammatory accelerated transit: Involvement of CB1 receptors and TRPV1 channels. Br. J. Pharmacol. 2014, 171, 4026–4037. [Google Scholar] [CrossRef] [Green Version]
- Marichal-Cancino, B.; González-Hernández, A.; MaassenVanDenBrink, A.; Ramírez-San Juan, E.; Villalón, C.M. Potential mechanisms involved in palmitoylethanolamide-induced vasodepressor effects in rats. J. Vasc. Res. 2020, 57, 152–163. [Google Scholar] [CrossRef]
- de Novellis, V.; Luongo, L.; Guida, F.; Cristino, L.; Palazzo, E.; Russo, R.; Marabese, I.; D’Agostino, G.; Calignano, A.; Rossi, F.; et al. Effects of intra-ventrolateral periaqueductal grey palmitoylethanolamide on thermoceptive threshold and rostral ventromedial medulla cell activity. Eur. J. Pharmacol. 2012, 676, 41–50. [Google Scholar] [CrossRef]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.-O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- Ross, R.A. The enigmatic pharmacology of GPR55. Trends Pharmacol. Sci. 2008, 30, 156–163. [Google Scholar] [CrossRef]
- Rinne, P.; Guillamat-Prats, R.; Rami, M.; Bindila, L.; Ring, L.; Lyytikäinen, L.P.; Raitoharju, E.; Oksala, N.; Lehtimäki, T.; Weber, C.; et al. Palmitoylethanolamide promotes a proresolving macrophage phenotype and attenuates atherosclerotic plaque formation. Arter. Thromb. Vasc. Biol. 2018, 38, 2562–2575. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, A.G.; Miskelly, M.G.; Moore, C.B.T.; Nesbit, M.A.; Christie, K.A.; Owolabi, A.I.; Flatt, P.R.; McKillop, A.M. CRISPR/Cas9 gene editing demonstrates metabolic importance of GPR55 in the modulation of GIP release and pancreatic beta cell function. Peptides 2020, 125, 170251. [Google Scholar] [CrossRef] [PubMed]
- Overton, H.; Babbs, A.; Doel, S.; Fyfe, M.; Gardner, L.; Griffin, G.; Jackson, H.; Proctor, M.; Rasamison, C.; Tang-Christensen, M.; et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006, 3, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauffer, L.M.; Iakoubov, R.; Brubaker, P.L. GPR119 Is Essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes 2009, 58, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- De Filippis, D.; Luongo, L.; Cipriano, M.; Palazzo, E.; Cinelli, M.P.; de Novellis, V.; Maione, S.; Iuvone, T. Palmitoylethanolamide reduces granuloma-induced hyperalgesia by modulation of mast cell activation in rats. Mol. Pain 2011, 7, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Filippis, D.; Russo, A.; De Stefano, D.; Cipriano, M.; Esposito, D.; Grassia, G.; Carnuccio, R.; Russo, G.; Iuvone, T. Palmitoylethanolamide inhibits rMCP-5 expression by regulating MITF activation in rat chronic granulomatous inflammation. Eur. J. Pharmacol. 2014, 725, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Facci, L.; Dal Toso, R.; Romanello, S.; Buriani, A.; Skaper, S.; Leon, A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc. Natl. Acad. Sci. USA 1995, 92, 3376–3380. [Google Scholar] [CrossRef] [Green Version]
- Granberg, M.; Fowler, C.J.; Jacobsson, S.O.P. Effects of the cannabimimetic fatty acid derivatives 2-arachidonoylglycerol, anandamide, palmitoylethanolamide and methanandamide upon IgE-dependent antigen-induced beta-hexosaminidase, serotonin and TNFα release from rat RBL-2H3 basophilic leukaemic cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2001, 364, 66–73. [Google Scholar] [CrossRef]
- Fowler, C.J.; Sandberg, M.; Tiger, G. Effects of water-soluble cigarette smoke extracts upon the release of ß-hexosaminidase from RBL-2H3 basophilic leukaemia cells in response to substance P, compound 48/80, concanavalin A and antigen stimulation. Inflamm. Res. 2003, 52, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, C.M.S.; Foreman, J.C.; Jordan, C.C.; Oehme, P.; Rénner, H.; Stewart, J.M. The effects of substance P on histamine and 5-hydroxytryptamine release in the rat. J. Physiol. 1982, 330, 393–411. [Google Scholar] [CrossRef]
- Abramo, F.; Lazzarini, G.; Pirone, A.; Lenzi, C.; Albertini, S.; della Valle, M.F.; Schievano, C.; Vannozzi, I.; Miragliotta, V. Ultramicronized palmitoylethanolamide counteracts the effects of compound 48/80 in a canine skin organ culture model. Vet. Dermatol. 2017, 28, 456-e104. [Google Scholar] [CrossRef] [Green Version]
- Nestmann, E.R. Safety of micronized palmitoylethanolamide (microPEA): Lack of toxicity and genotoxic potential. Food Sci. Nutr. 2016, 5, 292–309. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; Lippman-Hand, A. If nothing goes wrong, Is everything all right? Interpreting zero numerators. JAMA 1983, 249, 1743–1745. [Google Scholar] [CrossRef] [PubMed]
- Kahlich, R.; Klíma, J.; Cihla, F.; Franková, V.; Masek, K.; Rosicky, M.; Matousek, F.; Bruthans, J. Studies on prophylactic efficacy of N-2-hydroxyethyl palmitamide (Impulsin) in acute respiratory infections. Serologically controlled field trials. J. Hyg. Epidemiol. Microbiol. Immunol. 1979, 23, 11–24. [Google Scholar]
- Cruccu, G.; Stefano, G.D.; Marchettini, P.; Truini, A. micronized palmitoylethanolamide: A post hoc analysis of a controlled study in patients with low back pain—Sciatica. CNS Neurol. Disord. Drug Targets 2019, 18, 491–495. [Google Scholar] [CrossRef]
- Stockings, E.; Campbell, G.; Hall, W.D.; Nielsen, S.; Zagic, D.; Rahman, R.; Murnion, B.; Farrell, M.; Weier, M.; Degenhardt, L. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: A systematic review and meta-analysis of controlled and observational studies. Pain 2018, 159, 1932–1954. [Google Scholar] [CrossRef] [PubMed]
- Steels, E.; Venkatesh, R.; Steels, E.; Vitetta, G.; Vitetta, L. A double-blind randomized placebo controlled study assessing safety, tolerability and efficacy of palmitoylethanolamide for symptoms of knee osteoarthritis. Inflammopharmacology 2019, 27, 475–485. [Google Scholar] [CrossRef]
- Andresen, S.R.; Bing, J.; Hansen, R.M.; Biering-Sørensen, F.; Johannesen, I.L.; Hagen, E.M.; Rice, A.S.C.; Nielsen, J.F.; Bach, F.W.; Finnerup, N.B. Ultramicronized palmitoylethanolamide in spinal cord injury neuropathic pain: A randomized, double-blind, placebo-controlled trial. Pain 2016, 157, 2097–2103. [Google Scholar] [CrossRef] [Green Version]
- Ghazizadeh-Hashemi, M.; Ghajar, A.; Shalbafan, M.R.; Ghazizadeh-Hashemi, F.; Afarideh, M.; Malekpour, F.; Ghaleiha, A.; Ardebili, M.E.; Akhondzadeh, S. Palmitoylethanolamide as adjunctive therapy in major depressive disorder: A double-blind, randomized and placebo-controlled trial. J. Affect. Disord. 2018, 232, 127–133. [Google Scholar] [CrossRef]
- Strobbe, E.; Cellini, M.; Campos, E.C. Effectiveness of palmitoylethanolamide on endothelial dysfunction in ocular hypertensive patients: A randomized, placebo-controlled cross-over study. Investig. Opthalmology Vis. Sci. 2013, 54, 968–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gugliandolo, E.; Peritore, A.F.; Piras, C.; Cuzzocrea, S.; Crupi, R. Palmitoylethanolamide and related aliamides: Prohomeostatic lipid compounds for animal health and wellbeing. Vet. Sci. 2020, 7, 78. [Google Scholar] [CrossRef]
- Bruun, S.; Gouveia-Figueira, S.; Domellöf, M.; Husby, S.; Neergaard Jacobsen, L.; Michaelsen, K.F.; Fowler, C.J.; Zachariassen, G. Satiety factors oleoylethanolamide, stearoylethanolamide, and palmitoylethanolamide in mother’s milk are strongly associated with infant weight at four months of age-data from the Odense child cohort. Nutrients 2018, 10, 1747. [Google Scholar] [CrossRef]
| Reference | Model | Species Strain Genus | Wt (g) | PEA Dose | PPAR-α Involvement | ||
|---|---|---|---|---|---|---|---|
| Aldossary et al. [58] | Inflammatory pain. Complete Freund’s adjuvant hind paw injections, Von Frey paw withdrawal | R | S-D a | Male | 180–250 | 50 µg i.pl. | Effect mimicked by WY12643 and reduced by GW6471 |
| Alsalem et al. [59] | Osteoarthritis. Monosodium iodoacetate (MIA) in knee joint. Von Frey paw withdrawal | R | S-D | Male | 180–250 | 50 µg Intra-articular injection | GW6471 reversed anti-nociceptive effects of PEA |
| Borrelli et al. [60] | 2,4-dinitrobenzene-sulfonic acid induced colitis | M | ICR | Male | 25–30 | 0.1–10 mg⋅kg−1 i.p or p.o | GW6471 reversed anti-inflammatory effects of PEA (as did GPR55 and CB1 antagonists) |
| Costa et al. [61] | Neuropathic pain. Chronic constriction injury of sciatic nerve.Thermal hyperalgesia | M | C57BL/6J | Male | 25–30 | 10 mg/kg i.p. | GW6471 reversed PEA-induced anti-hyperalgesia (as did antagonists for CB1, PPAR-γ and TRPV1) |
| D’Agostino et al. [62] | Carrageenan-induced paw oedema | M | Swiss | Male | 20–25 | 0.01–1 µg i.c.v | Effect mimicked by GW7647 |
| D’Agostino et al. [63] | Carrageenan-induced paw hyperalgesia. Paw withdrawal. | M | Swiss | Male | 20–25 | 0.1–1 µg i.c.v | Effect mimicked by GW7647 |
| Di Cesare Mannelli et al. [64] | Peripheral neuropathy. Chronic constriction injury of sciatic nerve; mechanical allodynia and hyperalgesia | M | B6.129S4-SvJae-P paratm1Gonz | Male | - | 30 mg⋅kg−1 –0.3 mL s.c. | PPAR-α−/− mice |
| Di Paola et al. [65] | Inflammation after renal ischaemia–reperfusion injury | M | CD1 | - | 25–30 | 10 mg/kg i.p. | PPAR-α−/− mice. |
| Di Paola et al. [66] | Model of myocardial ischemia reperfusion injury | R | Wistar | Male | 250–300 | 10 mg/kg i.p. | PPAR-α−/− mice |
| Donvito et al. [67] | Paclitaxel-induced allodynia | M | ICR | Male | 18–35 | 30 mg/kg i.p. | Antagonism by GW6471 |
| Esposito et al. [68] | Inflammatory model of Parkinson’s disease | M | - | Male | 20–27 | 10 mg/kg, i.p | PPAR-α−/− mice |
| Esposito et al. [69] | Dextran sodium sulphate-induced colitis | M | CD1 | Male | 6 weeks old | 2, 10 or 50 mg/kg i.p. | Antagonism by MK866 |
| Impellizzeri et al. [70] | Streptozotocin-induced diabetic peripheral neuropathy | M | CD1 | Male | 18–22 | 10 mg/kg i.p. | PPAR-α−/− mice |
| Lo Verme et al. [46] | Carrageenan-induced paw oedema and phorbol ester-induced ear oedema | M | C57BL6 | Male | 25–30 g | 10 mg/kg i.p | PPAR-α−/− mice Also mimicked by PPAR-α agonists OEA, GW7647, and Wy-14643 |
| LoVerme et al. [47] | Sciatic nerve ligation, arthritis induced by Freund’s adjuvant, Carrageenan-induced paw oedema | M + R | Swiss mice and S-D rats | Male | - | 20 mg/kg s.c., 50 µg i.pl or 30 mg/kg i.p. | PPAR-α−/− mice Mimicked by GW7647 |
| Paterniti et al. [71] | Spinal cord injury (SCI) | M | - | - | 20–27 | 10 mg/kg i.p. | PPAR-α−/− mice. Also involvement of PPARs -δ and -γ |
| Sarnelli et al. [72] | Dextran sodium sulphate-induced colitis. Inflammation-associated angiogenesis | M | CD1 | Male | - | 2 and 10 mg/kg | PPAR-α−/− mice |
| Vaia et al. [55] | Model of contact allergic dermatitis | M | C57BL/6J | Female | 25–30 | 5 mg/kg i.p. | Ear scratches but not ear thickness was reduced by GW6471 |
| Ye et al. [73] | Pathological neovascularisation and fibrosis in oxygen induced retinopathy model | M | C57BL/6J | - | - | 30 mg/kg i.p. | PPAR-α−/− mice |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rankin, L.; Fowler, C.J. The Basal Pharmacology of Palmitoylethanolamide. Int. J. Mol. Sci. 2020, 21, 7942. https://doi.org/10.3390/ijms21217942
Rankin L, Fowler CJ. The Basal Pharmacology of Palmitoylethanolamide. International Journal of Molecular Sciences. 2020; 21(21):7942. https://doi.org/10.3390/ijms21217942
Chicago/Turabian StyleRankin, Linda, and Christopher J. Fowler. 2020. "The Basal Pharmacology of Palmitoylethanolamide" International Journal of Molecular Sciences 21, no. 21: 7942. https://doi.org/10.3390/ijms21217942