Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model
Abstract
:1. Introduction
2. Results
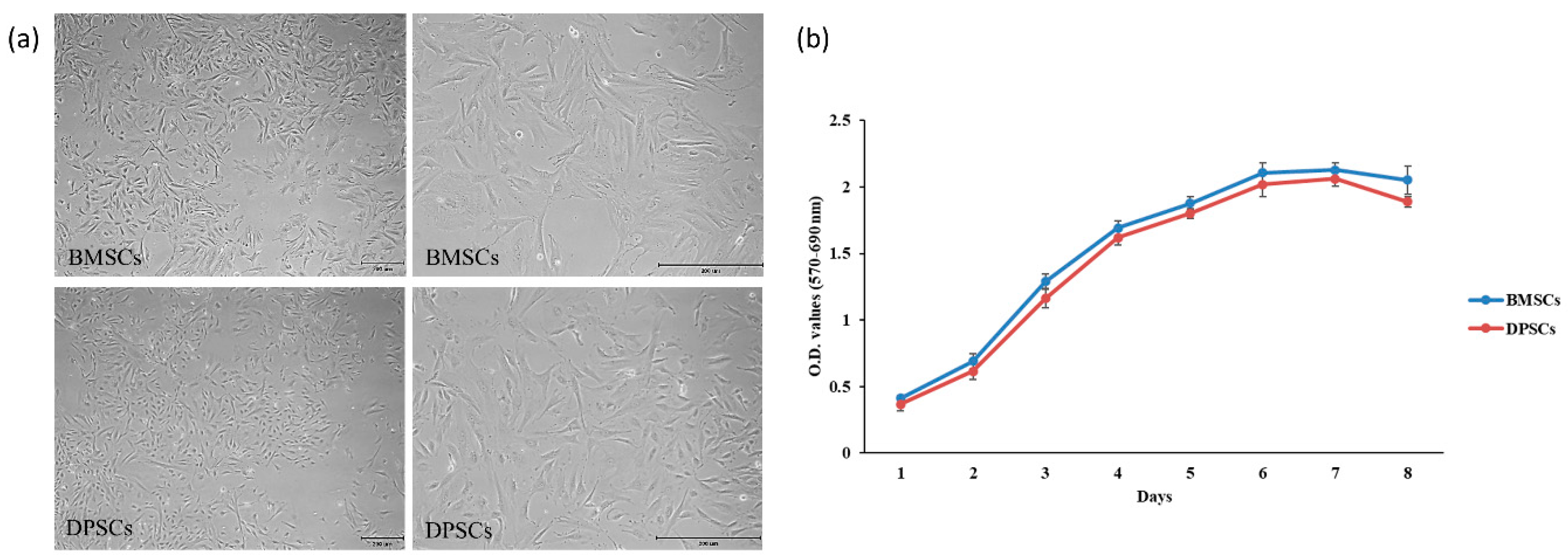
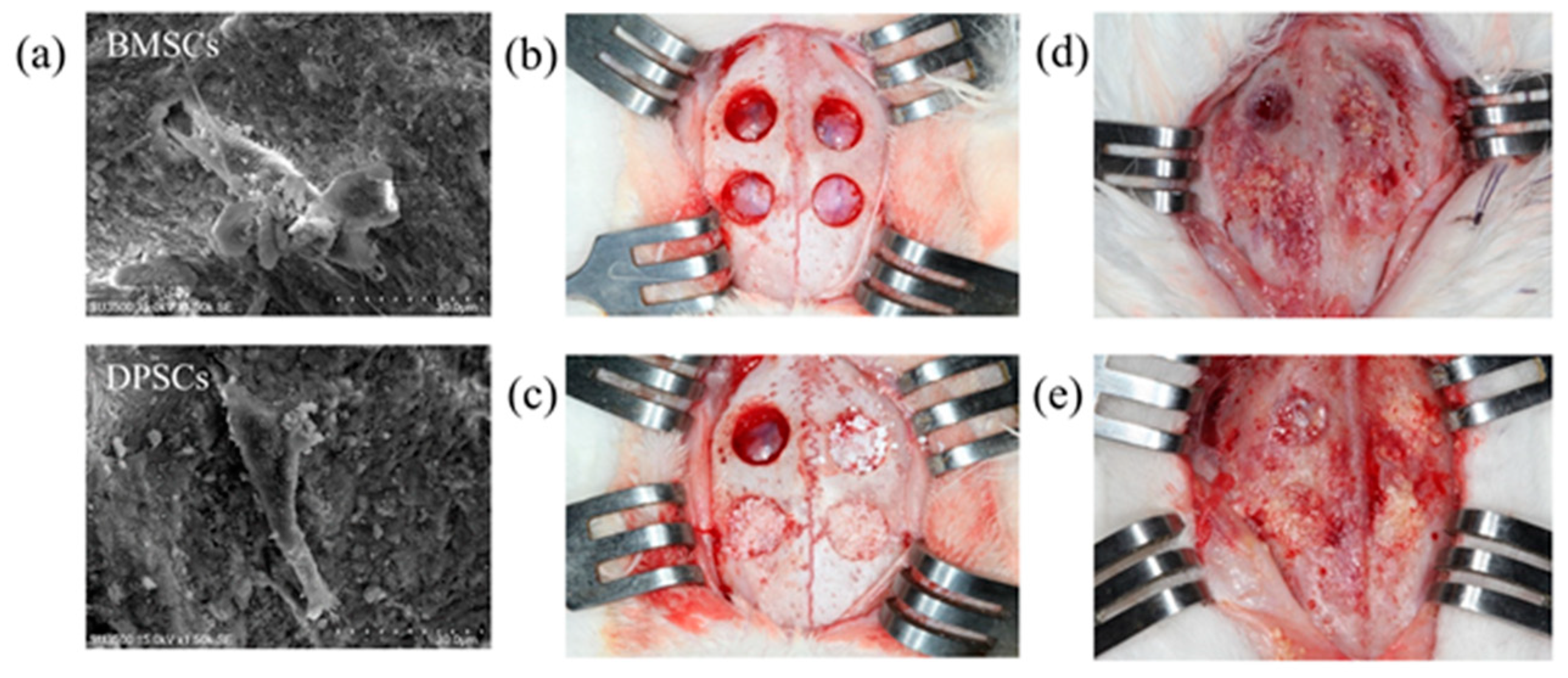
2.1. Characterization and Proliferation of BMSCs and DPSCs
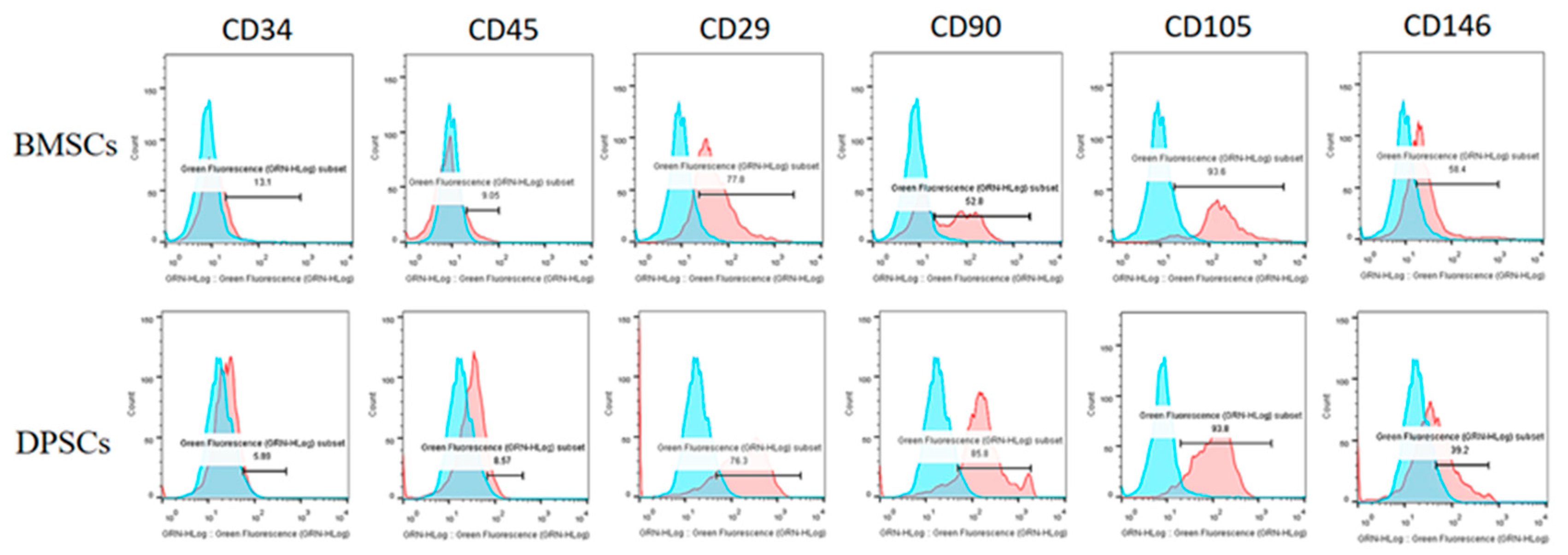
2.2. Fluorescence-Activated Cell Sorting Analysis (FACS) of BMSCs and DPSCs
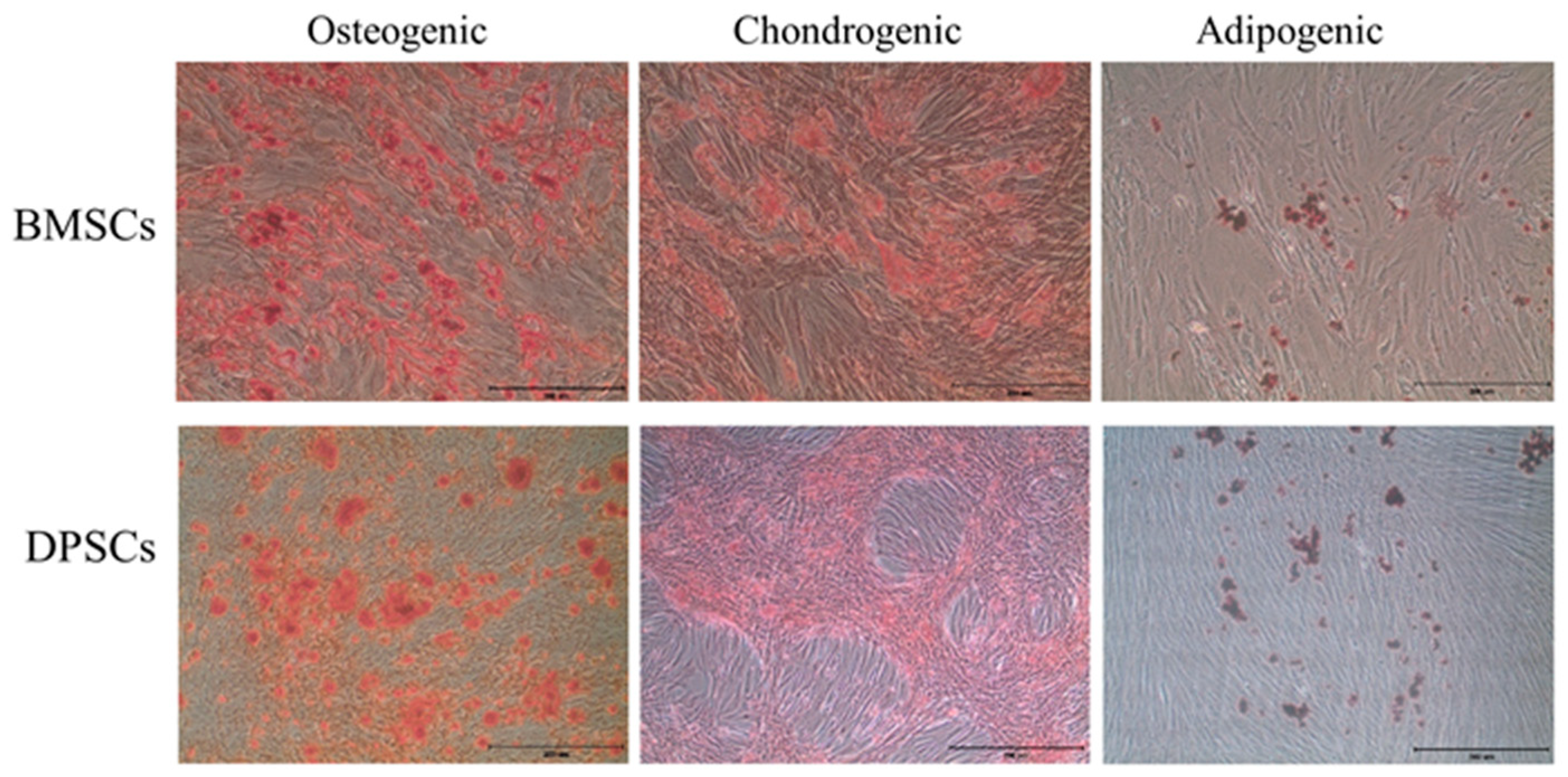
2.3. Trilineage Differentiation Capacities of BMSCs and DPSCs
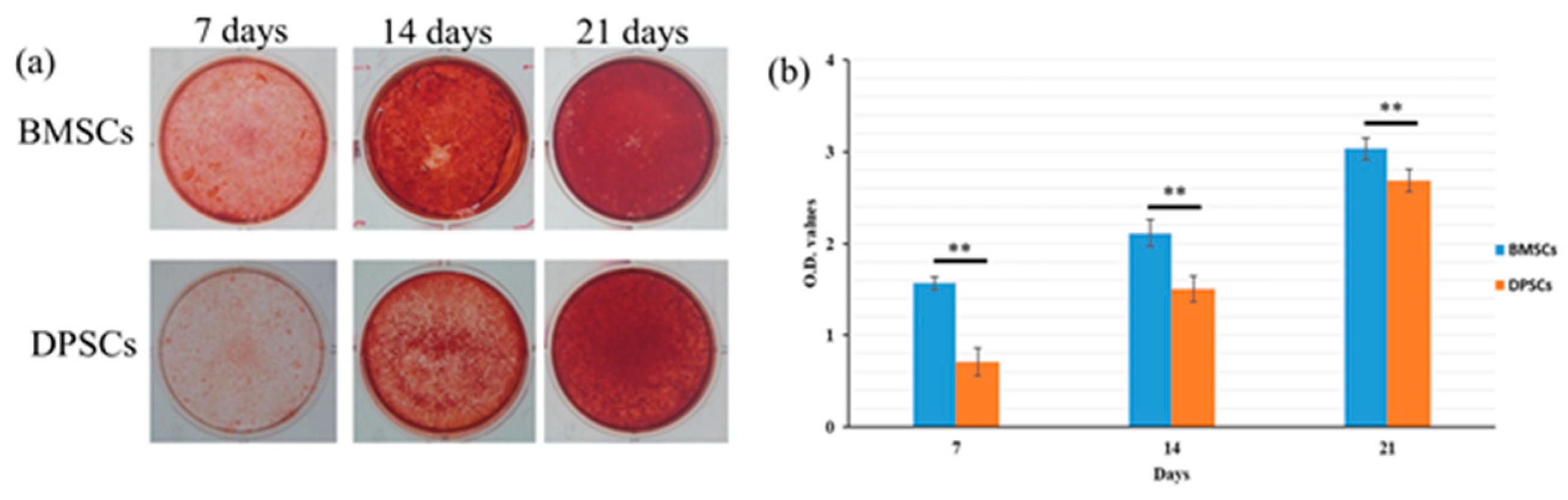
2.4. Osteogenesis and Gene Expression of BMSCs and DPSCs
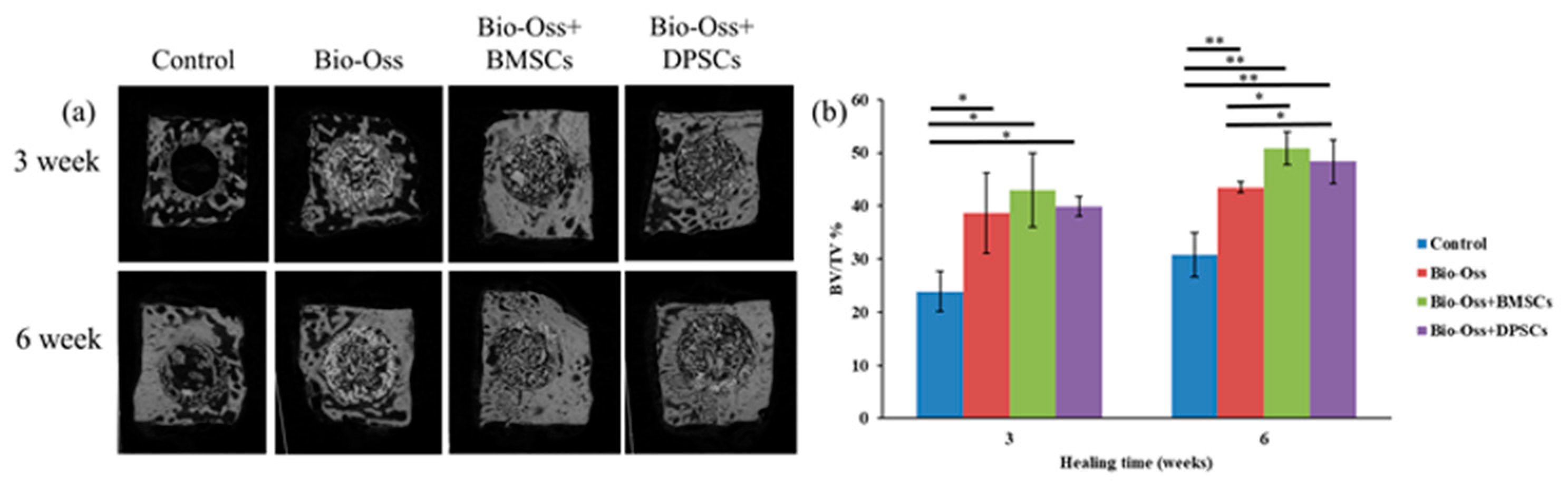
2.5. Micro-CT Measurements
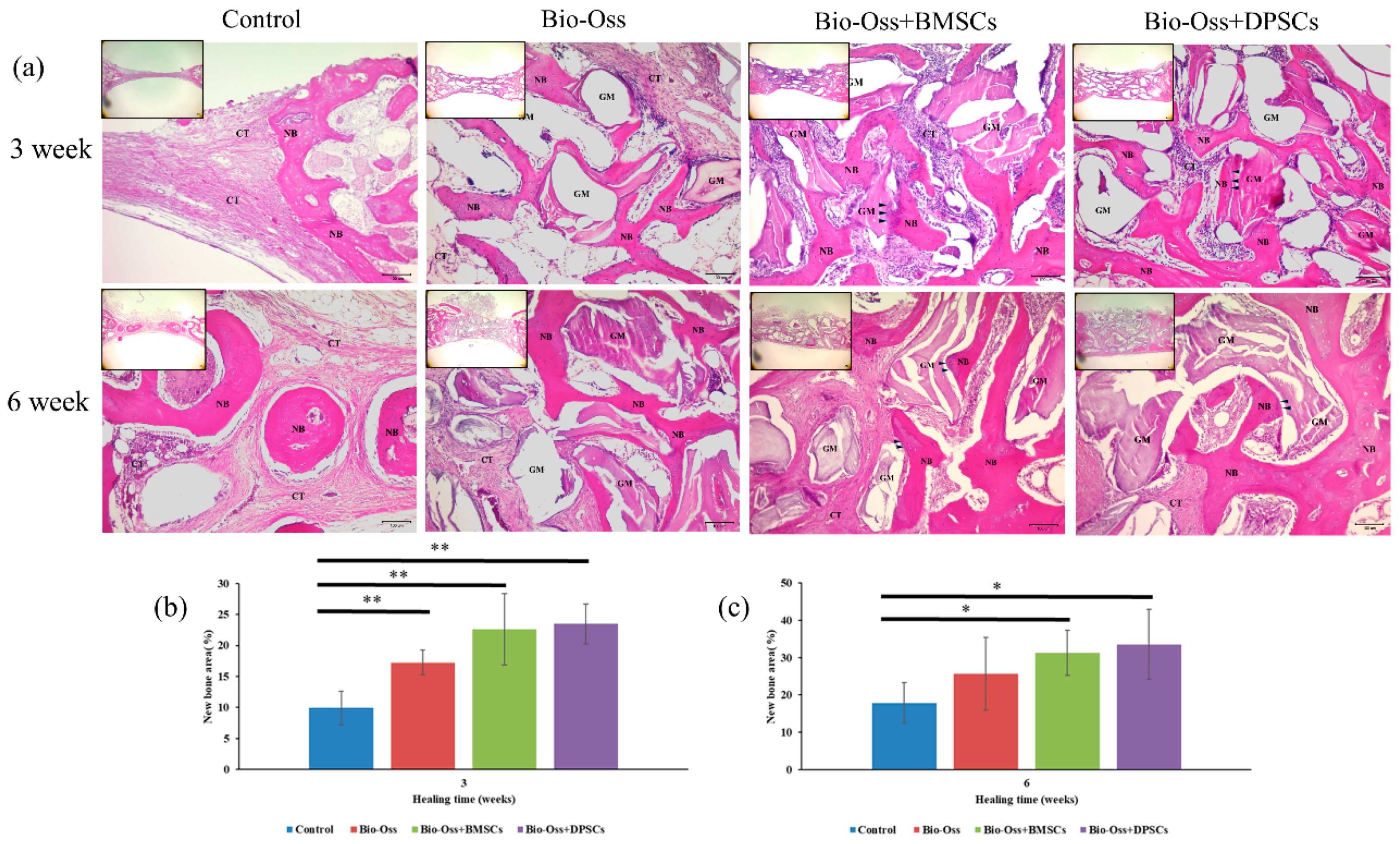
2.6. Histological Observations and Histomorphometric Analysis
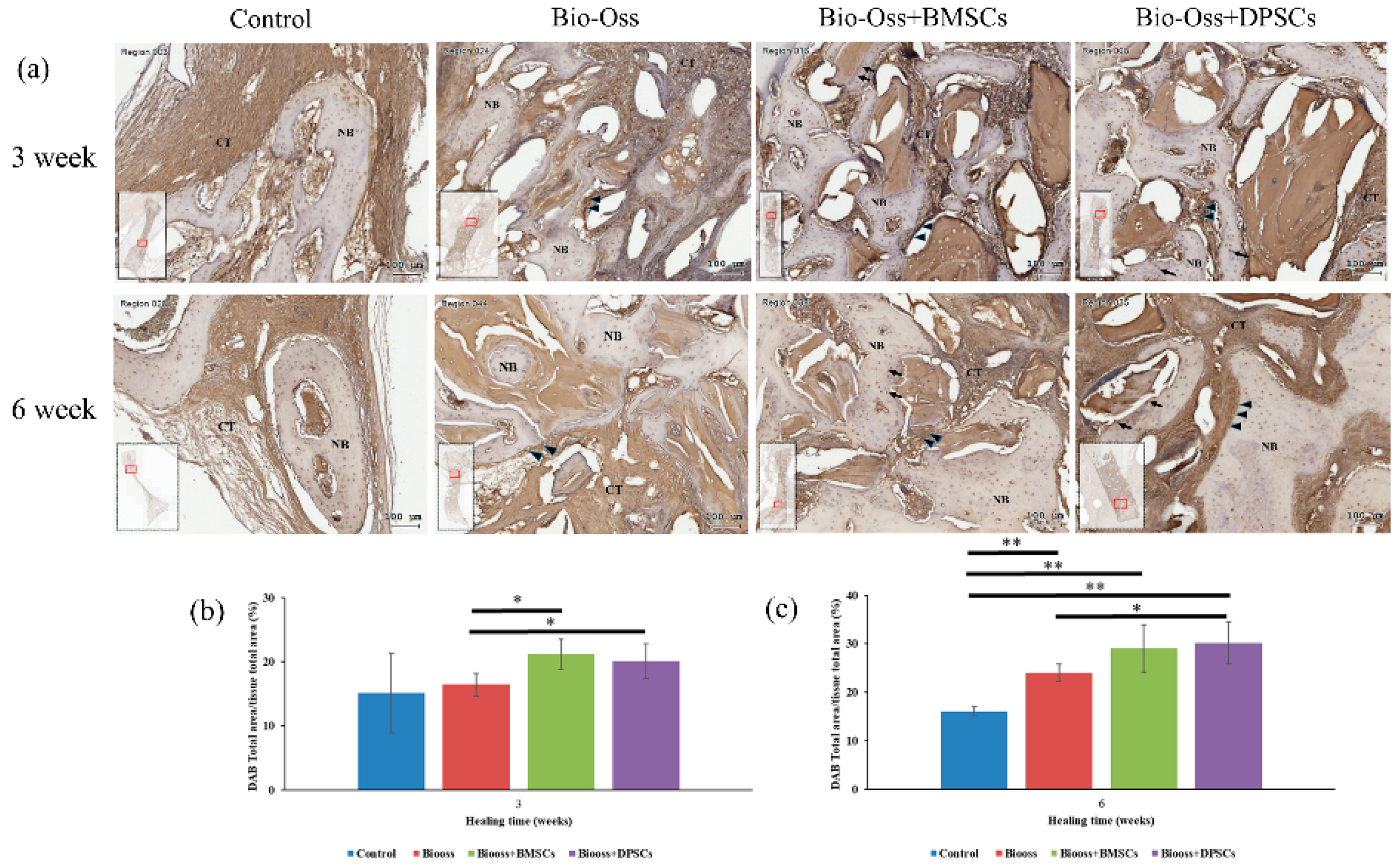
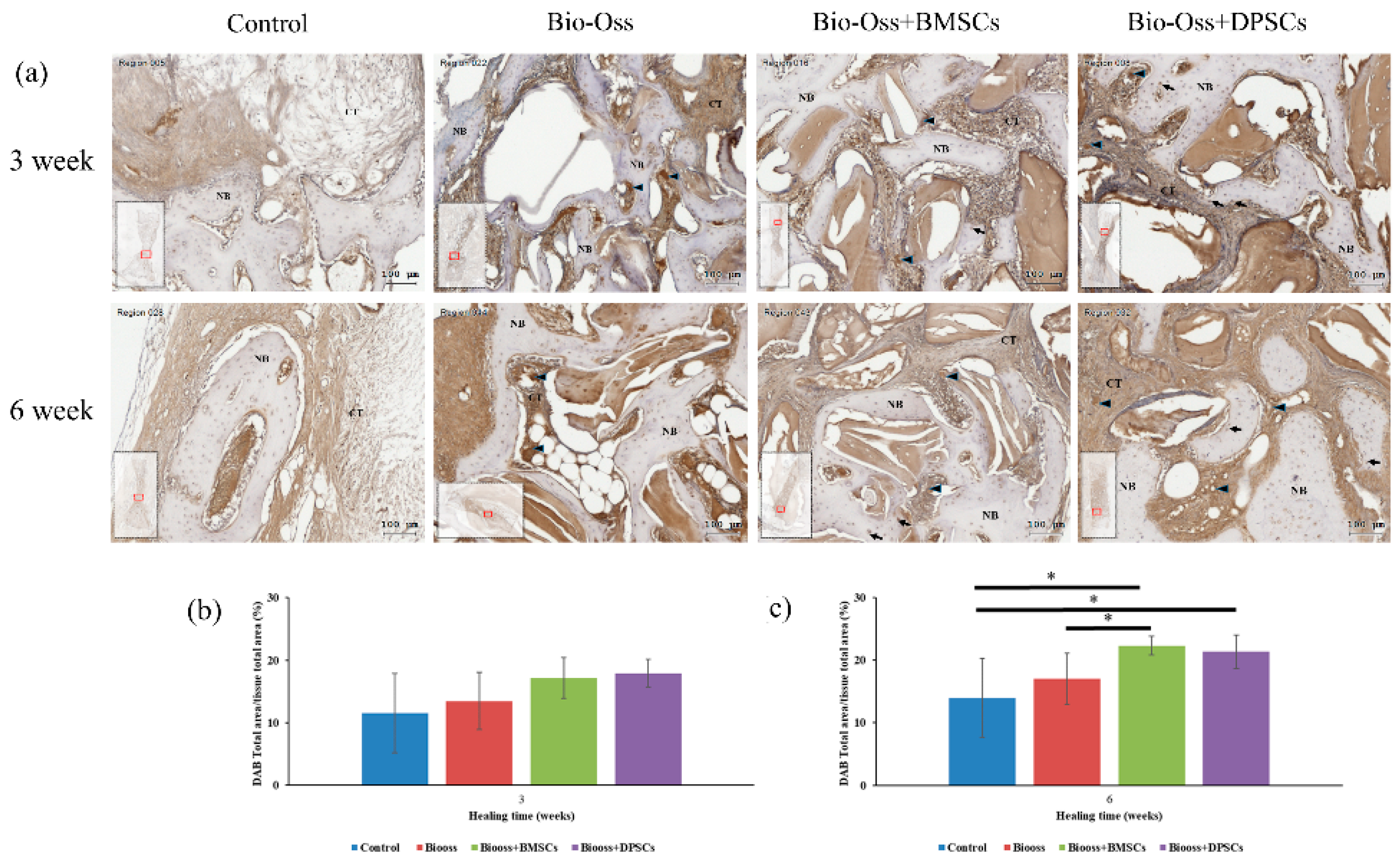
2.7. Immunohistochemical Evaluations
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics
4.2. Isolation and Culture of BMSCs and DPSCs
4.3. Flow Cytometric Assays
4.4. Osteogenic, Chondrogenic, and Adipogenic Differentiation in Vitro
4.5. Cell Proliferation Assay
4.6. Alkaline Phosphatase (ALP) Activity Assay
4.7. Calcium Deposition Assay
4.8. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
4.9. Animal Study Design and Surgical Procedures
4.10. Micro-Computed Tomography Measurements
4.11. Histology and Histomorphometric Analysis
4.12. Immunohistochemical Analysis
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gaihre, B.; Uswatta, S.; Jayasuriya, A.C. Reconstruction of Craniomaxillofacial Bone Defects Using Tissue-Engineering Strategies with Injectable and Non-Injectable Scaffolds. J. Funct. Biomater. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Pilipchuk, S.P.; Plonka, A.B.; Monje, A.; Taut, A.D.; Lanis, A.; Kang, B.; Giannobile, W.V. Tissue engineering for bone regeneration and osseointegration in the oral cavity. Dent. Mater. 2015, 31, 317–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eppley, B.L.; Pietrzak, W.S.; Blanton, M.W. Allograft and alloplastic bone substitutes: A review of science and technology for the craniomaxillofacial surgeon. J. Craniofac. Surg. 2005, 16, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Proussaefs, P.; Lozada, J. The use of intraorally harvested autogenous block grafts for vertical alveolar ridge augmentation: A human study. Int. J. Periodontics Restor. Dent. 2005, 25, 351–363. [Google Scholar]
- Frenken, J.W.; Bouwman, W.F.; Bravenboer, N.; Zijderveld, S.A.; Schulten, E.A.; ten Bruggenkate, C.M. The use of Straumann Bone Ceramic in a maxillary sinus floor elevation procedure: A clinical, radiological, histological and histomorphometric evaluation with a 6-month healing period. Clin. Oral. Implants Res. 2010, 21, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Tvedesøe, C.; Rölfing, J.H.; Foldager, C.B.; Lysdahl, H.; Kraft, D.C.; Chen, M.; Baas, J.; Le, D.Q.; Bünger, C.E. Dental pulp-derived stromal cells exhibit a higher osteogenic potency than bone marrow-derived stromal cells in vitro and in a porcine critical-size bone defect model. SICOT J. 2016, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Kaigler, D.; Pagni, G.; Park, C.H.; Braun, T.M.; Holman, L.A.; Yi, E.; Tarle, S.A.; Bartel, R.L.; Giannobile, W.V. Stem cell therapy for craniofacial bone regeneration: A randomized, controlled feasibility trial. Cell Transplant. 2013, 22, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.I.; Kanczler, J.; Tare, R.; Kassem, M.; Oreffo, R.O. Concise review: Bridging the gap: Bone regeneration using skeletal stem cell-based strategies—Where are we now? Stem Cells 2014, 32, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.T.; Liang, A.; Liu, S. Concise reviews: Characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 2015, 33, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Sun, Y.; Wang, B.; Xiong, Y.; Lin, W.; Wei, Q.; Wang, H.; He, W.; Wang, B.; et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 2017, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Abdel Meguid, E.; Ke, Y.; Ji, J.; El-Hashash, A.H.K. Stem cells applications in bone and tooth repair and regeneration: New insights, tools, and hopes. J. Cell. Physiol. 2018, 233, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Codispoti, B.; Paduano, F.; Nuzzolese, M.; Makeeva, I. Strategic Tools in Regenerative and Translational Dentistry. Int. J. Mol. Sci. 2019, 20, 1879. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Karbaat, L.; Wu, L.; Leijten, J.; Both, S.K.; Karperien, M. Trophic Effects of Mesenchymal Stem Cells in Tissue Regeneration. Tissue Eng. Part B 2017, 23, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, X.; Wang, S.; Xu, L.; Zhang, M.; Wang, G.; Jin, Y.; Zhang, X.; Jiang, X. Comparison of the use of adipose tissue-derived and bone marrow-derived stem cells for rapid bone regeneration. J. Dent. Res. 2013, 92, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Oh, S.Y.; Lee, J.S.; Park, S.Y.; Choi, E.Y.; Cho, K.S.; Kim, C.S. In vivo bone formation by human alveolar-bone-derived mesenchymal stem cells obtained during implant osteotomy using biphasic calcium phosphate ceramics or Bio-Oss as carriers. J. Biomed. Mater. Res. Part B 2016, 104, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, N.; Wagner, A.; Gehwolf, R.; Heimel, P.; Tempfer, H.; Korntner, S.; Augat, P.; Resch, H.; Redl, H.; Betz, O.; et al. Comparing the osteogenic potential of bone marrow and tendon-derived stromal cells to repair a critical-sized defect in the rat femur. J. Tissue Eng. Regen. Med. 2017, 11, 2014–2023. [Google Scholar] [CrossRef]
- Janko, M.; Sahm, J.; Schaible, A.; Brune, J.C.; Bellen, M.; Schroder, K.; Seebach, C.; Marzi, I.; Henrich, D. Comparison of three different types of scaffolds preseeded with human bone marrow mononuclear cells on the bone healing in a femoral critical size defect model of the athymic rat. J. Tissue Eng. Regen. Med. 2018, 12, 653–666. [Google Scholar] [CrossRef]
- Yu, B.H.; Zhou, Q.; Wang, Z.L. Periodontal ligament versus bone marrow mesenchymal stem cells in combination with Bio-Oss scaffolds for ectopic and in situ bone formation: A comparative study in the rat. J. Biomater. Appl. 2014, 29, 243–253. [Google Scholar] [CrossRef]
- Ling, L.E.; Feng, L.; Liu, H.C.; Wang, D.S.; Shi, Z.P.; Wang, J.C.; Luo, W.; Lv, Y. The effect of calcium phosphate composite scaffolds on the osteogenic differentiation of rabbit dental pulp stem cells. J. Biomed. Mater. Res. Part A 2015, 103, 1732–1745. [Google Scholar] [CrossRef]
- La Noce, M.; Paino, F.; Spina, A.; Naddeo, P.; Montella, R.; Desiderio, V.; De Rosa, A.; Papaccio, G.; Tirino, V.; Laino, L. Dental pulp stem cells: State of the art and suggestions for a true translation of research into therapy. J. Dent. 2014, 42, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Chamieh, F.; Collignon, A.M.; Coyac, B.R.; Lesieur, J.; Ribes, S.; Sadoine, J.; Llorens, A.; Nicoletti, A.; Letourneur, D.; Colombier, M.L.; et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci. Rep. 2016, 9, 38814. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Troya, M.; Zalduendo, M. Progress in the use of dental pulp stem cells in regenerative medicine. Cytotherapy 2018, 20, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, M.; Shakesheff, K.M.; White, L.J. Dental pulp stem cells: Function, isolation and applications in regenerative medicine. J. Tissue Eng. Regen. Med. 2015, 9, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Lew, W.Z.; Huang, Y.C.; Huang, K.Y.; Lin, C.T.; Tsai, M.T.; Huang, H.M. Static magnetic fields enhance dental pulp stem cell proliferation by activating the p38 mitogen-activated protein kinase pathway as its putative mechanism. J. Tissue Eng. Regen. Med. 2018, 12, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthi, M.; Bakkar, M.; Jordan, J.; Tran, S.D. Osteogenic Potential of Dental Mesenchymal Stem Cells in Preclinical Studies: A Systematic Review Using Modified ARRIVE and CONSORT Guidelines. Stem Cells Int. 2015, 2015, 378368. [Google Scholar] [CrossRef] [PubMed]
- Mortada, I.; Mortada, R. Dental pulp stem cells and osteogenesis: An update. Cytotechnology 2018, 70, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Annibali, S.; Bellavia, D.; Ottolenghi, L.; Cicconetti, A.; Cristalli, M.P.; Quaranta, R.; Pilloni, A. Micro-CT and PET analysis of bone regeneration induced by biodegradable scaffolds as carriers for dental pulp stem cells in a rat model of calvarial “critical size” defect: Preliminary data. J. Biomed. Mater. Res. Part B 2014, 102, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Asutay, F.; Polat, S.; Gül, M.; Subaşı, C.; Kahraman, S.A.; Karaöz, E. The effects of dental pulp stem cells on bone regeneration in rat calvarial defect model: Micro-computed tomography and histomorphometric analysis. Arch. Oral Biol. 2015, 60, 1729–1735. [Google Scholar] [CrossRef]
- Wongsupa, N.; Nuntanaranont, T.; Kamolmattayakul, S.; Thuaksuban, N. Assessment of bone regeneration of a tissue-engineered bone complex using human dental pulp stem cells/poly(ε-caprolactone)-biphasic calcium phosphate scaffold constructs in rabbit calvarial defects. J. Mater. Sci. Mater. Med. 2017, 28, 77. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Walboomers, X.F.; van Osch, G.J.; van den Dolder, J.; Jansen, J.A. Hard tissue formation in a porous HA/TCP ceramic scaffold loaded with stromal cells derived from dental pulp and bone marrow. Tissue Eng. Part A 2008, 14, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Isobe, Y.; Koyama, N.; Nakao, K.; Osawa, K.; Ikeno, M.; Yamanaka, S.; Okubo, Y.; Fujimura, K.; Bessho, K. Comparison of human mesenchymal stem cells derived from bone marrow, synovial fluid, adult dental pulp, and exfoliated deciduous tooth pulp. Int. J. Oral Maxillofac. Surg. 2016, 45, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Alge, D.L.; Zhou, D.; Adams, L.L.; Wyss, B.K.; Shadday, M.D.; Woods, E.J.; Gabriel, T.M.; Goebel, W.S. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J. Tissue Eng. Regen. Med. 2010, 4, 73–81. [Google Scholar] [PubMed]
- Davies, O.G.; Cooper, P.R.; Shelton, R.M.; Smith, A.J.; Scheven, B.A. A comparison of the in vitro mineralisation and dentinogenic potential of mesenchymal stem cells derived from adipose tissue, bone marrow and dental pulp. J. Bone Miner. Metab. 2015, 33, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Ando, T.; Hayashi, Y.; Kihara, T.; Hiraki, T.; Tsuka, Y.; Abe, T.; Kaku, M.; et al. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 876–882. [Google Scholar] [CrossRef]
- Tamaki, Y.; Nakahara, T.; Ishikawa, H.; Sato, S. In vitro analysis of mesenchymal stem cells derived from human teeth and bone marrow. Odontology 2013, 101, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Kunimatsu, R.; Nakajima, K.; Awada, T.; Tsuka, Y.; Abe, T.; Ando, K.; Hiraki, T.; Kimura, A.; Tanimoto, K. Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 501, 193–198. [Google Scholar] [CrossRef]
- Aghajani, F.; Hooshmand, T.; Khanmohammadi, M.; Khanjani, S.; Edalatkhah, H.; Zarnani, A.H.; Kazemnejad, S. Comparative Immunophenotypic Characteristics, Proliferative Features, and Osteogenic Differentiation of Stem Cells Isolated from Human Permanent and Deciduous Teeth with Bone Marrow. Mol. Biotechnol. 2016, 58, 415–427. [Google Scholar] [CrossRef]
- Liu, H.C.; Ling-Ling, E.; Wang, D.S.; Su, F.; Wu, X.; Shi, Z.P.; Lv, Y.; Wang, J.Z. Reconstruction of alveolar bone defects using bone morphogenetic protein 2 mediated rabbit dental pulp stem cells seeded on nano-hydroxyapatite/collagen/poly(L-lactide). Tissue Eng. Part A 2011, 17, 2417–2433. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura, S.; Ito, K.; Sugito, T.; Yoshimi, R.; Nagasaka, T.; Ueda, M. A feasibility of useful cell-based therapy by bone regeneration with deciduous tooth stem cells, dental pulp stem cells, or bone-marrow-derived mesenchymal stem cells for clinical study using tissue engineering technology. Tissue Eng. Part A 2010, 16, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Eitoku, M.; Suganuma, N. Mesenchymal stem/stromal cell function in modulating cell death. Stem Cell Res. Ther. 2019, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Singer, N.G.; Caplan, A.I. Mesenchymal stem cells: Mechanisms of inflammation. Annu. Rev. Pathol. 2011, 6, 457–478. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.L.; Ahmad, T.S.; Selvaratnam, L.; Kamarul, T. Isolation, characterization and the multi-lineage differentiation potential of rabbit bone marrow-derived mesenchymal stem cells. J. Anat. 2013, 222, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chang, Y.L.; Lo, Y.J.; Feng, S.W.; Huang, Y.C.; Tsai, H.Y.; Lin, C.T.; Fan, K.H.; Huang, H.M. Bone Healing Improvements Using Hyaluronic Acid and Hydroxyapatite/Beta-Tricalcium Phosphate in Combination: An Animal Study. Biomed. Res. Int. 2016, 2016, 8301624. [Google Scholar] [CrossRef]
- Chan, Y.H.; Lew, W.Z.; Lu, E.; Loretz, T.; Lu, L.; Lin, C.T.; Feng, S.W. An evaluation of the biocompatibility and osseointegration of novel glass fiber reinforced composite implants: In vitro and in vivo studies. Dent. Mater. 2018, 34, 470–485. [Google Scholar] [CrossRef]
- Kang, H.; Shih, Y.R.; Nakasaki, M.; Kabra, H.; Varghese, S. Small molecule-driven direct conversion of human pluripotent stem cells into functional osteoblasts. Sci. Adv. 2016, 2, e1600691. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Kobayashi, E.; Schaller, B.; Mottini, M.; Miron, R.J.; Saulacic, N. Effect of recombinant human bone morphogenic protein 9 (rhBMP9) loaded onto bone grafts versus barrier membranes on new bone formation in a rabbit calvarial defect model. J. Biomed. Mater. Res. Part A 2017, 105, 2655–2661. [Google Scholar] [CrossRef] [PubMed]

| Primers Used for RT-PCR Analysis | ||||
|---|---|---|---|---|
| Gene | Type | Primers | Accession | Product Length |
| Runx2 | Forward | 5′-TCAGGCATGTCCCTCGGTAT-3′ | XM_017345160 | 54 |
| Reverse | 5′-TGGCAGGTAGGTATGGTAGTGG-3’ | |||
| ALP | Forward | 5′-ACTGTGGACTACCTCTTG-3′ | XM_017346489 | 76 |
| Reverse | 5′-GGTCAGTGATGTTGTTCC-3′ | |||
| Osteocalcin | Forward | 5′-ACTCTTGTCGCCCTGCTG-3′ | XM_002715383 | 116 |
| Reverse | 5′-CTGCCCTCCCTCTTGGAC-3′ | |||
| GAPDH | Forward | 5′-GCCTGGAGAAAGCTGCTAAGT-3′ | NM_001082253 | 133 |
| Reverse | 5′– GAGTGGGTGGCACTGTTGAA-3′ | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-C.; Chan, Y.-H.; Hsieh, S.-C.; Lew, W.-Z.; Feng, S.-W. Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model. Int. J. Mol. Sci. 2019, 20, 5015. https://doi.org/10.3390/ijms20205015
Lee Y-C, Chan Y-H, Hsieh S-C, Lew W-Z, Feng S-W. Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model. International Journal of Molecular Sciences. 2019; 20(20):5015. https://doi.org/10.3390/ijms20205015
Chicago/Turabian StyleLee, Yu-Chieh, Ya-Hui Chan, Sung-Chih Hsieh, Wei-Zhen Lew, and Sheng-Wei Feng. 2019. "Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model" International Journal of Molecular Sciences 20, no. 20: 5015. https://doi.org/10.3390/ijms20205015





