Comparison of Theiler’s Murine Encephalomyelitis Virus Induced Spinal Cord and Peripheral Nerve Lesions Following Intracerebral and Intraspinal Infection
Abstract
:1. Introduction
2. Results
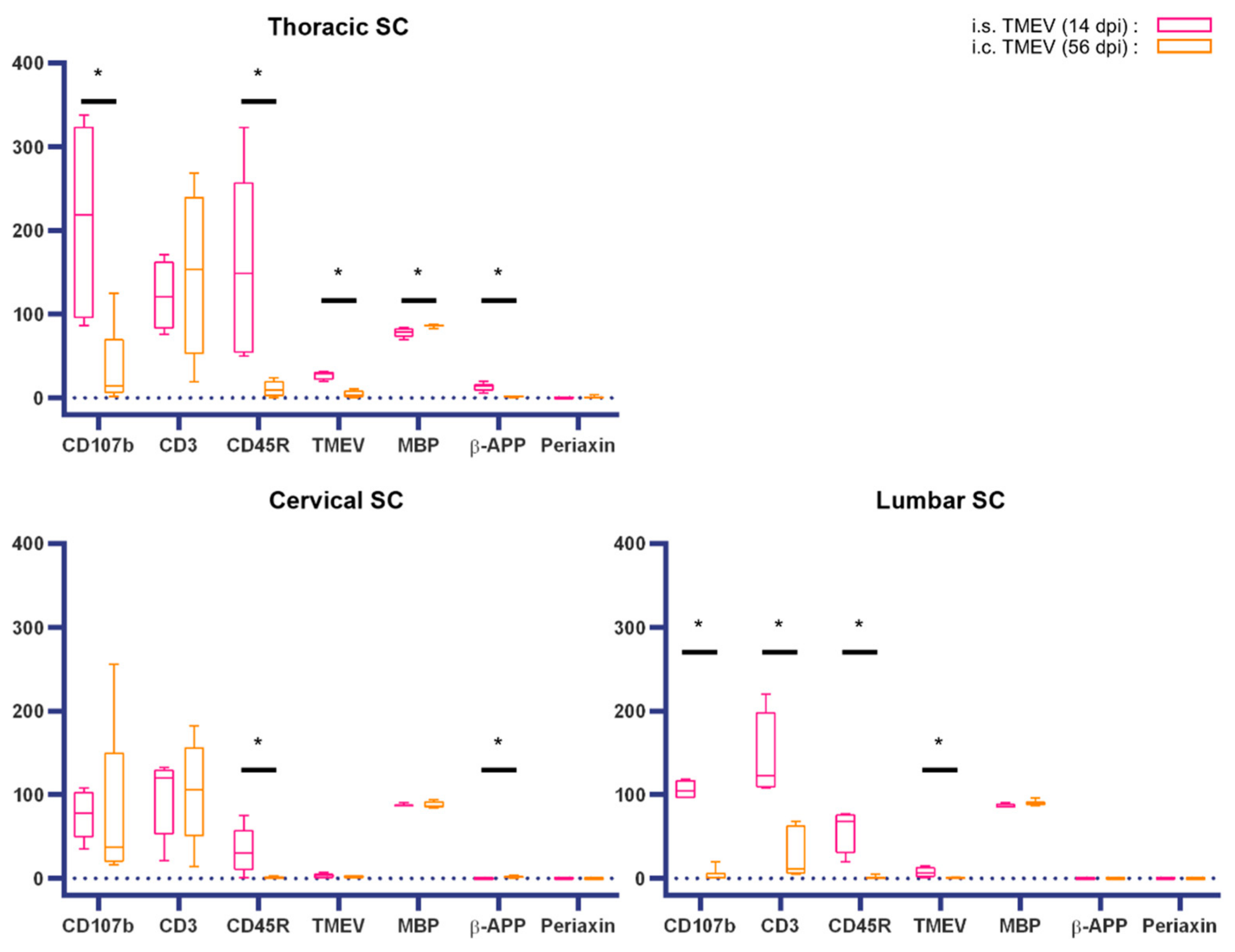
2.1. Intraspinal TMEV Infection
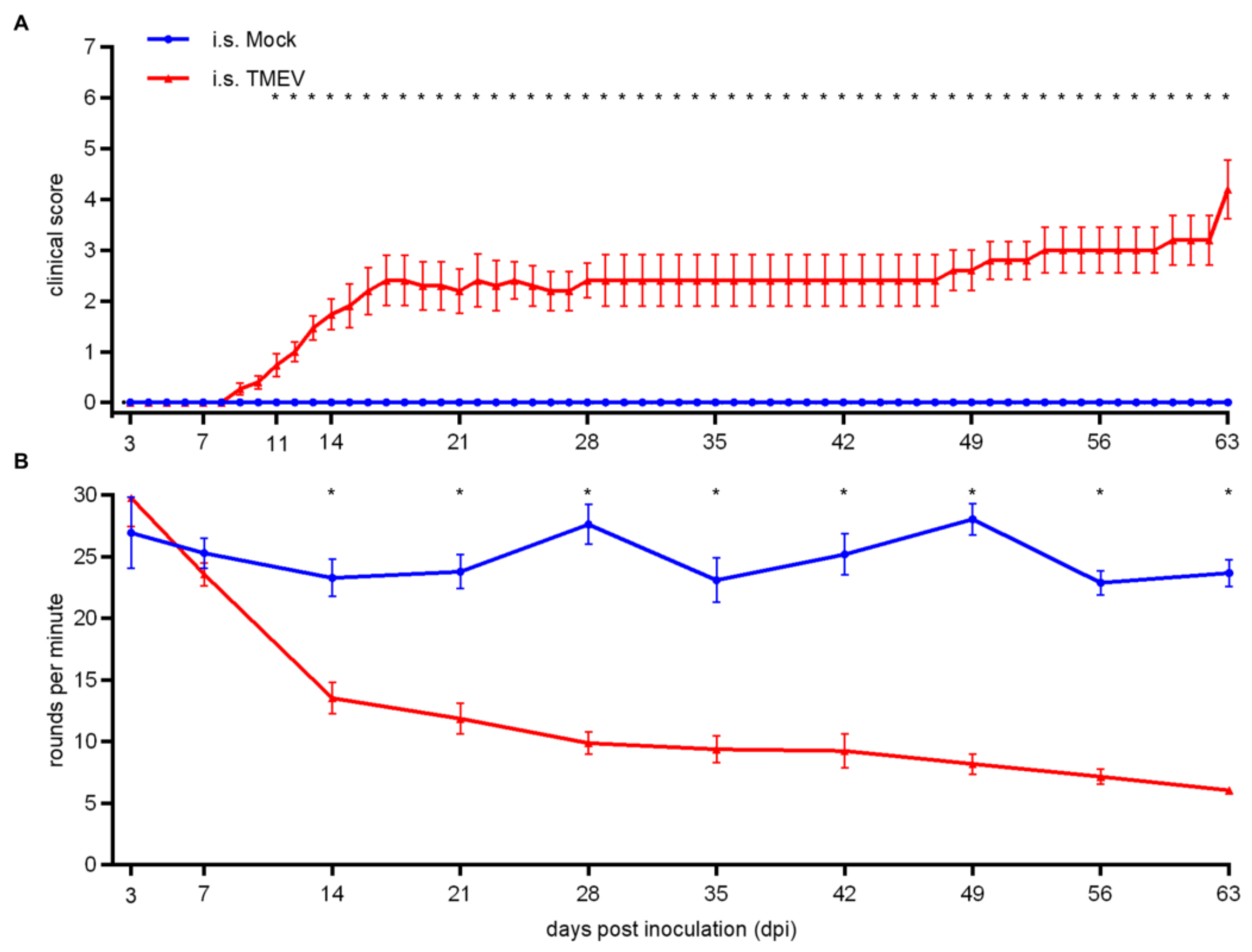
2.1.1. Clinical Investigation
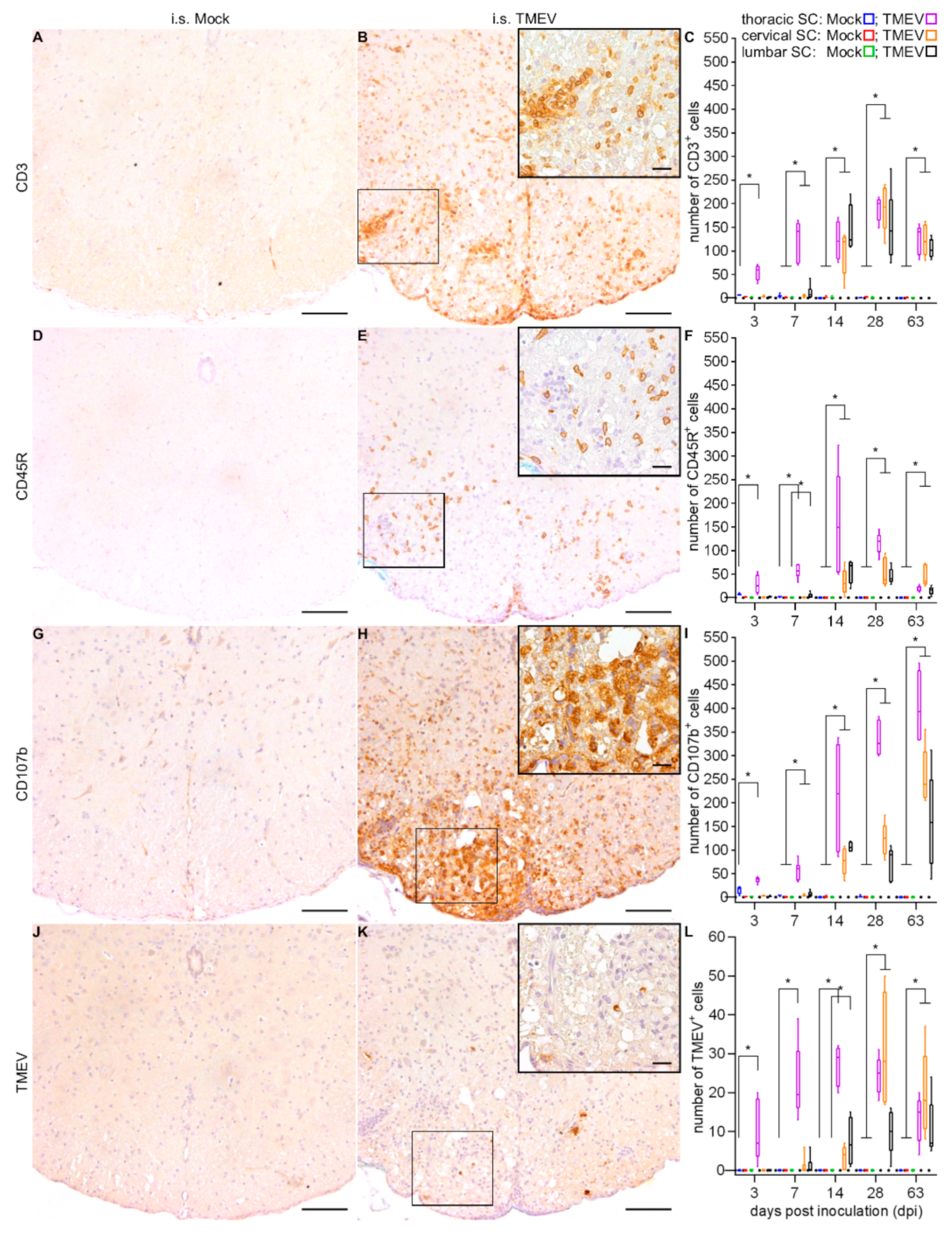
2.1.2. Spinal Cord Lesions
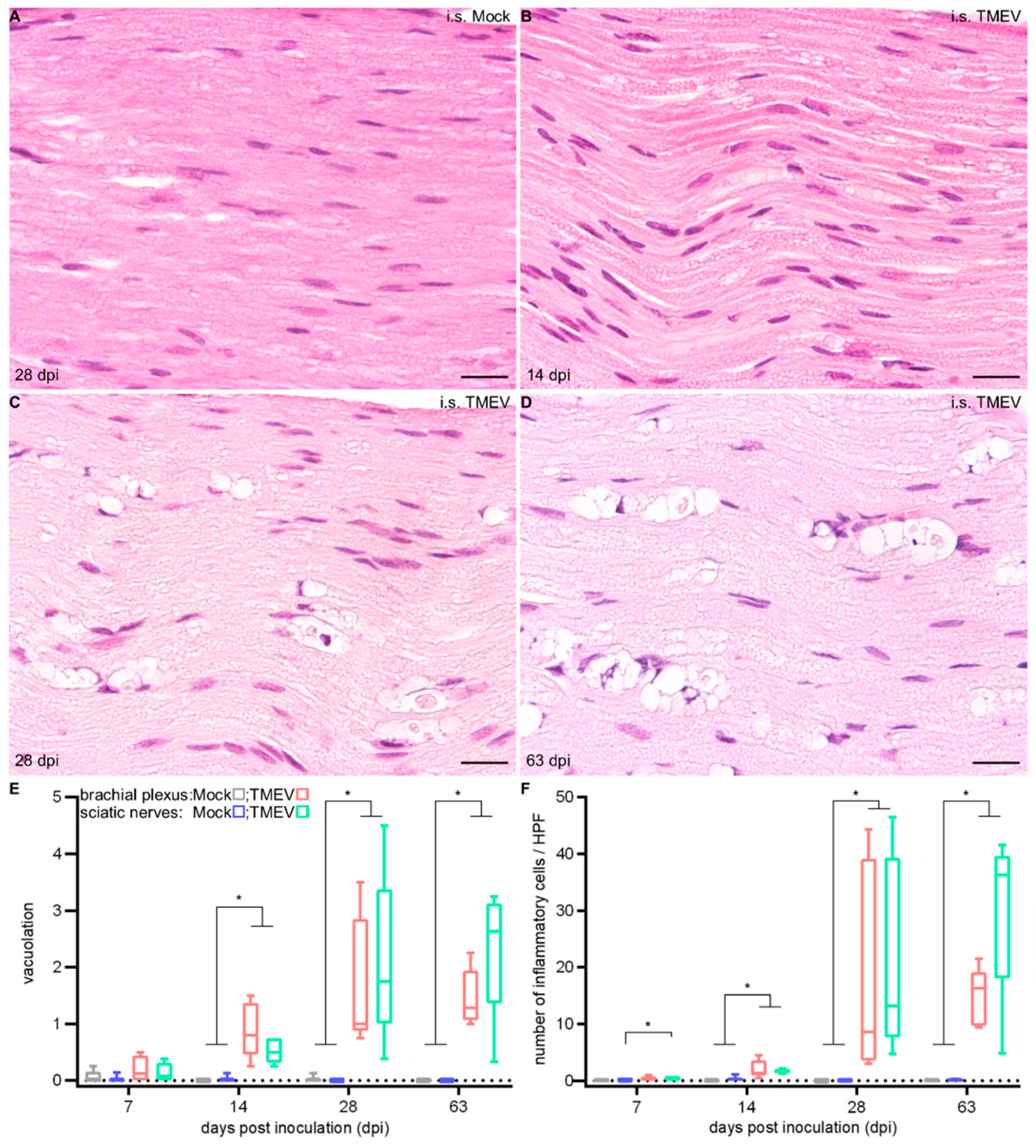
2.1.3. Peripheral Nerve Lesions
2.2. Intracerebral TMEV Infection
2.2.1. Clinical Investigation
2.2.2. Spinal Cord Lesions
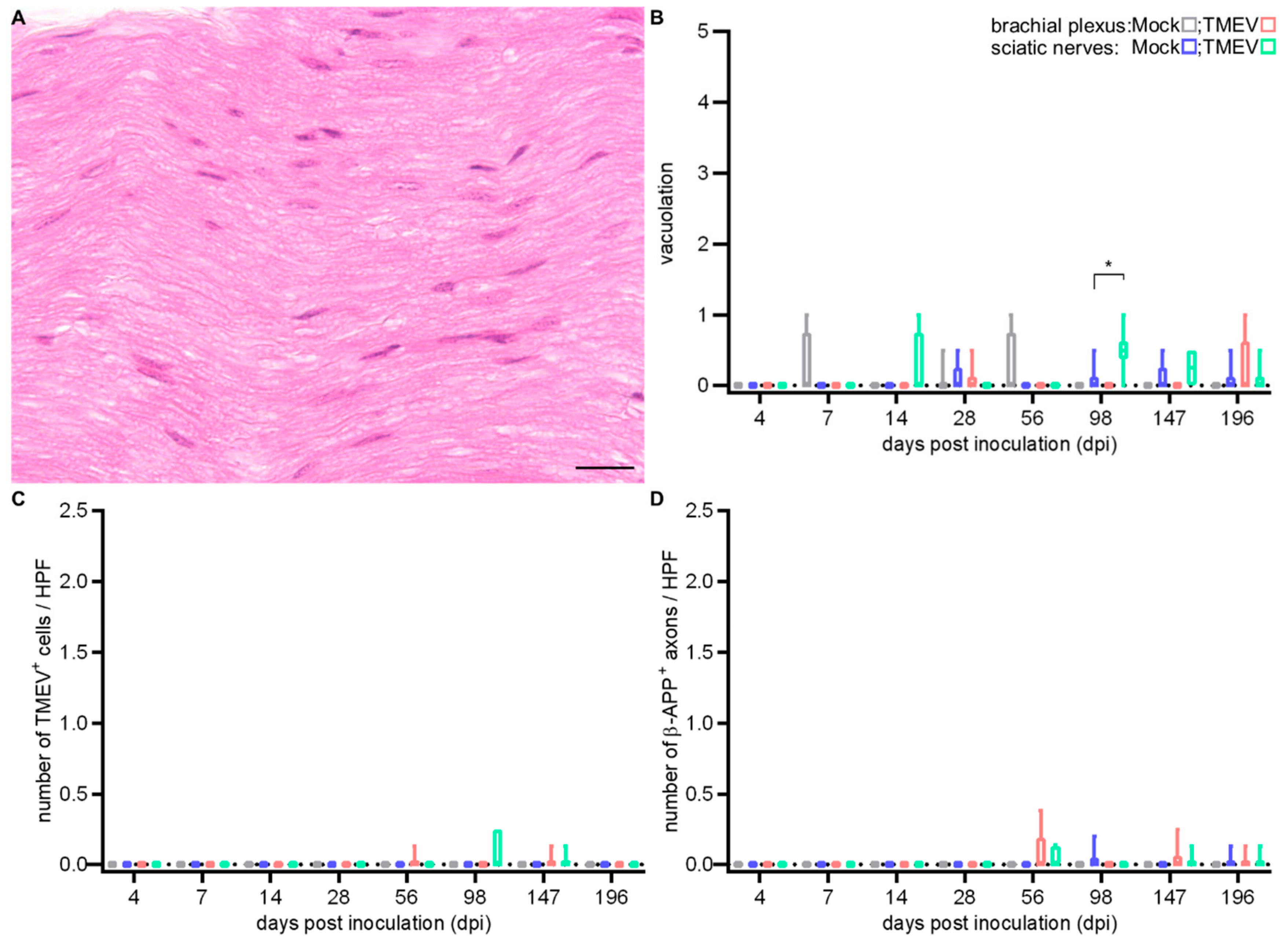
2.2.3. Peripheral Nerve Lesions
3. Discussion
4. Materials and Methods
4.1. Experimental Animals, Virus Infection and Tissue Processing
4.2. Clinical Investigations
4.3. Immunohistochemistry
4.4. Histological Examination
4.5. Statistical Analysis
4.6. Ethics Statement
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| β-APP | β-amyloid precursor protein |
| CNS | central nervous system |
| Crl | Charles River Laboratories |
| DA strain | Daniel’s strain |
| FFPE | formalin-fixed paraffin-embedded tissue |
| HE | hematoxylin and eosin |
| HPF | high power field |
| I.c. | Intracerebral |
| I.s. | Intraspinal |
| MBP | myelin basic protein |
| MS | Multiple sclerosis |
| NG2 | nerve/glial antigen 2 |
| PFU | plaque forming units |
| PN | peripheral nerves |
| PNS | peripheral nervous system |
| ROI | region of interest |
| SJL | Swiss Jim Lambert |
| SC | spinal cord |
| TMEV | Theiler′s murine encephalomyelitis virus |
| TMEV-IDD | Theiler´s murine encephalomyelitis virus-induced demyelinating disease |
References
- Pevear, D.C.; Calenoff, M.; Rozhon, E.; Lipton, H.L. Analysis of the complete nucleotide sequence of the picornavirus Theiler’s murine encephalomyelitis virus indicates that it is closely related to cardioviruses. J. Virol. 1987, 61, 1507–1516. [Google Scholar] [PubMed]
- Dal Canto, M.C.; Lipton, H.L. Primary demyelination in Theilers virus-infection. An ultrastructural study. Lab. Invest. 1975, 33, 626–637. [Google Scholar] [PubMed]
- Lindsley, M.D.; Rodriguez, M. Characterization of the inflammatory response in the central nervous system of mice susceptible or resistant to demyelination by Theiler’s virus. J. Immunol. 1989, 142, 2677–2682. [Google Scholar] [PubMed]
- Lipton, H.L.; Dal Canto, M.C. The TO strains of Theiler’s viruses cause “slow virus-like” infections in mice. Ann. Neurol. 1979, 6, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Mecha, M.; Carrillo-Salinas, F.J.; Mestre, L.; Feliu, A.; Guaza, C. Viral models of multiple sclerosis: Neurodegeneration and demyelination in mice infected with Theiler’s virus. Prog. Neurobiol. 2013, 101–102, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, I.; Sato, F.; Omura, S.; Fujita, M.; Sakiyama, N.; Park, A.M. Three immune-mediated disease models induced by Theiler’s virus: Multiple sclerosis, seizures and myocarditis. Clin. Exp. Neuroimmunol. 2016, 7, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, I.; Hansmann, F.; Ciurkiewicz, M.; Löscher, W.; Beineke, A. Facets of Theiler’s Murine Encephalomyelitis Virus-Induced Diseases: An Update. Int. J. Mol. Sci. 2019, 20, 448. [Google Scholar] [CrossRef]
- Dal Canto, M.C.; Kim, B.S.; Miller, S.D.; Melvold, R.W. Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelination: A model for human multiple sclerosis. Methods 1996, 10, 453–461. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Trapp, B.D.; Nave, K.A. Multiple sclerosis: An immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008, 31, 247–269. [Google Scholar] [CrossRef]
- He, D.; Guo, R.; Zhang, F.; Zhang, C.; Dong, S.; Zhou, H. Rituximab for relapsing-remitting multiple sclerosis. Cochrane Database Syst. Rev. 2013, 12, CD009130. [Google Scholar] [CrossRef] [PubMed]
- Iannazzo, S.; Iliza, A.C.; Perrault, L. Disease-Modifying Therapies for Multiple Sclerosis: A Systematic Literature Review of Cost-Effectiveness Studies. Pharmacoeconomics 2018, 36, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Ozakbas, S.; Cinar, B.P.; Oz, D.; Kosehasanogullari, G.; Kursun, B.B.; Kahraman, T. Monthly Pulse Methylprednisolone Therapy is Effective in Preventing Permanent Disease Progression in Secondary Progressive Multiple Sclerosis. Noro Psikiyatr. Ars. 2019, 56, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Ciotti, J.R.; Cross, A.H. Disease-Modifying Treatment in Progressive Multiple Sclerosis. Curr. Treat. Options Neurol. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Leitzen, E.; Jin, W.; Herder, V.; Beineke, A.; Elmarabet, S.A.; Baumgärtner, W.; Hansmann, F. Comparison of Reported Spinal Cord Lesions in Progressive Multiple Sclerosis with Theiler’s Murine Encephalomyelitis Virus Induced Demyelinating Disease. Int. J. Mol. Sci. 2019, 20, 989. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, I.; Li, L.; Li, D.; Klein, S.; Elmarabet, S.A.; Deschl, U.; Kalkuhl, A.; Baumgartner, W.; Ulrich, R.; Beineke, A. Dynamic changes and molecular analysis of cell death in the spinal cord of SJL mice infected with the BeAn strain of Theiler’s murine encephalomyelitis virus. Apoptosis 2018, 23, 170–186. [Google Scholar] [CrossRef]
- Ulrich, R.; Seeliger, F.; Kreutzer, M.; Germann, P.G.; Baumgärtner, W. Limited remyelination in Theiler’s murine encephalomyelitis due to insufficient oligodendroglial differentiation of nerve/glial antigen 2 (NG2)-positive putative oligodendroglial progenitor cells. Neuropathol. Appl. Neurobiol. 2008, 34, 603–620. [Google Scholar] [CrossRef]
- Oleszak, E.L.; Chang, J.R.; Friedman, H.; Katsetos, C.D.; Platsoucas, C.D. Theiler’s virus infection: A model for multiple sclerosis. Clin. Microbiol. Rev. 2004, 17, 174–207. [Google Scholar] [CrossRef]
- Lipton, H.L.; Dal Canto, M.C. Susceptibility of inbred mice to chronic central nervous system infection by Theiler’s murine encephalomyelitis virus. Infect. Immun. 1979, 26, 369–374. [Google Scholar]
- Brahic, M.; Bureau, J.F.; Michiels, T. The genetics of the persistent infection and demyelinating disease caused by Theiler’s virus. Annu. Rev. Microbiol. 2005, 59, 279–298. [Google Scholar] [CrossRef]
- Drescher, K.M.; Tracy, S. Establishment of a model to examine the early events involved in the development of virus-induced demyelinating lesions. Ann. N. Y. Acad. Sci. 2007, 1103, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Leitzen, E.; Raddatz, B.B.; Jin, W.; Goebbels, S.; Nave, K.A.; Baumgärtner, W.; Hansmann, F. Virus-triggered spinal cord demyelination is followed by a peripheral neuropathy resembling features of Guillain-Barre Syndrome. Sci. Rep. 2019, 9, 4588. [Google Scholar] [CrossRef] [PubMed]
- Theiler, M. Spontaneous Encephalomyelitis of Mice, a New Virus Disease. J. Exp. Med. 1937, 65, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, D.; Young, C.R.; Storts, R.; Ting, J.W.; Welsh, C.J. A comparison of the neurotropism of Theiler’s virus and poliovirus in CBA mice. Microb. Pathog. 2006, 41, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Theiler, M.; Gard, S. Encephalomyelitis of Mice: I. Characteristics and Pathogenesis of the Virus. J. Exp. Med. 1940, 72, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Rustigian, R.; Pappenheimer, A.M. Myositis in mice following intramuscular injection of viruses of the mouse encephalomyelitis group and of certain other neurotropic viruses. J. Exp. Med. 1949, 89, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Drescher, K.M.; Tracy, S.M. Injection of the sciatic nerve with TMEV: A new model for peripheral nerve demyelination. Virology 2007, 359, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Martinat, C.; Jarousse, N.; Prevost, M.C.; Brahic, M. The GDVII strain of Theiler’s virus spreads via axonal transport. J. Virol. 1999, 73, 6093–6098. [Google Scholar]
- Panos, M.; Christophi, G.P.; Rodriguez, M.; Scarisbrick, I.A. Differential expression of multiple kallikreins in a viral model of multiple sclerosis points to unique roles in the innate and adaptive immune response. Biol. Chem. 2014, 395, 1063–1073. [Google Scholar] [CrossRef] [Green Version]
- Lassmann, H.; Bradl, M. Multiple sclerosis: Experimental models and reality. Acta Neuropathol. 2017, 133, 223–244. [Google Scholar] [CrossRef]
- Tsunoda, I.; Kuang, L.Q.; Libbey, J.E.; Fujinami, R.S. Axonal injury heralds virus-induced demyelination. Am. J. Pathol. 2003, 162, 1259–1269. [Google Scholar] [CrossRef]
- Dal Canto, M.C.; Calenoff, M.A.; Miller, S.D.; Vanderlugt, C.L. Lymphocytes from mice chronically infected with Theiler’s murine encephalomyelitis virus produce demyelination of organotypic cultures after stimulation with the major encephalitogenic epitope of myelin proteolipid protein. Epitope spreading in TMEV infection has functional activity. J. Neuroimmunol 2000, 104, 79–84. [Google Scholar] [PubMed]
- Katz-Levy, Y.; Neville, K.L.; Padilla, J.; Rahbe, S.; Begolka, W.S.; Girvin, A.M.; Olson, J.K.; Vanderlugt, C.L.; Miller, S.D. Temporal development of autoreactive Th1 responses and endogenous presentation of self myelin epitopes by central nervous system-resident APCs in Theiler’s virus-infected mice. J. Immunol. 2000, 165, 5304–5314. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.D.; Vanderlugt, C.L.; Begolka, W.S.; Pao, W.; Yauch, R.L.; Neville, K.L.; Katz-Levy, Y.; Carrizosa, A.; Kim, B.S. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat. Med. 1997, 3, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.K.; Miller, S.D. The Role of T Cells and the Innate Immune System in the Pathogenesis of Theiler’s Virus Demyeliating Disease. In Experimental Models of Multiple Sclerosis; Lavi, E., Constantinescu, C.S., Eds.; Springer: Boston, MS, USA, 2005; pp. 645–657. [Google Scholar]
- Croxford, J.L.; Olson, J.K.; Anger, H.A.; Miller, S.D. Initiation and exacerbation of autoimmune demyelination of the central nervous system via virus-induced molecular mimicry: Implications for the pathogenesis of multiple sclerosis. J. Virol. 2005, 79, 8581–8590. [Google Scholar] [CrossRef] [PubMed]
- Croxford, J.L.; Olson, J.K.; Miller, S.D. Epitope spreading and molecular mimicry as triggers of autoimmunity in the Theiler’s virus-induced demyelinating disease model of multiple sclerosis. Autoimmun. Rev. 2002, 1, 251–260. [Google Scholar] [CrossRef]
- Lipton, H.L.; Dal Canto, M.C. Theiler’s virus-induced demyelination: Prevention by immunosuppression. Science 1976, 192, 62–64. [Google Scholar] [CrossRef]
- Ure, D.R.; Rodriguez, M. Preservation of neurologic function during inflammatory demyelination correlates with axon sparing in a mouse model of multiple sclerosis. Neuroscience 2002, 111, 399–411. [Google Scholar] [CrossRef]
- Lipton, H.L. Theiler’s virus infection in mice: An unusual biphasic disease process leading to demyelination. Infect. Immun. 1975, 11, 1147–1155. [Google Scholar]
- Huang, W.; Zhao, N.; Bai, X.; Karram, K.; Trotter, J.; Goebbels, S.; Scheller, A.; Kirchhoff, F. Novel NG2-CreERT2 knock-in mice demonstrate heterogeneous differentiation potential of NG2 glia during development. Glia 2014, 62, 896–913. [Google Scholar] [CrossRef]
- Nishiyama, A.; Boshans, L.; Goncalves, C.M.; Wegrzyn, J.; Patel, K.D. Lineage, fate, and fate potential of NG2-glia. Brain Res. 2016, 1638, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Komitova, M.; Suzuki, R.; Zhu, X. Polydendrocytes (NG2 cells): Multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 2009, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Leone, D.P.; Genoud, S.; Atanasoski, S.; Grausenburger, R.; Berger, P.; Metzger, D.; Macklin, W.B.; Chambon, P.; Suter, U. Tamoxifen-inducible glia-specific Cre mice for somatic mutagenesis in oligodendrocytes and Schwann cells. Mol. Cell. Neurosci. 2003, 22, 430–440. [Google Scholar] [CrossRef]
- Levine, J. The reactions and role of NG2 glia in spinal cord injury. Brain Res. 2016, 1638, 199–208. [Google Scholar] [CrossRef]
- Zawadzka, M.; Rivers, L.E.; Fancy, S.P.; Zhao, C.; Tripathi, R.; Jamen, F.; Young, K.; Goncharevich, A.; Pohl, H.; Rizzi, M.; et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 2010, 6, 578–590. [Google Scholar] [CrossRef]
- Rodriguez, J.P.; Coulter, M.; Miotke, J.; Meyer, R.L.; Takemaru, K.; Levine, J.M. Abrogation of beta-catenin signaling in oligodendrocyte precursor cells reduces glial scarring and promotes axon regeneration after CNS injury. J. Neurosci. 2014, 34, 10285–10297. [Google Scholar] [CrossRef]
- Sun, Y.; Lehmbecker, A.; Kalkuhl, A.; Deschl, U.; Sun, W.; Rohn, K.; Tzvetanova, I.D.; Nave, K.A.; Baumgärtner, W.; Ulrich, R. STAT3 represents a molecular switch possibly inducing astroglial instead of oligodendroglial differentiation of oligodendroglial progenitor cells in Theiler’s murine encephalomyelitis. Neuropathol. Appl. Neurobiol. 2015, 41, 347–370. [Google Scholar] [CrossRef]
- Weller, A.H.; Magliato, S.A.; Bell, K.P.; Rosenberg, N.L. Spontaneous myopathy in the SJL/J mouse: Pathology and strength loss. Muscle Nerve 1997, 20, 72–82. [Google Scholar] [CrossRef]
- Chow, E.Y.; Ho, F.C. Age-related changes in the morphology and immunophenotype of spontaneous lymphomas of SJL/N mice. J. Pathol. 1988, 156, 331–339. [Google Scholar] [CrossRef]
- Dhib-Jalbut, S. Pathogenesis of myelin/oligodendrocyte damage in multiple sclerosis. Neurology 2007, 68, S13–S21; discussion S43–S54. [Google Scholar] [CrossRef]
- Holmoy, T.; Hestvik, A.L. Multiple sclerosis: Immunopathogenesis and controversies in defining the cause. Curr. Opin. Infect. Dis. 2008, 21, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Lisak, R.P. Neurodegeneration in multiple sclerosis: Defining the problem. Neurology 2007, 68, S5–S12; discussion S43–S54. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Tsirka, S.E. Animal Models of MS Reveal Multiple Roles of Microglia in Disease Pathogenesis. Neurol. Res. Int. 2011, 2011, 383087. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.L.; Jin, F.; Pirko, I.; Johnson, A.J. Theiler’s murine encephalomyelitis virus as an experimental model system to study the mechanism of blood-brain barrier disruption. J. Neurovirol. 2014, 20, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Kreutzer, M.; Seehusen, F.; Kreutzer, R.; Pringproa, K.; Kummerfeld, M.; Claus, P.; Deschl, U.; Kalkul, A.; Beineke, A.; Baumgärtner, W.; et al. Axonopathy is associated with complex axonal transport defects in a model of multiple sclerosis. Brain Pathol. 2012, 22, 454–471. [Google Scholar] [CrossRef]
- Tsunoda, I.; Fujinami, R.S. Inside-Out versus Outside-In models for virus induced demyelination: Axonal damage triggering demyelination. Springer Semin. Immunopathol. 2002, 24, 105–125. [Google Scholar] [CrossRef]
- Libbey, J.E.; Lane, T.E.; Fujinami, R.S. Axonal pathology and demyelination in viral models of multiple sclerosis. Discov. Med. 2014, 18, 79–89. [Google Scholar]
- Navarrete-Talloni, M.J.; Kalkuhl, A.; Deschl, U.; Ulrich, R.; Kummerfeld, M.; Rohn, K.; Baumgärtner, W.; Beineke, A. Transient peripheral immune response and central nervous system leaky compartmentalization in a viral model for multiple sclerosis. Brain Pathol. 2010, 20, 890–901. [Google Scholar] [CrossRef]
- Lassmann, H.; Brück, W.; Lucchinetti, C.F. The immunopathology of multiple sclerosis: An overview. Brain Pathol. 2007, 17, 210–218. [Google Scholar] [CrossRef]
- Fitzner, D.; Simons, M. Chronic progressive multiple sclerosis - pathogenesis of neurodegeneration and therapeutic strategies. Curr. Neuropharmacol. 2010, 8, 305–315. [Google Scholar] [CrossRef]
- Andersson, K.B.; Winer, L.H.; Mork, H.K.; Molkentin, J.D.; Jaisser, F. Tamoxifen administration routes and dosage for inducible Cre-mediated gene disruption in mouse hearts. Transgenic Res. 2010, 19, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, F.; Herder, V.; Kalkuhl, A.; Haist, V.; Zhang, N.; Schaudien, D.; Deschl, U.; Baumgärtner, W.; Ulrich, R. Matrix metalloproteinase-12 deficiency ameliorates the clinical course and demyelination in Theiler’s murine encephalomyelitis. Acta Neuropathol. 2012, 124, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, F.; Jungwirth, N.; Zhang, N.; Skripuletz, T.; Stein, V.M.; Tipold, A.; Stangel, M.; Baumgärtner, W. Beneficial and detrimental impact of transplanted canine adipose-derived stem cells in a virus-induced demyelinating mouse model. Vet. Immunol. Immunopathol. 2018, 202, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Kummerfeld, M.; Meens, J.; Haas, L.; Baumgärtner, W.; Beineke, A. Generation and characterization of a polyclonal antibody for the detection of Theiler’s murine encephalomyelitis virus by light and electron microscopy. J. Virol. Methods 2009, 160, 185–188. [Google Scholar] [CrossRef] [PubMed]





| 1st Antibody | Pre-Treatment | Blocking Serum | 2nd Antibody | ||
|---|---|---|---|---|---|
| Antigen, Target | Product Name | Dilution | |||
| CD3, T lymphocytes | Dako A0452 | 1:250 | microwave, citrate buffer | goat | goat anti-rabbit |
| CD107b, microglia/macrophages | BioRad MCA2293 | 1:400 | microwave, citrate buffer | rabbit | rabbit anti-rat |
| CD45R, B lymphocytes | BD Bioscience 553085 | 1:1000 | microwave, citrate buffer | - | - |
| Capsid protein 1 (VP1), TMEV | [65] | 1:2000 | - | goat | goat anti-rabbit |
| Myelin basic protein (MBP), myelin | Chemicon AB980 GeneTex GTX32733 | 1:500 1:250 | - - | goat | goat anti-rabbit |
| Beta-amyloid precursor protein (β-APP), axonal damage | Merck/Millipore MAB348 | 1:2000 | microwave, citrate buffer | goat | goat anti-mouse |
| Periaxin, Schwann cells | Sigma HPA001868 | 1:5000 | microwave, citrate buffer | goat | goat anti-rabbit |
| LivingColor DsRed, Tdtomato | Takara/Clontech 632496 | 1:500 | microwave, citrate buffer | goat | goat anti-rabbit |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, W.; Leitzen, E.; Goebbels, S.; Nave, K.-A.; Baumgärtner, W.; Hansmann, F. Comparison of Theiler’s Murine Encephalomyelitis Virus Induced Spinal Cord and Peripheral Nerve Lesions Following Intracerebral and Intraspinal Infection. Int. J. Mol. Sci. 2019, 20, 5134. https://doi.org/10.3390/ijms20205134
Jin W, Leitzen E, Goebbels S, Nave K-A, Baumgärtner W, Hansmann F. Comparison of Theiler’s Murine Encephalomyelitis Virus Induced Spinal Cord and Peripheral Nerve Lesions Following Intracerebral and Intraspinal Infection. International Journal of Molecular Sciences. 2019; 20(20):5134. https://doi.org/10.3390/ijms20205134
Chicago/Turabian StyleJin, Wen, Eva Leitzen, Sandra Goebbels, Klaus-Armin Nave, Wolfgang Baumgärtner, and Florian Hansmann. 2019. "Comparison of Theiler’s Murine Encephalomyelitis Virus Induced Spinal Cord and Peripheral Nerve Lesions Following Intracerebral and Intraspinal Infection" International Journal of Molecular Sciences 20, no. 20: 5134. https://doi.org/10.3390/ijms20205134





