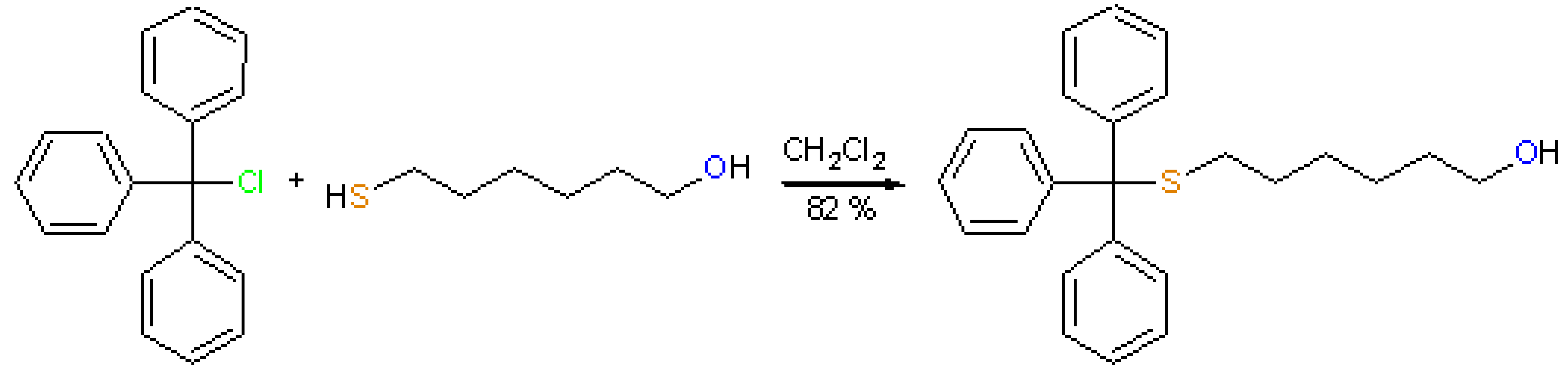
6-S-Trityl-mercapto-hexan-1-ol is an important compound, because its O-β-cyanoethyl-N,N-diisopropyl phosphoramidite derivative is the most widely used building block for the synthesis of 5'-thiol modified oligonucleotides. The synthesis of this compound has been previously described from triphenylmethanethiol and 6-bromo-hexan-1-ol [1]. We have investigated the direct reaction of 6-mercapto-hexan-1-ol and trityl chloride. These reagents are cheaper and also readily commercially available. Moreover, the commonly used bases (e.g. pyridine and triethylamine) in the tritylation reaction give rise to the formation of large amounts of undesired side products which hampers the purification of the desired compound. On the other hand, if the reaction is carried out without any base [2] the desired compound is formed almost quantitavely; thus, the thiol group can be tritylated selectively. To a stirred solution of 6-mercapto-hexan-1-ol (2 ml, 15 mmol) in dichloromethane (20 ml), was added dropwise over 30 min a solution of trityl chloride (2.8 g, 10 mmol) in dichloromethane (50 ml) at room temperature. According to TLC (dichloromethane), all the trityl chloride was converted in 15 min. The reaction mixture was extracted with 0.1 M NaOH (in order to remove the unreacted mercaptohexanol from the mixture) then the organic layer was dried over MgSO4, evaporated and the resulting yellow oil was crystallized from toluene/light petroleum ether to give 3.1 g (8.2 mmol, 82%) of the title compound.
Mp.: 73.5-74.5 °C.
1H NMR (CDCl3, 500 MHz): 1.26 (m, 5H, upon deuteration 4H, C(4)H2-C(3)H2, OH); 1.40 (m, 2H, C(5)H2); 1.48 (m, 2H, C(2)H2); 2.15 (t, J=7.3 Hz, 2H, C(6)H2); 3.58 (t, J=6.6 Hz, 2H, C(1)H2); 7.20 (m, 3H, aromatic CH); 7.27 (m, 6H, aromatic CH); 7.40(m, 6H, aromatic CH).
13C NMR (CDCl3, 125 MHz, assignment based onJ-modulated spin-echo, HMQC and HMBC experiments): 25.97 (C-3); 29.25 (C-5); 29.42 (C-4); 32.60 (C-6); 33.23 (C-2); 63.57 (C-1); 67.13 (Ph3CS); 127.20; 128.49; 130.31 (aromatic C's); 145.77 (aromatic Cq).
Nano ESI-MS (LiCl additive in acetonitrile [3], m/z, %): 383 (100, [M+Li]+).
Anal. calcd. for C25H28OS (376.555): C, 79.74; H,7.49; S, 8.52; found C, 79.59; H, 7.35; S, 8.49 %.
Supplementary materials
Supplementary File 1Supplementary File 2References
- Kumar, P.; Gupta, K. C. Chem. Lett. 1996, 635–636.
- Culvenor, C. C.; Davies, W; Savige, W. E. J. Chem. Soc. 1952, 4480–4485.
- Kele, Z.; Kupihár, Z.; Kovács, L.; Janáky, T.; Szabó, P. T. J. Mass Spectrom. 1999, 34, 1317–1321. [PubMed]
- Sample Availability: sample available from the authors.
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/