Personalized Treatment Response Assessment for Rare Childhood Tumors Using Microcalorimetry–Exemplified by Use of Carbonic Anhydrase IX and Aquaporin 1 Inhibitors
Abstract
:1. Introduction
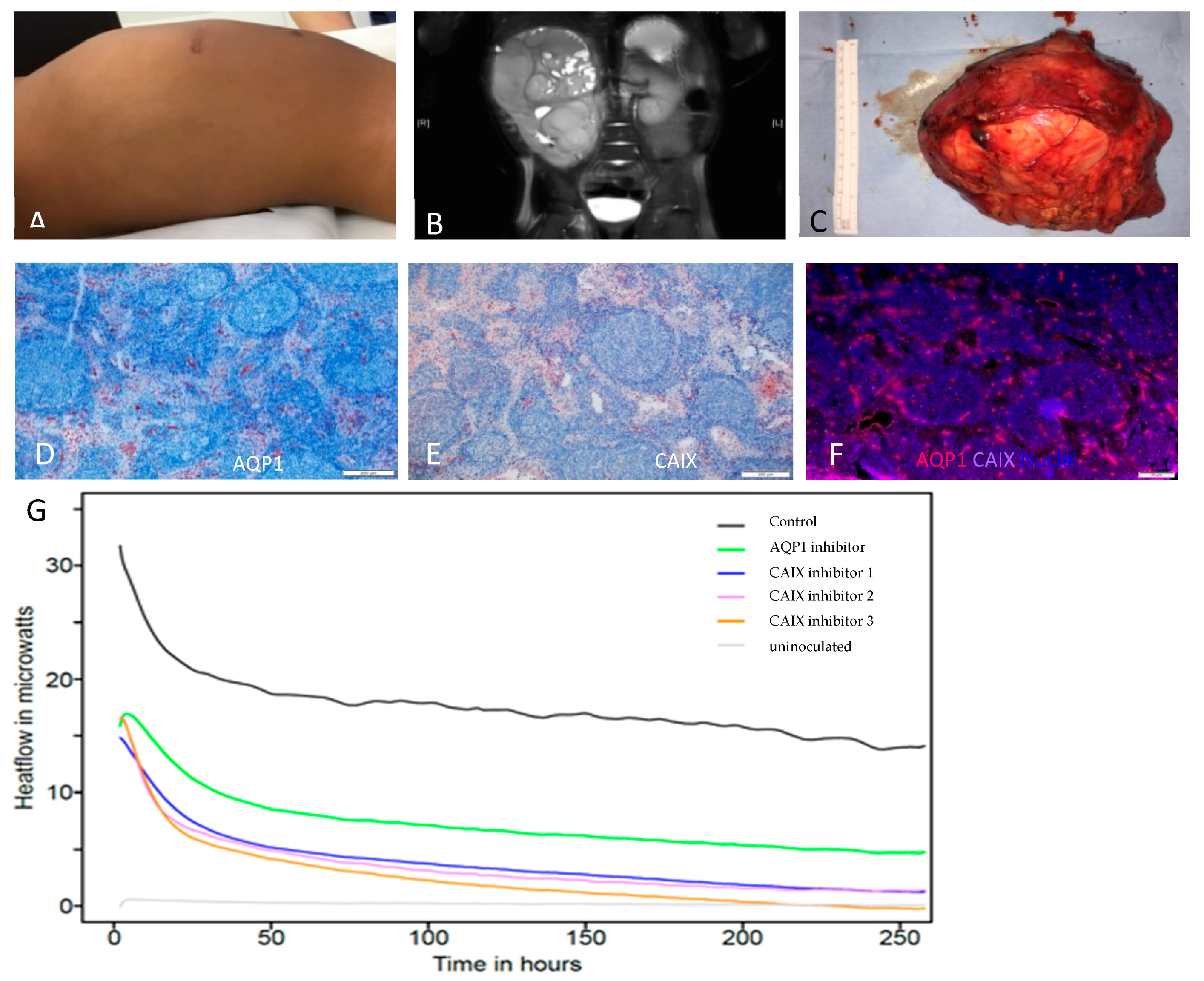
2. Results
3. Discussion
4. Materials and Methods
4.1. Tumor Slice Culture and Microcalorimetric Measurement
4.2. Isothermal Microcalorimetry
4.3. Immunostaining
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CAIX | Carbonic anhydrase IX |
| AQP1 | Aquaporin 1 |
References
- Wadso, I. Isothermal microcalorimetry in applied biology. Thermochim. Acta 2002, 394, 305–311. [Google Scholar] [CrossRef]
- Bonkat, G.; Braissant, O.; Widmer, A.F.; Frei, R.; Rieken, M.; Wyler, S.; Gasser, T.C.; Wirz, D.; Daniels, A.U.; Bachmann, A. Rapid detection of urinary tract pathogens using microcalorimetry: Principle, technique and first results. BJU Int. 2012, 110, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Muller, G.; Egli, A.; Widmer, A.; Frei, R.; Halla, A.; Wirz, D.; Gasser, T.C.; Bachmann, A.; Wagenlehner, F.; et al. Seven hours to adequate antimicrobial therapy in urosepsis using isothermal microcalorimetry. J. Clin. Microbiol. 2014, 52, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Galindo, F.G.; Rocculi, P.; Wadso, L.; Sjohlm, I. The potential of isothermal calorimetry in monitoring and predicting quality changes during processing and storage of minimally processed fruits and vegetables. Trends Food Sci. Tech. 2005, 16, 325–331. [Google Scholar] [CrossRef]
- Doostmohammadi, A.; Monshi, A.; Fathi, M.H.; Karbasi, S.; Braissant, O.; Daniels, A.U. Direct cytotoxicity evaluation of 63S bioactive glass and bone-derived hydroxyapatite particles using yeast model and human chondrocyte cells by microcalorimetry. J. Mater. Sci.-Mater. Med. 2011, 22, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Keiser, J.; Meister, I.; Bachmann, A.; Wirz, D.; Gopfert, B.; Bonkat, G.; Wadso, I. Isothermal microcalorimetry accurately detects bacteria, tumorous microtissues, and parasitic worms in a label-free well-plate assay. Biotechnol. J. 2015, 10, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Kemp, R.B.; Guan, Y.H. The application of heat flux measurements to improve the growth of mammalian cells in culture. Acta 2000, 349, 23–30. [Google Scholar] [CrossRef]
- Braissant, O.; Wirz, D.; Gopfert, B.; Daniels, A.U. Biomedical Use of Isothermal Microcalorimeters. Sensors 2010, 10, 9369–9383. [Google Scholar] [CrossRef]
- Steliarova-Foucher, E.; Stiller, C.; Lacour, B.; Kaatsch, P. International Classification of Childhood Cancer, third edition. Cancer 2005, 103, 1457–1467. [Google Scholar] [CrossRef]
- Brok, J.; Treger, T.D.; Gooskens, S.L.; van den Heuvel-Eibrink, M.M.; Pritchard-Jones, K. Biology and treatment of renal tumours in childhood. Eur. J. Cancer 2016, 68, 179–195. [Google Scholar] [CrossRef] [Green Version]
- Furtwangler, R.; Gooskens, S.L.; van Tinteren, H.; de Kraker, J.; Schleiermacher, G.; Bergeron, C.; de Camargo, B.; Acha, T.; Godzinski, J.; Sandstedt, B.; et al. Clear cell sarcomas of the kidney registered on International Society of Pediatric Oncology (SIOP) 93-01 and SIOP 2001 protocols: A report of the SIOP Renal Tumour Study Group. Eur. J. Cancer 2013, 49, 3497–3506. [Google Scholar] [CrossRef] [PubMed]
- Argani, P.; Perlman, E.J.; Breslow, N.E.; Browning, N.G.; Green, D.M.; D’Angio, G.J.; Beckwith, J.B. Clear cell sarcoma of the kidney: A review of 351 cases from the National Wilms Tumor Study Group Pathology Center. Am. J. Surg. Pathol. 2000, 24, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, Y.; Kanai, F.; Ichimura, T.; Tateishi, K.; Tanaka, Y.; Ohta, M.; Seto, M.; Tada, M.; Ijichi, H.; Ikenoue, T.; et al. Identification of a suppressive mechanism for Hedgehog signaling through a novel interaction of Gli with 14-3-3. J. Biol. Chem. 2010, 285, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Ueno-Yokohata, H.; Okita, H.; Nakasato, K.; Akimoto, S.; Hata, J.; Koshinaga, T.; Fukuzawa, M.; Kiyokawa, N. Consistent in-frame internal tandem duplications of BCOR characterize clear cell sarcoma of the kidney. Nat. Genet. 2015, 47, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Ameis, H.M.; Drenckhan, A.; Freytag, M.; Izbicki, J.R.; Supuran, C.T.; Reinshagen, K.; Holland-Cunz, S.; Gros, S.J. Carbonic anhydrase IX correlates with survival and is a potential therapeutic target for neuroblastoma. J. Enzym. Inhib. Med. Chem. 2015, 1–6. [Google Scholar] [CrossRef]
- Drenckhan, A.; Freytag, M.; Supuran, C.T.; Sauter, G.; Izbicki, J.R.; Gros, S.J. CAIX furthers tumour progression in the hypoxic tumour microenvironment of esophageal carcinoma and is a possible therapeutic target. J. Enzym. Inhib. Med. Chem. 2018, 33, 1024–1033. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 2012, 33, 207–214. [Google Scholar] [CrossRef]
- Lou, Y.; McDonald, P.C.; Oloumi, A.; Chia, S.; Ostlund, C.; Ahmadi, A.; Kyle, A.; Auf dem Keller, U.; Leung, S.; Huntsman, D.; et al. Targeting tumor hypoxia: Suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011, 71, 3364–3376. [Google Scholar] [CrossRef]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef]
- Yoshida, T.; Hojo, S.; Sekine, S.; Sawada, S.; Okumura, T.; Nagata, T.; Shimada, Y.; Tsukada, K. Expression of aquaporin-1 is a poor prognostic factor for stage II and III colon cancer. Mol. Clin. Oncol. 2013, 1, 953–958. [Google Scholar] [CrossRef] [Green Version]
- Abreu-Rodriguez, I.; Sanchez Silva, R.; Martins, A.P.; Soveral, G.; Toledo-Aral, J.J.; Lopez-Barneo, J.; Echevarria, M. Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of Hif-1alpha. PLoS ONE 2011, 6, e28385. [Google Scholar] [CrossRef] [PubMed]
- Fiaschi, T.; Giannoni, E.; Taddei, M.L.; Cirri, P.; Marini, A.; Pintus, G.; Nativi, C.; Richichi, B.; Scozzafava, A.; Carta, F.; et al. Carbonic anhydrase IX from cancer-associated fibroblasts drives epithelial-mesenchymal transition in prostate carcinoma cells. Cell Cycle 2013, 12, 1791–1801. [Google Scholar] [CrossRef] [Green Version]
- Boros, L.G.; D’Agostino, D.P.; Katz, H.E.; Roth, J.P.; Meuillet, E.J.; Somlyai, G. Submolecular regulation of cell transformation by deuterium depleting water exchange reactions in the tricarboxylic acid substrate cycle. Med. Hypotheses 2016, 87, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lane, A.N.; Ricketts, C.J.; Sourbier, C.; Wei, M.H.; Shuch, B.; Pike, L.; Wu, M.; Rouault, T.A.; Boros, L.G.; et al. Metabolic reprogramming for producing energy and reducing power in fumarate hydratase null cells from hereditary leiomyomatosis renal cell carcinoma. PLoS ONE 2013, 8, e72179. [Google Scholar] [CrossRef] [PubMed]
- Boros, L.G.; Collins, T.Q.; Somlyai, G. What to eat or what not to eat-that is still the question. Neuro-Oncol. 2017, 19, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Yeung, P.; Sin, H.S.; Chan, S.; Chan, G.C.; Chan, B.P. Microencapsulation of Neuroblastoma Cells and Mesenchymal Stromal Cells in Collagen Microspheres: A 3D Model for Cancer Cell Niche Study. PLoS ONE 2015, 10, e0144139. [Google Scholar] [CrossRef]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinska, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Maelandsmo, G.M. , et al. Patient-derived xenograft models: An emerging platform for translational cancer research. Cancer Discov. 2014, 4, 998–1013. [Google Scholar] [CrossRef]
- Chip, S.; Zhu, X.; Kapfhammer, J.P. The analysis of neurovascular remodeling in entorhino-hippocampal organotypic slice cultures. J. Vis. Exp. 2014, e52023. [Google Scholar] [CrossRef]
- Wang, S.; Brunne, B.; Zhao, S.; Chai, X.; Li, J.; Lau, J.; Failla, A.V.; Zobiak, B.; Sibbe, M.; Westbrook, G.L.; et al. Trajectory Analysis Unveils Reelin’s Role in the Directed Migration of Granule Cells in the Dentate Gyrus. J. Neurosci. 2018, 38, 137–148. [Google Scholar] [CrossRef]
- Gros, S.J.; Dohrmann, T.; Peldschus, K.; Schurr, P.G.; Kaifi, J.T.; Kalinina, T.; Reichelt, U.; Mann, O.; Strate, T.G.; Adam, G.; et al. Complementary use of fluorescence and magnetic resonance imaging of metastatic esophageal cancer in a novel orthotopic mouse model. Int. J. Cancer 2010, 126, 2671–2681. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gros, S.J.; Holland-Cunz, S.G.; Supuran, C.T.; Braissant, O. Personalized Treatment Response Assessment for Rare Childhood Tumors Using Microcalorimetry–Exemplified by Use of Carbonic Anhydrase IX and Aquaporin 1 Inhibitors. Int. J. Mol. Sci. 2019, 20, 4984. https://doi.org/10.3390/ijms20204984
Gros SJ, Holland-Cunz SG, Supuran CT, Braissant O. Personalized Treatment Response Assessment for Rare Childhood Tumors Using Microcalorimetry–Exemplified by Use of Carbonic Anhydrase IX and Aquaporin 1 Inhibitors. International Journal of Molecular Sciences. 2019; 20(20):4984. https://doi.org/10.3390/ijms20204984
Chicago/Turabian StyleGros, Stephanie J., Stefan G. Holland-Cunz, Claudiu T. Supuran, and Olivier Braissant. 2019. "Personalized Treatment Response Assessment for Rare Childhood Tumors Using Microcalorimetry–Exemplified by Use of Carbonic Anhydrase IX and Aquaporin 1 Inhibitors" International Journal of Molecular Sciences 20, no. 20: 4984. https://doi.org/10.3390/ijms20204984




