Abstract
A strategy for constructing trifluoromethylated spiroisoxazolones has been developed. This approach relies on the 1,3-dipolar cycloaddition of CF3-substituted nitrile imines, generated in situ from trifluoroacetyl hydrazonoyl bromides and K2CO3, with the exocyclic double bond of 4-benzylidene-3-methylisoxazol-5(4H)-ones. The reaction provides a series of trifluoromethylated spiro(isoxazolone-pyrazoline) derivatives in moderate to high yields (up to 93%). The protocol exhibits broad substrate compatibility with respect to aromatic substituents on both reaction partners. To the best of our knowledge, the introduction of a trifluoromethyl group at the 3-position of the pyrazoline ring via nitrile imine cycloaddition chemistry has not been previously reported. The resulting products incorporate a valuable CF3-substituted pyrazoline pharmacophore spiro-fused to an isoxazolone core and may be of interest for medicinal chemistry programs.
1. Introduction
Spirocyclic compounds are ring systems characterized by two cyclic structures connected through a common spiro carbon atom. This unique architectural feature imparts high conformational rigidity and a stable three-dimensional configuration, endowing spiro scaffolds with physicochemical properties, metabolic behaviors, and biological activities distinct from those of planar aromatic systems or flexible chain structures [1,2,3]. In medicinal chemistry, the incorporation of a spirocyclic framework serves as a valuable strategy for optimizing key drug-like properties [4,5,6,7,8]. This includes the modulation of lipophilicity, enhancement of aqueous solubility, stabilization of bioactive conformations, improvement of target selectivity, and fine-tuning of pharmacokinetic profiles [9,10,11].
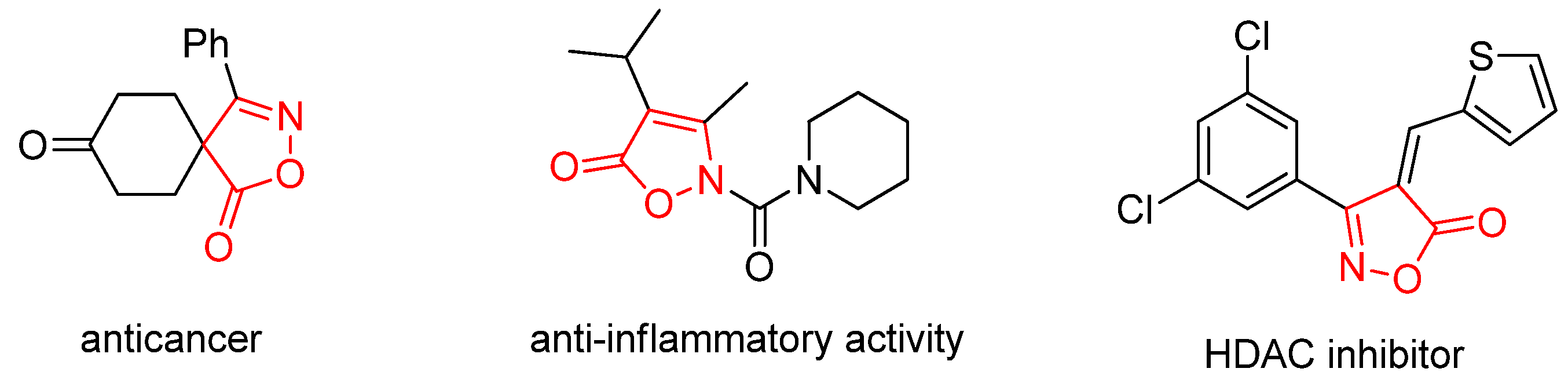
Isoxazolone and its derivatives represent an important class of five-membered heterocyclic compounds containing both nitrogen and oxygen atoms. They serve as crucial pharmacophores and structural motifs found in numerous natural products and pharmaceuticals [12,13,14]. The distinctive architecture of the isoxazolone ring enables it to engage in diverse non-covalent interactions, notably as a hydrogen-bond acceptor (via its N and O atoms), π-π stacking (through its unsaturated ring system), and hydrophilic interactions. This capacity to interact with various protein targets underpins the broad spectrum of biological activities exhibited by isoxazolone-based compounds, leading to their widespread application in medicinal and agrochemical fields. A multitude of both natural and synthetic isoxazolone derivatives have been investigated as active agents for various therapeutic purposes, including anticancer, anti-inflammatory, antidiabetic, and antiviral applications (Figure 1) [15,16,17]. In addition, incorporating a trifluoromethyl group serves as a strategic medicinal chemistry tactic to simultaneously enhance key pharmacological properties. Its strong electron-withdrawing effect and high lipophilicity improve metabolic stability, membrane permeability, and target binding affinity, while its role as a versatile bioisostere allows for the fine-tuning of molecular interactions, a principle validated by numerous successful therapeutics [18,19,20,21].
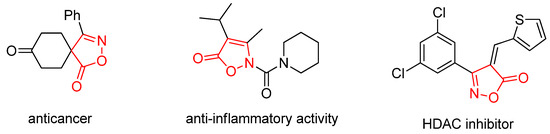
Figure 1.
Examples of biologically isoxazolone derivatives.
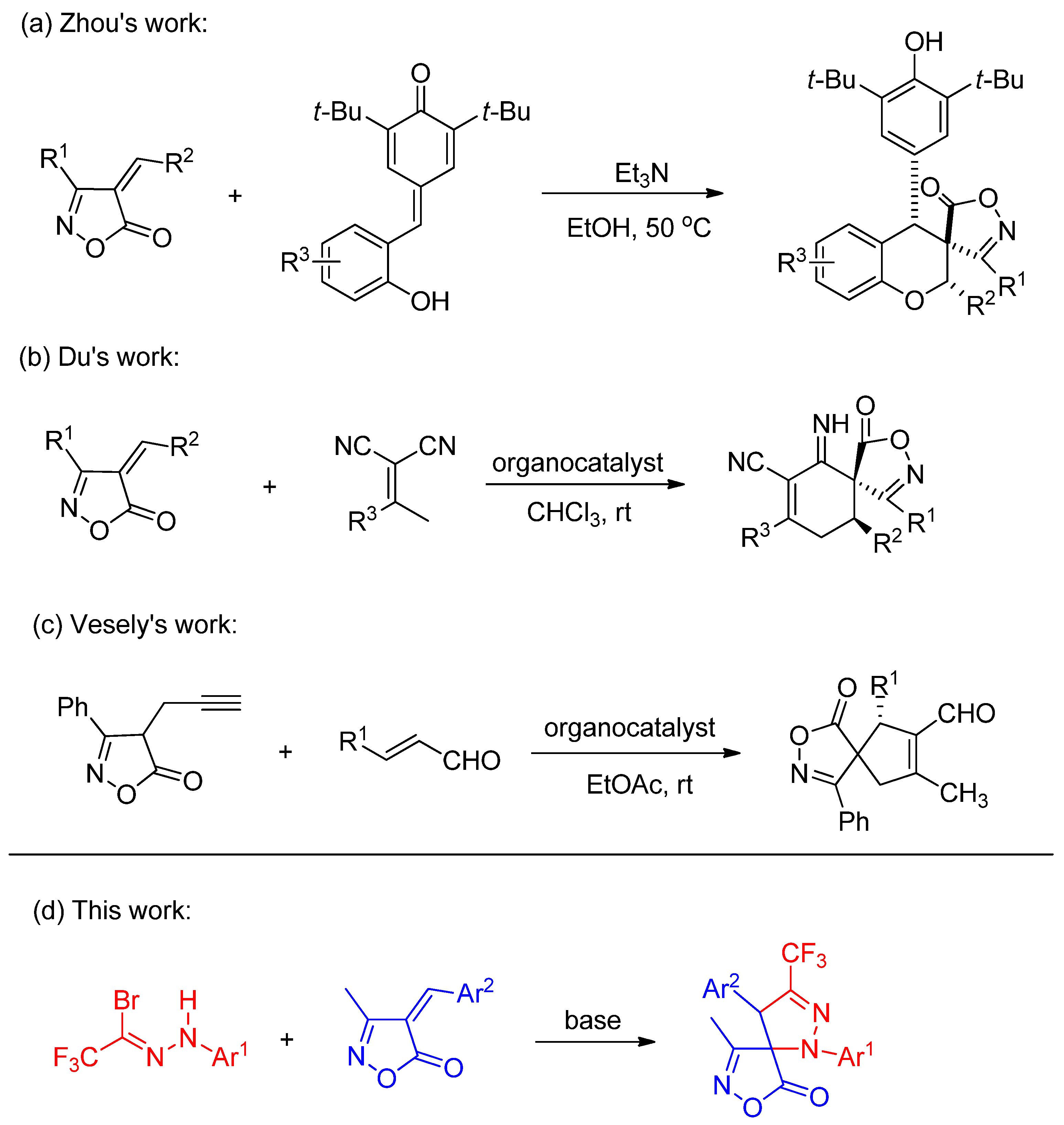
Given the considerable potential demonstrated by spiroisoxazolone compounds in medicinal chemistry, recent years have witnessed growing research interest in the multi-site modification of isoxazolones [22,23,24]. In 2019, Zhou and co-workers reported a series of spiroisoxazolone-chromane derivatives via an addition-annulation reaction between ortho-hydroxyphenyl-substituted p-quinone analogs and 4-alkylideneisoxazol-5-ones (Scheme 1a) [25]. Subsequently, in 2021, Du’s team described an asymmetric Michael addition-annulation cascade between 4-alkylideneisoxazol-5-ones and dicyanoalkenes, leading to a series of isoxazolone-based spirocyclic derivatives (Scheme 1b) [26]. More recently, in 2025, Vesely’s group achieved the synthesis of chiral spiroisoxazolones through an asymmetric Conia-ene-type cascade reaction of α,β-unsaturated aldehydes with propargyl-substituted isoxazolones (Scheme 1c) [27]. Motivated by these advancements and the fact that the introduction of a trifluoromethyl group at the pyrazoline 3-position via nitrile imine cycloaddition remains unexplored, we report herein the 1,3-dipolar cycloaddition of in situ-generated CF3-substituted nitrile imines (from trifluoroacetyl hydrazonoyl bromides) with 4-benzylidene-3-methylisoxazol-5(4H)-ones.
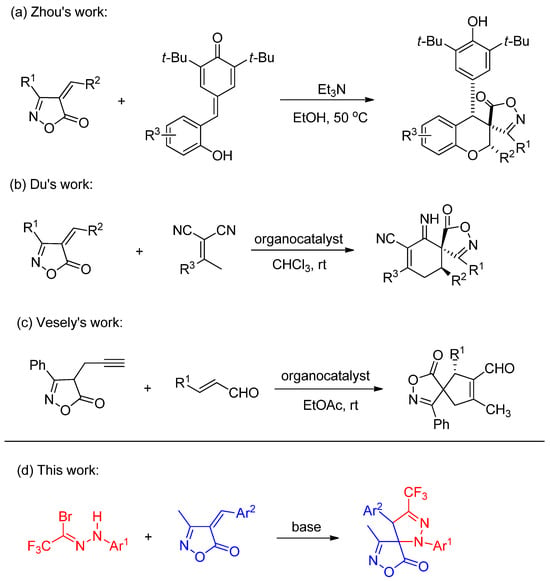
Scheme 1.
Examples of spiroisoxazolidinone synthesis and our work. (a) addition-annulation reaction [25]; (b) asymmetric Michael addition-annulation cascade [26]; (c) asymmetric Conia-ene-type cascade reaction [27]; (d) our research plan.
2. Results and Discussion
2.1. Optimization of Reaction Conditions
The 1,3-dipolar cycloaddition of trifluoromethyl-substituted nitrile imines with 4-benzylidene-3-methylisoxazol-5(4H)-ones was investigated. The required nitrile imines were generated in situ from trifluoroacetyl hydrazonoyl bromides by treatment with K2CO3. Using 4-benzylidene-3-methylisoxazol-5(4H)-one (1a) and 2,2,2-trifluoro-N′-phenylacetohydrazonoyl bromide (2a) as model substrates, the reaction in THF at room temperature for 24 h afforded a mixture of syn-3a and anti-3a in 52% combined yield with a diastereomeric ratio of 1.7:1 (Table 1, entry 1). To improve the reaction efficiency, we screened various solvents. Gratifyingly, the use of 1,2-dichloroethane (DCE) significantly enhanced the yield to 78% (Table 1, entry 3). Solvent polarity may affect dipole stability, with DCE proving optimal due to its low polarity in this system. Subsequent evaluation of different bases confirmed K2CO3 as the most effective. The use of soluble bases such as triethylamine leads to rapid deprotonation of the hydrazonoyl bromide, resulting in a high transient concentration of the nitrile imine dipole, which promotes side processes like dimerization and decomposition, thereby reducing the yield and diastereoselectivity. In contrast, insoluble bases like K2CO3 facilitate slow and controlled generation of the dipole, maintaining a low stationary concentration that minimizes these undesired reactions and enhances the efficiency of the desired cycloaddition. The reaction temperature was also optimized, revealing that both elevating to 50 °C and lowering to 0 °C resulted in diminished yields. Finally, screening of substrate stoichiometry demonstrated that a molar ratio of 1a/2a/base = 1.2:1:1.2 further increased the yield to 92% (Table 1, entry 10). The use of 1.2 equivalents of the nitrile imine precursor provided optimal yields by compensating for its dimerization during the reaction, ensuring sufficient reactive dipole remains available for cycloaddition; however, increasing to 1.5 equivalents led to reduced yields, likely due to elevated dipole concentration exacerbating side reactions such as further dimerization. Consequently, the optimized conditions were established as follows: 1a/2a/base molar ratio of 1.2:1:1.2, K2CO3 as base, in 1,2-dichloroethane at room temperature for 24 h.

Table 1.
Optimization of reaction conditions a.
2.2. Substrate Scope
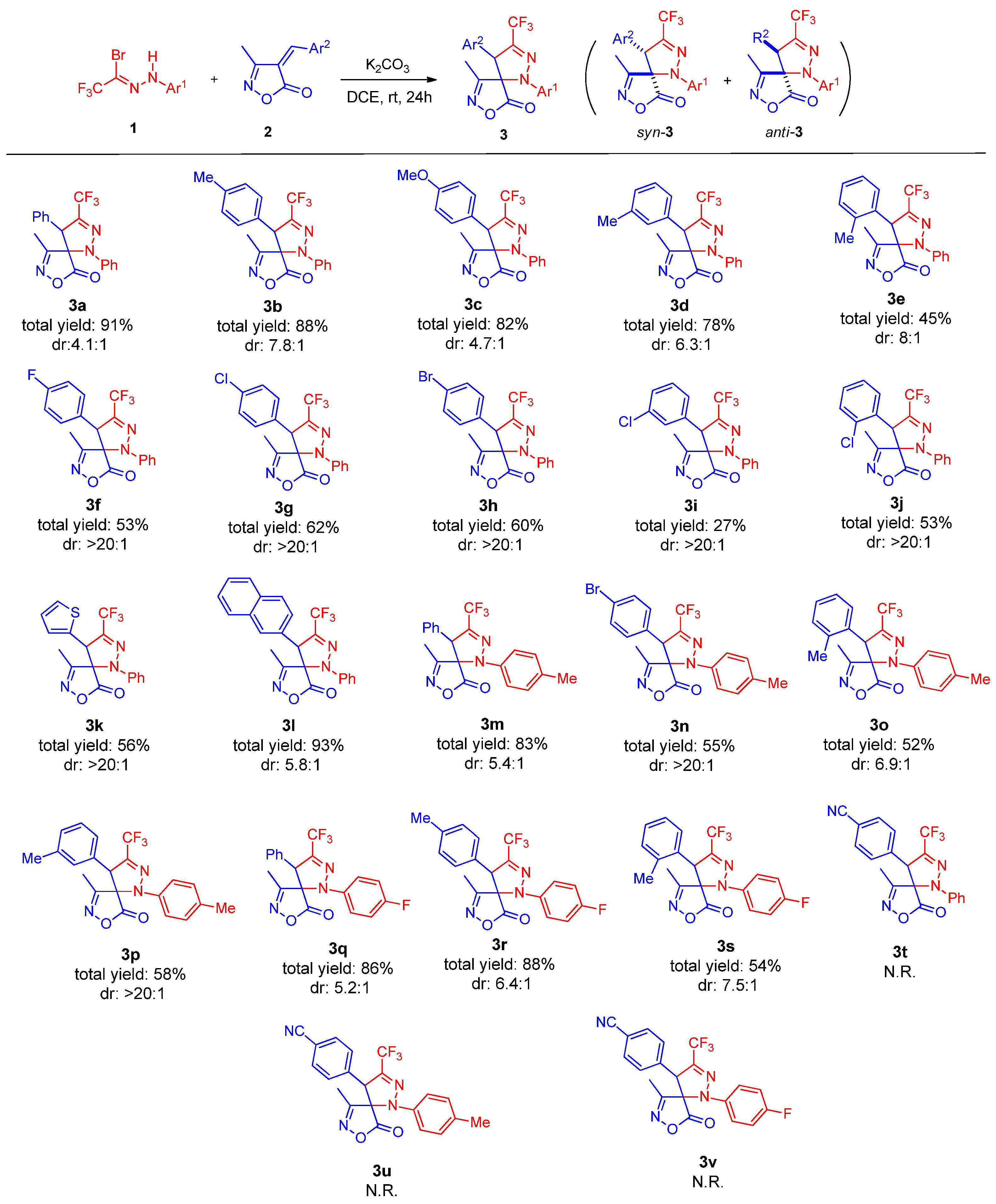
Under the optimized conditions, the substrate scope of this reaction was investigated, as shown in Scheme 2. We first explored the variation in the unsaturated isoxazolone component. The reaction proceeded efficiently when the aromatic ring (Ar2) bore electron-donating groups (e.g., methyl or methoxy) at the para- or meta-positions, affording products 3a–3c in 78–88% yields. In contrast, the presence of these substituents at the ortho-position led to a significant decrease in yield (3d, 45%), presumably due to steric hindrance. Substrates with electron-withdrawing groups (–F, –Br, –Cl) on the Ar2 aromatic ring were also well tolerated, affording products 3f–3g in moderately reduced yields. Notably, the reaction produced solely the syn-diastereomer. Replacing the phenyl ring with a 2-thienyl group was feasible, providing the product 3k in 56% yield. Remarkably, the introduction of a naphthyl group at Ar2 resulted in an excellent yield of 93% for product 3l. When a strongly electron-withdrawing 4-cyanophenyl group was introduced at the Ar2 (for compounds 3t–3v), no cycloaddition took place under the optimized conditions, even after heating at 50 °C for 48 h. TLC monitoring and 1H NMR analysis of the crude mixture showed no formation of any cycloadduct (neither the desired regioisomer nor its alternative). This complete lack of reactivity is attributed to the significantly reduced dipolarophilicity of the alkene caused by the 4-cyanophenyl substituent, a phenomenon analogous to that observed for 4-nitrophenyl-substituted 4-arylideneoxazol-5(4H)-ones in related nitrile imine cycloadditions [28].
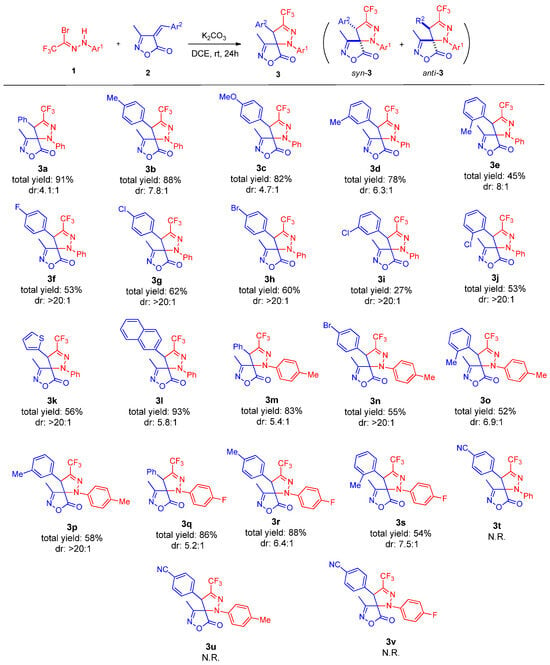
Scheme 2.
Substrate scope of arylidene isoxazol-5(4H)-one 1 and trifluoroacetyl bromohydrazone 2. Unless noted otherwise, the reactions were carried out with 1 (0.48 mmol), 2 (0.40 mmol), and K2CO3 (0.48 mmol) in DCE (2.0 mL) at rt for 24 h. The yields were isolated after column chromatography. Diastereomeric ratio was determined by 1H NMR analysis of the crude reaction mixture.
Subsequently, the scope concerning the trifluoromethyl bromohydrazone component (Ar1 aromatic ring) was examined. A variety of substituents were compatible with the reaction conditions. Both electron-donating (4-CH3) and electron-withdrawing groups (4-F) on the Ar1 aryl ring proceeded smoothly, furnishing the desired spirocyclic products 3m–3p or 3q–3s in yields comparable to those obtained with the parent phenyl-derived substrate.
2.3. Scaled-Up Synthesis
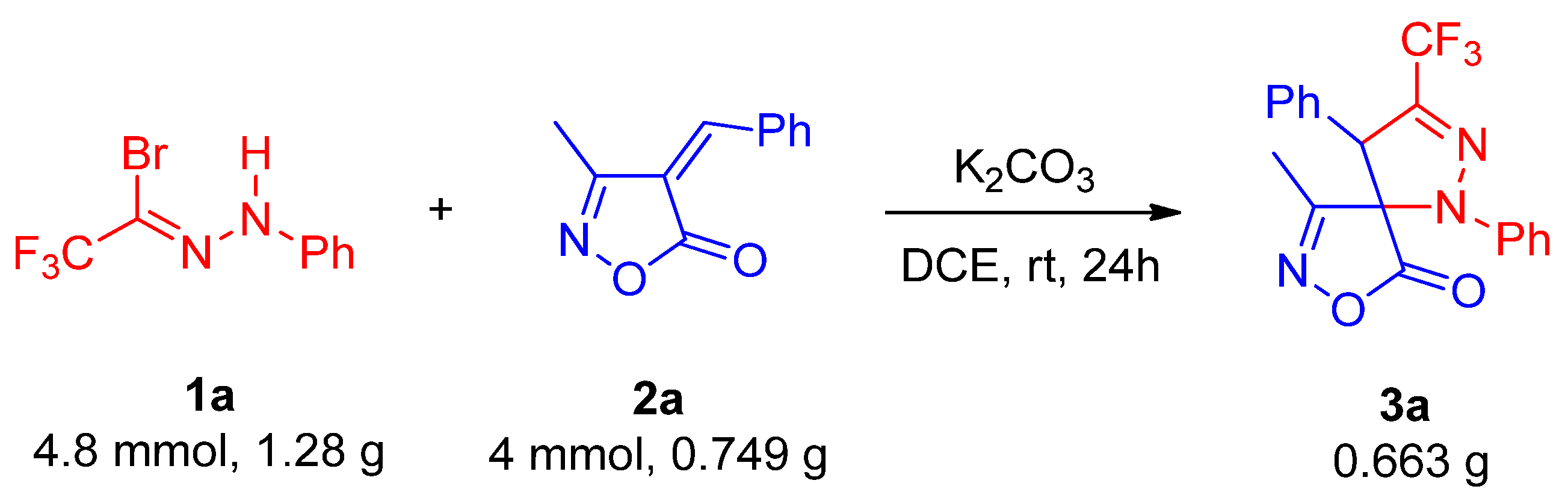
Subsequently, to evaluate the industrial applicability of this reaction, a gram-scale synthesis was performed by scaling up the reaction by tenfold, which afforded product 3a in 83% overall yield with 3.8:1 dr (Scheme 3).
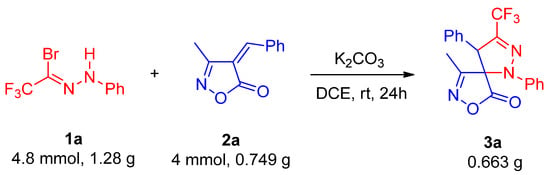
Scheme 3.
Gram-scale synthesis of 3a.
2.4. X-Ray Crystal Structure
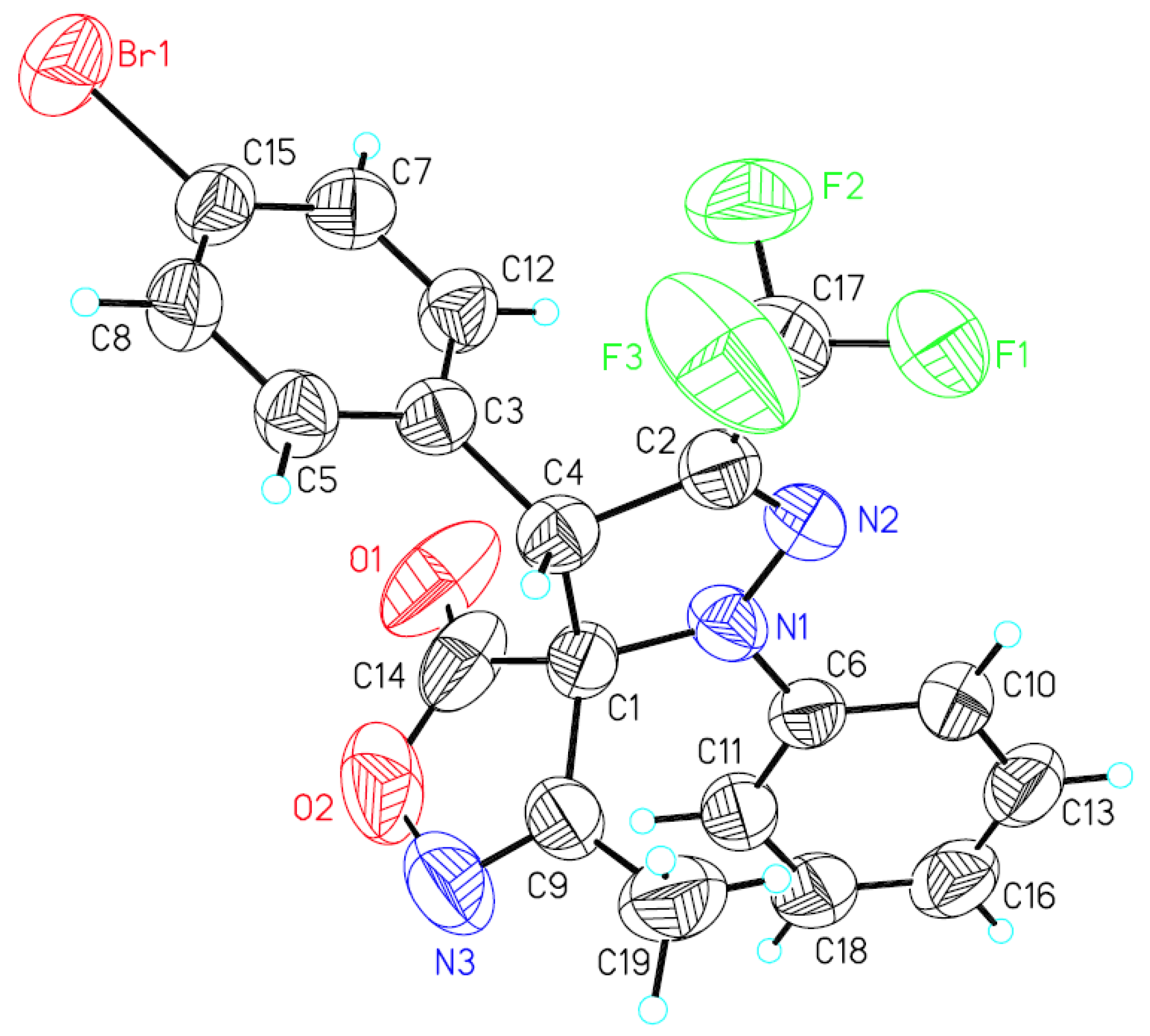
A single crystal of compound syn-3h was obtained by crystallization from petroleum ether and dichloromethane (8:1 v/v) at room temperature. The relative configuration of syn-3h was determined by X-ray crystallography analysis [29] as (rel-4S, 5R) (Figure 2), the relative configuration of other syn-products were assigned analogously.
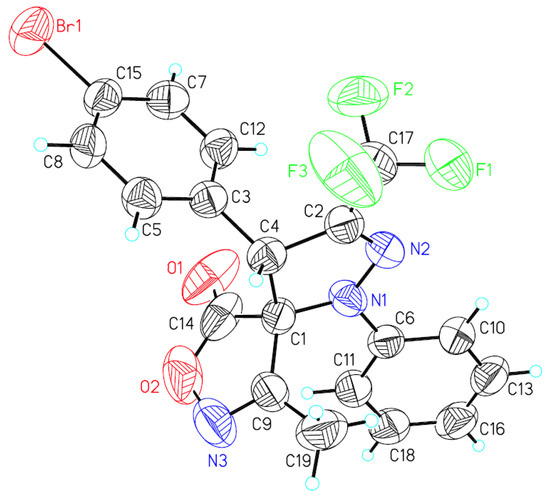
Figure 2.
X-ray crystal structure of syn-3h.
2.5. Reaction Mechanism
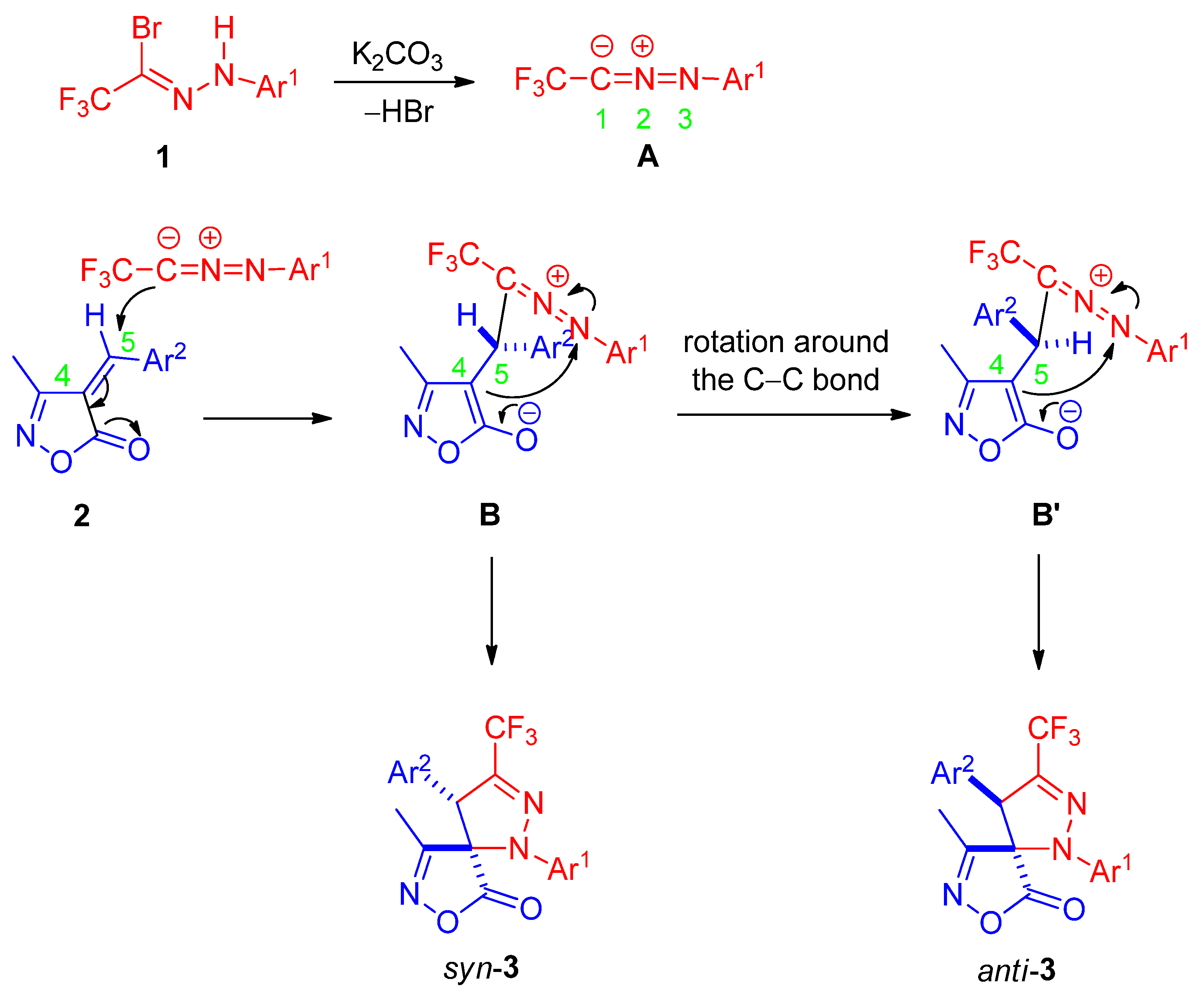
To investigate the origin of the observed diastereoselectivity, the starting isoxazolone 1a was monitored by 1H NMR in CDCl3 under two sets of conditions. In the absence of base, no formation of the (E)-isomer was detected at room temperature over 24 h. When 1.2 equivalents of anhydrous K2CO3 were added for 24 h to simulate the reaction conditions, the exocyclic double bond geometry remained exclusively (Z) throughout, with no evidence of isomerization. These results clearly indicate that the (Z)-configuration of the dipolarophile is configurationally stable under the cycloaddition conditions. Consequently, the stereochemical outcome must be controlled after the initial irreversible C–C bond-forming step rather than by pre-equilibration of the alkene conformers.
A plausible reaction mechanism consistent with these observations is proposed in Scheme 4. The reaction commences with K2CO3-promoted dehydrobromination of bromohydrazone 1 to generate nitrile imine intermediate A in situ. Regioselective [3+2] cycloaddition of A onto the (Z)-configured unsaturated isoxazolone 2 affords zwitterionic intermediate B. Rotation around the newly formed anionic C–C single bond in B can produce rotamer B′, differing in the spatial arrangement of the substituents. Subsequent intramolecular nucleophilic attack of the C4 enolate on the iminium nitrogen (N3) then yields the syn-diastereomer syn-3 predominantly from B, while the minor anti-diastereomer anti-3 is generated from B′.
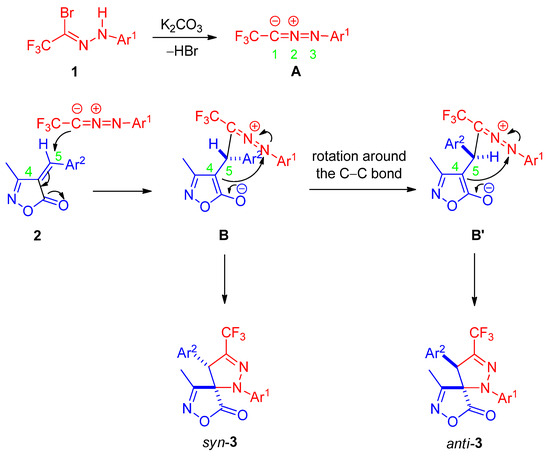
Scheme 4.
Proposed reaction mechanism.
3. Materials and Methods
3.1. General Information
Commercially available compounds were used without further purification. Solvents were dried according to standard procedures. Column chromatography was performed with silica gel (200–300 mesh) (Yantai, China). Melting points were determined with a WRX-4 melting-point apparatus (Shanghai, China) and are uncorrected. 1H NMR spectra were measured with a Bruker Ascend 500 MHz or 400 MHz spectrometer (Karlsurhe, Germany); chemical shifts were reported in δ (ppm) units relative to tetramethylsilane (TMS) as the internal standard. 13C NMR spectra were measured at 126 MHz with a 500 MHz spectrometer or at 101 MHz with a 400 MHz spectrometer; chemical shifts are reported in ppm relative to tetramethylsilane and referenced to the solvent peak (CDCl3, δC = 77.00). The 19F NMR spectra were measured at 471 MHz or 376 MHz. Proton coupling patterns are intricately characterized as diverse spectroscopic signatures, ranging from singlets (s) to doublets (d), triplets (t), quartets (q), multiplets (m), and broadened signals (br s). High-resolution mass spectra were acquired using the Agilent 6520 accurate-mass-Q-TOF MS system (Beijing, China), which incorporates an electrospray ionization (ESI+) source.
3.2. Experimental Materials
A series of unsaturated isoxazolones were synthesized according to literature procedures [30,31,32], while the trifluoromethyl bromohydrazones were synthesized according to literature procedures [33,34].
3.3. General Procedure for the Synthesis of Spiroisoxazolones 3
To a solution of K2CO3 (0.4 mmol) in 1,2-dichloroethane (2 mL) was added trifluoromethyl bromohydrazone 1 (0.48 mmol, 1.2 equiv) and unsaturated isoxazolone derivative 2 (0.4 mmol, 1.0 equiv). The reaction mixture was stirred at room temperature for 24 h. Upon completion, the solvent was removed under reduced pressure, and the residue was purified by flash chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 20:1, v/v) to afford product 3.
(rel-4S,5R)-9-Methyl-1,4-diphenyl-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3a). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 109.0 mg, 73% yield, yellow oil; 1H NMR (500 MHz, CDCl3): δ 7.41–7.36 (m, 3H, ArH), 7.32–7.28 (m, 2H, ArH), 7.19–7.17 (m, 2H, ArH), 7.07 (t, J = 7.5 Hz, 1H, ArH), 7.00 (dd, J1 = 9.0 Hz, J2 = 0.8 Hz, 2H, ArH), 4.85 (s, 1H, CH), 2.18 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 169.8, 165.5, 141.3, 138.5 (q, 2JC–F = 37.9 Hz), 130.1, 129.9, 129.4, 129.1, 128.4, 123.9, 119.9 (q, 1JC–F = 272.0 Hz), 114.9, 77.3, 60.6, 11.8 ppm; 19F NMR (471 MHz, CDCl3): δ −62.3 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C19H15F3N3O2 [M + H]+ 374.1111, found 374.1142.
(rel-4R,5R)-9-Methyl-1,4-diphenyl-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (anti-3a). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 26.9 mg, 18% yield, yellow solid; m.p. 192–194 °C; 1H NMR (500 MHz, CDCl3): δ 7.44 (t, J = 2.5 Hz, 3H, ArH), 7.33–7.29 (m, 2H, ArH), 7.23–7.21 (m, 2H, ArH), 7.09 (t, J = 7.5 Hz, 1H, ArH), 6.99 (d, J = 8.0 Hz, 2H, ArH), 5.16 (s, 1H, CH), 1.38 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 169.8, 165.5, 141.3, 138.5 (q, 2JC–F = 37.8 Hz), 130.0, 129.9, 129.3, 129.1, 128.4, 123.9, 119.9 (q, 1JC–F = 272.0 Hz), 114.9, 77.3, 60.5 11.8 ppm; 19F NMR (471 MHz, CDCl3): δ −62.3 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C19H15F3N3O2 [M + H]+ 374.1111, found 374.1141.
(rel-4S,5R)-9-Methyl-1-phenyl-4-(p-tolyl)-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3b). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 117.7 mg, 78% yield, yellow solid; m.p. 202–204 °C; 1H NMR (500 MHz, CDCl3): δ 7.32–7.29 (m, 2H, ArH), 7.19 (d, J = 8.0 Hz, 2H, ArH), 7.08–7.05 (m, 3H, ArH), 6.99 (d, J = 7.5 Hz, 2H, ArH), 4.83 (s, 1H, CH), 2.35 (s, 3H, CH3), 2.19 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 169.9, 165.6, 141.3, 140.2, 138.6 (q, 2JC–F = 37.6 Hz), 129.9, 129.8, 129.2, 125.3, 123.8, 119.9 (q, 1JC–F = 272.0 Hz), 114.9, 77.4, 60.4, 21.3, 11.8 ppm; 19F NMR (471 MHz, CDCl3): δ −62.3 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C20H17F3N3O2 [M + H]+ 388.1267, found 388.1255.
(rel-4R,5R)-9-Methyl-1-phenyl-4-(p-tolyl)-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (anti-3b). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 15.5 mg, 10% yield, yellow oil; 1H NMR (500 MHz, CDCl3): δ 7.31 (t, J = 7.8 Hz, 2H, ArH), 7.23 (d, J = 8.0 Hz, 2H, ArH), 7.10–7.06 (m, 3H, ArH), 6.98 (d, J = 8.0 Hz, 2H, ArH), 5.13 (s, 1H, CH), 2.37 (s, 3H, CH3), 1.41 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 174.5, 164.7, 141.3, 140.2, 138.9 (q, 2JC–F = 38.2 Hz), 130.2, 129.9, 128.6, 126.5, 124.1, 120.0 (q, 1JC–F = 272.3 Hz), 115.2, 77.3, 62.7, 21.2, 13.6 ppm; 19F NMR (471 MHz, CDCl3): δ −62.3 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C20H17F3N3O2 [M + H]+ 388.1267, found 388.1273.
(rel-4S,5R)-4-(4-Methoxyphenyl)-9-methyl-1-phenyl-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3c). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 108.1 mg, 67% yield, brown oil; 1H NMR (500 MHz, CDCl3): δ 7.32–7.29 (m, 2H, ArH), 7.11 (d, J = 9.0 Hz, 2H, ArH), 7.07 (t, J = 7.5 Hz, 1H, ArH), 6.99 (d, J = 8.0 Hz, 2H, ArH), 6.89 (d, J = 9.0 Hz, 2H, ArH), 4.84 (s, 1H, CH), 3.79 (s, 3H, OCH3), 2.18 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 170.0, 165.6, 160.8, 141.4, 138.7 (q, 2JC–F = 37.5 Hz), 130.7, 129.9, 123.2, 120.0, 119.9 (q, 1JC–F = 272.0 Hz), 114.8, 114.5, 77.3, 60.2, 55.2, 11.8 ppm; 19F NMR (471 MHz, CDCl3): δ −62.3 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C20H17F3N3O3 [M + H]+ 404.1217, found 404.1210.
(rel-4R,5R)-4-(4-Methoxyphenyl)-9-methyl-1-phenyl-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (anti-3c). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 24.2 mg, 15% yield, colorless oil; 1H NMR (500 MHz, CDCl3): δ 7.32–7.29 (m, 2H, ArH), 7.14 (d, J = 8.5 Hz 2H, ArH), 7.08 (t, J = 7.5 Hz, 1H, ArH), 6.98 (d, J = 7.5 Hz, 2H, ArH), 6.95–6.92 (m, 2H, ArH), 5.13 (s, 1H, CH), 3.83 (s, 3H, OCH3), 1.42 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 174.5, 164.8, 160.7, 141.3, 138.9 (q, 2JC–F = 38.1 Hz), 130.0, 129.9, 124.1, 121.3, 120.0 (q, 1JC–F = 272.1 Hz), 115.2, 114.9, 77.2, 62.4, 55.4, 13.6 ppm; 19F NMR (471 MHz, CDCl3): δ −62.3 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C20H17F3N3O3 [M + H]+ 404.1217, found 404.1210.
(rel-4S,5R)-9-Methyl-1-phenyl-4-(m-tolyl)-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3d). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 103.8 mg, 67% yield, yellow oil; 1H NMR (500 MHz, CDCl3): δ 7.32–7.28 (m, 2H, ArH), 7.26–7.24 (m, 1H, ArH), 7.20 (d, J = 7.5 Hz, 1H, ArH), 7.06 (t, J = 7.3 Hz, 1H, ArH), 7.00 (t, J = 8.5 Hz, 4H, ArH), 4.81 (s, 1H, CH), 2.34 (s, 3H, CH3), 2.18 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 169.8, 165.5, 141.3, 138.9, 138.6 (q, 2JC–F = 37.7 Hz), 130.8, 129.92, 129.86, 128.9, 128.3, 126.4, 123.9, 119.9 (q, 1JC–F = 272.0 Hz), 114.9, 77.3, 60.6, 21.3, 11.8 ppm; 19F NMR (471 MHz, CDCl3): δ −62.3 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C20H17F3N3O2 [M + H]+ 388.1267, found 388.1272.
(rel-4R,5R)-9-Methyl-1-phenyl-4-(m-tolyl)-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (anti-3d). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 17.0 mg, 11% yield, yellow oil; 1H NMR (500 MHz, CDCl3): δ 7.33–7.29 (m, Hz, 3H, ArH), 7.24 (d, J = 8.0 Hz, 2H, ArH), 7.08 (d, J = 7.5 Hz, 1H, ArH), 7.02–6.98 (m, 4H, ArH), 5.11 (s, 1H, CH), 2.37 (s, 3H, CH3), 1.40 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 174.5, 164.7, 141.3, 139.6, 138.9 (q, 2JC–F = 38.2 Hz), 130.8, 129.8, 129.5, 129.4, 129.3, 125.7, 124.1, 119.9 (q, 1JC–F = 271.6 Hz), 118.9, 115.3, 77.2, 62.8, 21.4, 13.5 ppm; 19F NMR (471 MHz, CDCl3): δ −61.8 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C20H17F3N3O2 [M + H]+ 388.1267, found 388.1264.
(rel-4S,5R)-9-Methyl-1-phenyl-4-(o-tolyl)-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3e). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 62.0 mg, 40% yield, yellow solid; m.p. 156–158 °C; 1H NMR (500 MHz, CDCl3): δ 7.32–7.26 (m, 4H, ArH), 7.24–7.22 (m, 1H, ArH), 7.16 (d, J = 7.0 Hz, 1H, ArH), 7.07 (t, J = 7.2 Hz 1H, ArH), 6.98 (d, J = 8.5 Hz, 2H, ArH), 5.16 (s, 1H, CH), 2.30 (s, 3H, CH3), 2.16 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 169.8, 165.6, 141.1, 139.2 (q, 2JC–F = 37.8 Hz), 135.6, 131.1, 130.5, 129.9, 129.8, 126.9, 126.5, 123.8, 119.9 (q, 1JC–F = 271.8 Hz), 114.7, 75.8, 56.1, 19.5, 11.8 ppm; 19F NMR (471 MHz, CDCl3): δ −62.3 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C20H17F3N3O2 [M + H]+ 388.1267, found 388.1264.
(rel-4R,5R)-9-Methyl-1-phenyl-4-(o-tolyl)-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (anti-3e). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 7.7 mg, 5% yield, yellow solid; m.p. 131–133 °C; 1H NMR (500 MHz, CDCl3): δ 7.34–7.26 (m, 5H, ArH), 7.17 (d, J = 8.0 Hz, 1H, ArH), 7.08 (t, J = 7.2 Hz, 1H, ArH), 6.98 (d, J = 8.0 Hz, 2H, ArH), 5.37 (s, 1H, CH), 2.31 (s, 3H, CH3), 1.31 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 174.3, 165.6, 141.4, 139.6 (q, 2JC–F = 38.0 Hz), 136.9, 131.9, 130.0, 129.8, 129.1, 128.3, 126.6, 124.1, 119.9 (q, 1JC–F = 272.2 Hz), 115.4, 75.7, 58.8, 19.6, 13.5 ppm; 19F NMR (471 MHz, CDCl3): δ −61.9 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C20H17F3N3O2 [M + H]+ 388.1267, found 388.1268.
(rel-4S,5R)-4-(4-Fluorophenyl)-9-methyl-1-phenyl-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3f). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 82.9 mg, 53% yield, yellow oil; 1H NMR (500 MHz, CDCl3): δ 7.33–7.29 (m, 2H, ArH), 7.19–7.16 (m, 2H, ArH), 7.09–7.06 (m, 3H, ArH), 6.99 (d, J = 8.0 Hz, 2H, ArH), 4.84 (s, 1H, CH), 2.17 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 169.9, 165.5, 163.6 (d, 1JC–F = 250.9 Hz), 141.1, 138.2 (q, 2JC–F = 37.9 Hz), 131.3 (d, 3JC–F = 8.7 Hz), 129.9, 124.2 (d, 4JC–F = 3.4 Hz), 124.1, 119.9 (q, 1JC–F = 272.0 Hz), 116.3 (d, 2JC–F = 22.2 Hz), 114.9, 77.2, 59.7, 11.8 ppm; 19F NMR (471 MHz, CDCl3): δ −62.3 (s, 3F), −110.2 (m, 1F) ppm. HRMS (ESI): m/z calcd. for C19H14F4N3O2 [M + H]+ 392.1017, found 392.0996.
(rel-4S,5R)-4-(4-Chlorophenyl)-9-methyl-1-phenyl-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3g). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 101.1 mg, 62% yield, yellow oil; 1H NMR (500 MHz, CDCl3): δ 7.37 (d, J = 7.5 Hz, 2H, ArH), 7.33–7.30 (m, 2H, ArH), 7.12 (d, J = 8.5 Hz, 2H, ArH), 7.09 (t, J = 7.5 Hz, 1H, ArH), 6.99 (d, J = 8.0 Hz, 2H, ArH), 4.81 (s, 1H, CH), 2.17 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 169.8, 165.4, 141.1, 138.0 (q, 2JC–F = 37.9 Hz), 136.4, 130.7, 129.9, 129.4, 126.9, 124.1, 119.8 (q, 1JC–F = 272.7 Hz), 115.0, 77.1, 59.7, 11.8 ppm; 19F NMR (471 MHz, CDCl3): δ −62.1 (s, 3F), ppm. HRMS (ESI): m/z calcd. for C19H14ClF3N3O2 [M + H]+408.0721, found 408.0712.
(rel-4S,5R)-4-(4-Bromophenyl)-9-methyl-1-phenyl-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3h). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 108.5 mg, 60% yield, yellow solid; m.p. 233–235 °C; 1H NMR (500 MHz, CDCl3): δ 7.37 (d, J = 8.5 Hz, 2H, ArH), 7.33–7.29 (m, 2H, ArH), 7.12 (d, J = 8.5 Hz 2H, ArH), 7.09 (t, J = 7.5 Hz, 1H, ArH), 6.99 (d, J = 8.0 Hz 2H, ArH), 4.81 (s, 1H, CH), 2.18 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 169.8, 165.3, 141.1, 137.9 (q, 2JC–F = 38.0 Hz), 132.4, 130.9, 129.9, 127.4, 124.6, 124.1, 119.8 (q, 1JC–F = 272.0 Hz), 116.6, 77.1, 59.8, 11.8 ppm; 19F NMR (471 MHz, CDCl3): δ −62.3 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C19H14BrF3N3O2 [M + H]+ 452.0216, found 452.0209.
(rel-4S,5R)-4-(3-Chlorophenyl)-9-methyl-1-phenyl-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3i). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 44.0 mg, 27% yield, yellow oil; 1H NMR (500 MHz, CDCl3): δ 7.14 (d, J = 8.0 Hz, 2H, ArH), 7.07 (t, J = 7.8 Hz, 2H, ArH), 6.94–6.86 (m, 3H, ArH), 6.80 (s, 1H, ArH), 6.74 (d, J = 7.5 Hz, 1H, ArH), 6.04 (s, 1H, CH), 2.54 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 169.4, 142.4, 140.8 (q, 2JC–F = 37.1 Hz), 135.6, 133.4, 128.7, 128.5, 128.4, 127.6, 126.9, 125.6, 125.2, 119.7 (q, 1JC–F = 272.7 Hz), 119.4, 105.0, 77.2, 22.5 ppm; 19F NMR (471 MHz, CDCl3): δ −70.1 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C19H14ClF3N3O2 [M + H]+ 408.0721, found 408.0707.
(rel-4S,5R)-4-(2-Chlorophenyl)-9-methyl-1-phenyl-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3j). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 86.4 mg, 53% yield, yellow oil; 1H NMR (500 MHz, CDCl3): δ 7.54–7.51 (m, 1H, ArH), 7.42–7.35 (m, 2H, ArH), 7.32–7.28 (m, 3H, ArH), 7.10 (t, J = 7.2 Hz, 1H, ArH), 6.99 (d, J = 8.5 Hz, 2H, ArH), 5.67 (s, 1H, CH), 1.34 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 174.0, 163.7, 141.0, 138.1 (q, 2JC–F = 38.3 Hz), 134.7, 131.3, 130.8, 130.3, 129.8, 128.6, 127.3, 124.4, 119.8 (q, 1JC–F = 272.1 Hz), 115.8, 75.6, 58.6, 13.3 ppm; 19F NMR (471 MHz, CDCl3): δ −61.6 (s, 3F), ppm. HRMS (ESI): m/z calcd. for C19H14ClF3N3O2 [M + H]+ 408.0721, found 408.0722.
(rel-4S,5R)-9-Methyl-1-phenyl-4-(thiophen-3-yl)-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3k). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 85.0 mg, 56% yield, yellow oil; 1H NMR (500 MHz, CDCl3): δ 7.36 (d, J = 5.0 Hz, 1H, ArH), 7.30 (d, J = 8.0 Hz, 2H, ArH), 7.09–7.04 (m, 3H, ArH), 7.00 (d, J = 8.0 Hz, 2H, ArH), 5.17 (s, 1H, CH), 2.18 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 169.6, 165.2, 141.3, 137.5 (q, 2JC–F = 38.1 Hz), 130.3, 129.9, 128.5, 128.2, 127.8, 124.2, 119.7 (q, 1JC–F = 272.0 Hz), 115.2, 77.8, 54.6, 11.8 ppm; 19F NMR (471 MHz, CDCl3): δ −62.2 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C17H13F3N3O2S [M + H]+ 380.0675, found 380.0663.
(rel-4S,5R)-9-Methyl-4-(naphthalen-1-yl)-1-phenyl-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3l). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 133.8 mg, 79% yield, yellow solid; m.p. 197–199 °C; 1H NMR (500 MHz, CDCl3): δ 7.93–7.90 (m, 2H, ArH), 7.64 (d, J = 8.0 Hz, 1H, ArH), 7.60–7.51 (m, 3H, ArH), 7.41 (d, J = 7.5 Hz, 1H, ArH), 7.30 (t, J = 8.0 Hz, 2H, ArH), 7.07 (t, J = 7.5 Hz, 1H, ArH), 7.00 (d, J = 8.0 Hz, 2H, ArH), 5.71 (s, 1H, CH), 2.26 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 169.8, 165.1, 141.1, 138.9 (q, 2JC–F = 38.0 Hz), 134.1, 130.8, 130.6, 129.9, 129.7, 129.1, 127.6, 126.3, 125.2, 124.1, 123.9, 121.0, 120.0 (q, 1JC–F = 272.1 Hz), 114.9, 75.9, 55.0, 11.9 ppm. 19F NMR (471 MHz, CDCl3): δ −61.9 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C23H17F3N3O2 [M + H]+424.1267, found 424.1269.
(rel-4R,5R)-9-Methyl-4-(naphthalen-1-yl)-1-phenyl-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (anti-3l). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 23.7 mg, 14% yield, colorless oil; 1H NMR (500 MHz, CDCl3): δ 7.94 (t, J = 7.5 Hz, 2H, ArH), 7.77 (d, J = 8.5 Hz, 1H, ArH), 7.66–7.58 (m, 2H, ArH), 7.51 (t, J = 7.8 Hz, 1H, ArH), 7.43 (d, J = 7.0 Hz, 2H, ArH), 7.32–7.28 (m, 2H, ArH), 7.08 (t, J = 7.5 Hz, 1H, ArH), 7.00 (d, J = 8.0 Hz, 1H, ArH), 5.97 (s, 1H, CH), 1.12 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 174.7, 164.8, 141.3, 139.2 (q, 2JC–F = 38.0 Hz), 134.1, 131.0, 130.9, 129.8, 129.4, 128.1, 127.5, 127.0, 125.9, 124.7, 124.2, 121.8, 120.0 (q, 1JC–F = 272.2 Hz), 115.6, 76.2, 57.9, 13.5 ppm. 19F NMR (471 MHz, CDCl3): δ −61.4 (s, 3F), ppm. HRMS (ESI): m/z calcd. for C23H17F3N3O2 [M + H]+ 424.1267, found 424.1272.
(rel-4S,5R)-9-Mmethyl-4-phenyl-1-(p-tolyl)-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3m). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 108.5 mg, 70% yield, white solid; m.p. 195–197 °C; 1H NMR (400 MHz, CDCl3): δ 7.42–7.38 (m, 3H, ArH), 7.20–7.17 (m, 2H, ArH), 7.11 (d, J = 8.0 Hz, 2H, ArH), 6.90 (d, J = 8.8 Hz, 2H, ArH), 4.84 (s, 1H, CH), 2.29 (s, 3H, CH3), 2.20 (s, 3H, CH3) ppm; 13C NMR (101 MHz, CDCl3): δ 169.9, 165.5, 139.0, 138.1 (q, 2JC–F = 37.8 Hz), 133.8, 130.3, 130.0, 129.4, 129.1, 128.5, 120.0 (q, 1JC–F = 272.3 Hz), 115.9, 77.7, 60.4, 20.6, 11.8 ppm; 19F NMR (376 MHz, CDCl3): δ −62.2 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C20H17F3N3O2 [M + H]+ 388.1267, found 388.1254.
(rel-4R,5R)-9-Methyl-4-phenyl-1-(p-tolyl)-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (anti-3m). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 20.1 mg, 13% yield, yellow oil; 1H NMR (400 MHz, CDCl3): δ 7.44–7.42 (m, 3H, ArH), 7.23–7.21 (m, 2H, ArH), 7.10 (d, J = 8.0 Hz, 2H, ArH), 6.89 (d, J = 8.8 Hz, 2H, ArH), 5.14 (s, 1H, CH), 2.29 (s, 3H, CH3), 1.37 (s, 3H, CH3) ppm; 13C NMR (101MHz, CDCl3): δ 174.5, 164.5, 138.9,138.4 (q, 2JC–F = 38.1 Hz), 134.2, 130.3, 130.0, 129.7, 129.6, 128.7, 120.0 (q, 1JC–F = 272.4 Hz), 115.8, 77.6, 62.6, 20.6, 13.5 ppm; 19F NMR (376 MHz, CDCl3): δ −61.8 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C20H17F3N3O2 [M + H]+ 388.1267, found 388.1256.
(rel-4S,5R)-4-(4-Bromophenyl)-9-methyl-1-(p-tolyl)-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3n). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 102.6 mg, 55% yield, yellow oil; 1H NMR (500 MHz, CDCl3): δ 7.52 (d, J = 8.0 Hz, 2H, ArH), 7.11 (d, J = 8.0 Hz, 2H, ArH), 7.06 (d, J = 8.0 Hz, 2H, ArH), 6.89 (d, J = 8.5 Hz, 2H, ArH), 4.78 (s, 1H, CH), 2.29 (s, 3H, CH3), 2.18 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 169.9, 165.3, 138.8, 137.6 (q, 2JC–F = 37.8 Hz), 134.1, 132.4, 130.9, 130.4, 127.5, 124.5, 119.9 (q, 1JC–F = 272.0 Hz), 115.4, 77.5, 59.7, 20.6, 11.8 ppm; 19F NMR (471 MHz, CDCl3): δ −62.2 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C20H16BrF3N3O2 [M + H]+ 466.0373, found 466.0316.
(rel-4S,5R)-9-Methyl-4-(o-tolyl)-1-(p-tolyl)-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3o). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 72.3 mg, 45% yield, yellow oil; 1H NMR (500 MHz, CDCl3): δ 7.29–7.23 (m, 2H, ArH), 7.21 (d, J = 8.0 Hz, 1H, ArH), 7.16 (d, J = 7.5 Hz, 1H, ArH), 7.10 (d, J = 8.5 Hz, 2H, ArH), 6.89 (d, J = 8.5 Hz, 2H, ArH), 5.14 (s, 1H, CH), 2.29 (s, 3H, CH3), 2.28 (s, 3H, CH3), 2.16 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 170.0, 165.7, 138.9, 138.8 (q, 2JC–F = 37.6 Hz), 135.6, 133.7, 131.1, 130.5, 130.3, 129.7, 127.0, 126.5, 119.9 (q, 1JC–F = 271.9 Hz), 115.1, 76.2, 56.0, 20.6, 19.5, 11.8 ppm; 19F NMR (471 MHz, CDCl3): δ −62.2 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C21H19F3N3O2 [M + H]+ 402.1424, found 402.1429.
(rel-4R,5R)-9-Mmethyl-4-(o-tolyl)-1-(p-tolyl)-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (anti-3o). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 11.2 mg, 7% yield, yellow oil; 1H NMR (500 MHz, CDCl3): δ 7.34–7.28 (m, 2H, ArH), 7.27–7.25 (m, 1H, ArH), 7.17 (d, J = 7.5 Hz, 1H, ArH), 7.10 (d, J = 9.0 Hz, 2H, ArH), 6.88 (d, J = 8.5 Hz, 2H, ArH), 5.34 (s, 1H, CH), 2.30 (s, 3H, CH3), 2.29 (s, 3H, CH3), 1.31 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 174.4, 165.6, 139.3 (q, 2JC–F = 38.0 Hz), 139.1, 136.8, 134.2, 131.8, 130.3, 129.9, 129.1, 128.5, 126.6, 119.9 (q, 1JC–F = 272.4 Hz), 116.1, 76.1, 58.6, 20.6, 19.6, 13.5 ppm; 19F NMR (471 MHz, CDCl3): δ −61.8 (s, 3F), ppm. HRMS (ESI): m/z calcd. for C21H19F3N3O2 [M + H]+ 402.1424, found 402.1429.
(rel-4S,5R)-9-Methyl-4-(m-tolyl)-1-(p-tolyl)-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3p). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 93.1 mg, 58% yield, yellow oil; 1H NMR (500 MHz, CDCl3): δ 7.27 (t, J = 7.8 Hz, 1H, ArH), 7.20 (d, J = 7.5 Hz, 1H, ArH), 7.10 (d, J = 8.5 Hz, 2H, ArH), 6.98 (d, J = 9.5 Hz, 2H, ArH), 6.90 (d, J = 8.5 Hz, 2H, ArH), 4.80 (s, 1H, CH), 2.34 (s, 3H, CH3), 2.28 (s, 3H, CH3), 2.19 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 169.9, 165.5, 139.1, 138.9, 138.2 (q, 2JC–F =37.8 Hz), 133.7, 130.8, 130.3, 129.9, 128.9, 128.4, 126.5, 120.0 (q, 1JC–F = 271.8 Hz), 115.3, 77.7, 60.5, 21.3, 20.6, 11.8 ppm; 19F NMR (471 MHz, CDCl3): δ −62.2 (s, 3F) ppm. HRMS (ESI): m/z calcd. for C21H19F3N3O2 [M + H]+ 402.1424, found 402.1420.
(rel-4S,5R)-1-(4-Fluorophenyl)-9-methyl-4-phenyl-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3q). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 112.7 mg, 72% yield, yellow oil; 1H NMR (400 MHz, CDCl3): δ 7.42–7.36 (m, 3H, ArH), 7.19–7.17 (m, 2H, ArH), 7.04–6.96 (m, 4H, ArH), 4.87 (s, 1H, CH), 2.21 (s, 3H, CH3) ppm; 13C NMR (101 MHz, CDCl3): δ 169.8, 165.2, 159.4 (d, 1J = 245.1 Hz), 138.9 (q, 2J = 38.0 Hz), 137.6 (d, 4J = 2.4 Hz), 130.1, 129.3, 129.1, 128.2, 119.8 (q, 1J = 272.6 Hz), 117.2 (d, 3J = 8.1 Hz), 116.7 (d, 2J = 22.9 Hz), 78.0, 60.6, 11.8 ppm; 19F NMR (376 MHz, CDCl3): δ −62.3 (s, 3F), 118.4 (m, 1F) ppm. HRMS (ESI): m/z calcd. for C19H14F4N3O2 [M + H]+ 392.1017, found 392.0983.
(rel-4R,5R)-1-(4-Fluorophenyl)-9-methyl-4-phenyl-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (anti-3q). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 21.9 mg, 14% yield, yellow oil; 1H NMR (400 MHz, CDCl3): δ 7.46–7.44 (m, 3H, ArH), 7.23–7.21 (m, 2H, ArH), 7.04–6.96 (m, 4H, ArH), 5.15 (s,1H, CH), 1.39 (s, 3H, CH3) ppm; 13C NMR (101 MHz, CDCl3): δ 174.2, 164.3, 159.7 (d, 1J = 245.5 Hz), 139.4 (q, 2JC-F = 38.3 Hz), 137.5 (d, 4J = 2.7 Hz), 130.1, 129.7, 129.5, 128.7, 119.8 (q, 1JC-F = 272.7 Hz), 117.9 (d, 3J = 8.2 Hz), 116.6 (d, 2J = 23.0 Hz), 77.6, 62.6, 13.5 ppm; 19F NMR (376 MHz, CDCl3): δ −62.0 (s, 3F), 117.9 (m, 1F) ppm. HRMS (ESI): m/z calcd. for C19H14F4N3O2 [M + H]+ 392.1017, found 392.1004.
(rel-4S,5R)-1-(4-Fluorophenyl)-9-methyl-4-(p-tolyl)-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3r). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 123.2 mg, 76% yield, yellow oil; 1H NMR (500 MHz, CDCl3): δ 7.19 (d, J = 8.0 Hz, 2H, ArH), 7.06 (d, J = 8.0 Hz, 2H, ArH), 7.03–6.96 (m, 4H, ArH), 4.85 (s, 1H, CH), 2.34 (s, 3H, CH3), 2.21 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 169.8, 165.2, 159.4 (d, 1J = 244.9 Hz), 140.2, 139.1 (q, 2J = 37.8 Hz), 137.7 (4d, J = 2.5 Hz), 129.9, 129.2, 125.1, 119.8 (q, 1J = 272.2 Hz), 117.1 (d, 3J = 7.3 Hz), 116.6 (d, 2J = 22.9 Hz), 78.1, 60.4, 21.2, 11.8 ppm; 19F NMR (471 MHz, CDCl3): δ −62.3 (s, 3F), 118.6 (m, 1F) ppm. HRMS (ESI): m/z calcd. for C20H16F4N3O2 [M + H]+ 406.1173, found 406.1178.
(rel-4R,5R)-1-(4-Fluorophenyl)-9-methyl-4-(p-tolyl)-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (anti-3r). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 19.5 mg, 12% yield, yellow oil; 1H NMR (500 MHz, CDCl3): δ 7.24 (d, J = 8.0 Hz, 2H, ArH), 7.09 (d, J = 8.0 Hz, 2H, ArH), 7.04–6.95 (m, 4H, ArH), 5.12 (s, 1H, CH), 2.38 (s, 3H, CH3), 1.42 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 174.3, 164.4, 159.6 (d, 1J = 244.9 Hz), 140.3, 139.6 (q, 2J = 37.9 Hz), 137.6 (d, 4J = 2.6 Hz), 130.3, 128.6, 126.4, 119.9 (q, 1J = 272.3 Hz), 117.7 (d, 3J = 8.1 Hz), 116.6 (d, 2J = 23.1 Hz), 77.7, 62.5, 21.2, 13.6 ppm; 19F NMR (471 MHz, CDCl3): δ −62.0 (s, 3F), 118.1 (m, 1F) ppm. HRMS (ESI): m/z calcd. for C20H16F4N3O2 [M + H]+ 406.1173, found 406.1181.
(rel-4S,5R)-1-(4-Fluorophenyl)-9-methyl-4-(o-tolyl)-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (syn-3s). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 77.8 mg, 48% yield, yellow oil; 1H NMR (500 MHz, CDCl3): δ 7.30–7.25 (m, 2H, ArH), 7.24–7.21 (m, 1H, ArH), 7.15 (d, J = 7.5 Hz, 1H, ArH), 7.03–6.95 (m, 4H, ArH), 5.16 (s,1H, CH), 2.29 (s, 3H, CH3), 2.17 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 169.8, 165.4, 159.3 (d, 1J = 244.4 Hz), 139.6 (q, 2J = 38.0 Hz), 137.5 (d, 4J = 2.5 Hz), 135.6, 131.2, 130.5, 129.8, 126.8, 126.6, 119.8 (q, 1J = 272.2), 116.9 (d, 3J = 8.1 Hz), 116.7 (d, 2J = 23.2 Hz), 76.3, 56.2, 19.5, 11.8 ppm; 19F NMR (471 MHz, CDCl3): δ −62.7 (s, 3F), 118.7 (m, 1F) ppm. HRMS (ESI): m/z calcd. for C20H16F4N3O2 [M + H]+ 406.1173, found 406.1170.
(rel-4R,5R)-1-(4-Fluorophenyl)-9-methyl-4-(o-tolyl)-3-(trifluoromethyl)-7-oxa-1,2,8-triazaspiro[4.4]nona-2,8-dien-6-one (anti-3s). The synthesis of this compound was conducted using a standardized protocol, followed by purification through column chromatography on silica gel (200–300 mesh) with petroleum ether/ethyl acetate (20:1 v/v) as the eluent to give 9.7 mg, 6% yield, yellow oil; 1H NMR (500 MHz, CDCl3): δ 7.35–7.27 (m, 3H, ArH), 7.17 (d, J = 7.5 Hz, 1H, ArH), 7.04–6.95 (m, 4H, ArH), 5.36 (s, 1H, CH), 2.30 (s, 3H, CH3), 1.32 (s, 3H, CH3) ppm; 13C NMR (126 MHz, CDCl3): δ 174.1, 165.3, 159.7 (d, 1J = 245.1 Hz), 140.4 (q, 2J = 38.1 Hz), 137.7 (d, 4J = 2.6 Hz), 136.8, 131.9, 130.1, 129.0, 128.2, 126.7, 119.8 (q, 1J = 272.2 Hz), 118.1 (d, 3J = 8.2 Hz), 116.7 (d, 2J = 23.1 Hz), 76.3, 58.7, 19.6, 13.5 ppm; 19F NMR (471 MHz, CDCl3): δ −62.1 (s, 3F), 117.9 (m, 1F) ppm. HRMS (ESI): m/z calcd. for C20H16F4N3O2 [M + H]+ 406.1173, found 406.1170.
3.4. Procedure for the Scaled-Up Synthesis of Compound 3a
To a dried 50 mL round-bottom flask, trifluoromethyl bromohydrazone 1a (4,8 mmol, 1.28 g), unsaturated isoxazolone derivative 2a (4 mmol, 0.749 g), K2CO3 (4 mmol, 0.553 g) and 1,2-dichloroethane (20 mL) were added. The reaction mixture was stirred at room temperature for 24 h. Upon completion, the solvent was removed under reduced pressure, and the residue was purified by flash chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 20:1, v/v) to afford product 3a in 83% overall yield with 3.8:1 dr.
4. Conclusions
In summary, a [3+2] cycloaddition reaction between nitrile imines and unsaturated isoxazolones was developed. This protocol employs inexpensive, readily available, and environmentally benign K2CO3 as the base, affording a series of trifluoromethylated spiroisoxazolone derivatives in moderate to excellent yields. Furthermore, the reaction exhibits a broad substrate scope, as both the trifluoroacetyl bromohydrazones and unsaturated isoxazolones can be widely varied. This versatility enables the synthesis of products bearing diverse potential pharmacophores, thereby providing a valuable library of candidate compounds for new drug discovery.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules31010073/s1, Copies of 1H and 13C NMR spectra of new compounds.
Author Contributions
W.Z. wrote the preliminary manuscript and performed the experiments and acquired and analyzed the original data; D.-M.D. designed the research plan, supervised the experiments, modified all figures and schemes, analyzed and checked all the data, and revised this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article and Supplementary Materials.
Acknowledgments
We thank the Analysis and Testing Center of the Beijing Institute of Technology for the measurement of NMR and mass spectrometry.
Conflicts of Interest
There are no conflicts of interest to declare.
References
- Guardia, A.; Baiget, J.; Cacho, M.; Pérez, A.; Ortega-Guerra, M.; Nxumalo, W.; Khanye, S.D.; Rullas, J.; Ortega, F.; Jiménez, E.; et al. Easy-to-Synthesize Spirocyclic Compounds Possess Remarkable in Vivo Activity against Mycobacterium Tuberculosis. J. Med. Chem. 2018, 61, 11327–11340. [Google Scholar] [CrossRef]
- Peng, Y.; Huo, X.; Luo, Y.; Wu, L.; Zhang, W. Enantio- and Diastereodivergent Synthesis of Spirocycles through Dual-metal-catalyzed [3+2] Annulation of 2-Vinyloxiranes with Nucleophilic Dipoles. Angew. Chem. Int. Ed. 2021, 60, 24941–24949. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Meazza, M.; Guo, H.; Yang, J.W.; Rios, R. New Development in the Enantioselective Synthesis of Spiro Compounds. Chem. Soc. Rev. 2018, 47, 5946–5996. [Google Scholar] [CrossRef] [PubMed]
- Hiesinger, K.; Dar’in, D.; Proschak, E.; Krasavin, M. Spirocyclic Scaffolds in Medicinal Chemistry. J. Med. Chem. 2021, 64, 150–183. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, S.; Wu, S.; Cheng, Q.; Chen, Q.; Jiao, Y.; Zhang, Y.; Shi, F. Catalytic Asymmetric (3 + 3) Cycloaddition between Different 2-Indolylmethanols. Sci. China Chem. 2024, 67, 2629–2636. [Google Scholar] [CrossRef]
- Xie, X.; Huang, W.; Peng, C.; Han, B. Organocatalytic Asymmetric Synthesis of Six-membered Carbocycle-based Spiro Compounds. Adv. Synth. Catal. 2018, 360, 194–228. [Google Scholar] [CrossRef]
- Yasui, M.; Ohbu, H.; Ishikawa, M.; Yoshida, T.; Takeda, N.; Hirao, S.; Abe, T.; Ueda, M. Synthesis of Spiro[indole-3,3′-pyrrolidine]-2′-(thi)ones. J. Org. Chem. 2023, 88, 1093–1106. [Google Scholar] [CrossRef]
- James, M.J.; O’Brien, P.; Taylor, R.J.K.; Unsworth, W.P. Synthesis of Spirocyclic Indolenines. Chem.–Eur. J. 2016, 22, 2856–2881. [Google Scholar] [CrossRef]
- Carreira, E.M.; Fessard, T.C. Four-Membered Ring-Containing Spirocycles: Synthetic Strategies and Opportunities. Chem. Rev. 2014, 114, 8257–8322. [Google Scholar] [CrossRef]
- Kumar, P.; Singampalli, A.; Bandela, R.; Srimounika, B.; Rajyalakshmi, S.I.; Devi, A.; Nanduri, S.; Venkata Madhavi, Y. Spirocyclic Compounds: Potential Drug Leads in the Fight against Mycobacterium Tuberculosis. Future Med. Chem. 2025, 17, 819–837. [Google Scholar] [CrossRef]
- Natho, P.; Colella, M.; Luisi, R. Strained Spiro Heterocycles as Potential Bioisosteres: An Update on the Synthesis of Heteroatom-Containing Spiro[2.3]hexanes and Spiro[3.3]heptanes. Chem. Commun. 2025, 61, 6579–6594. [Google Scholar] [CrossRef] [PubMed]
- Ishioka, T.; Tanatani, A.; Nagasawa, K.; Hashimoto, Y. Anti-Androgens with Full Antagonistic Activity toward Human Prostate Tumor LNCaP Cells with Mutated Androgen Receptor. Bioorganic Med. Chem. Lett. 2003, 13, 2655–2658. [Google Scholar] [CrossRef] [PubMed]
- Anwar, T.; Nadeem, H.; Sarwar, S.; Naureen, H.; Ahmed, S.; Khan, A.; Arif, M. Investigation of Antioxidant and Anti-nociceptive Potential of Isoxazolone, Pyrazolone Derivatives, and Their Molecular Docking Studies. Drug Dev. Res. 2020, 81, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Anwer, K.E.; Sayed, G.H.; Ramadan, R.M. Synthesis, Spectroscopic, DFT Calculations, Biological Activities and Molecular Docking Studies of New Isoxazolone, Pyrazolone, Triazine, Triazole and Amide Derivatives. J. Mol. Struct. 2022, 1256, 132513. [Google Scholar] [CrossRef]
- Ouzounthanasis, K.A.; Glamočlija, J.; Ćirić, A.; Koumbis, A.E. Studies of the Synthesis of Fused Isoxazoline/Isoquinolinones and Evaluation of the Antifungal Activity of Isoxazole-like Benzamide and Isoquinolinone Hybrids. Molecules 2025, 30, 589. [Google Scholar] [CrossRef]
- Wazalwar, S.S.; Perdih, F. Synthesis, Single Crystal Study, Molecular Docking, and in Vitro Anticancer Screening of a Series of Isoxazolone Derivatives of 3-Chloro-3-phenylacrylaldehydes. J. Mol. Struct. 2025, 1322, 140282. [Google Scholar] [CrossRef]
- Deng, B.-L.; Hartman, T.L.; Buckheit, R.W.; Pannecouque, C.; De Clercq, E.; Cushman, M. Replacement of the Metabolically Labile Methyl Esters in the Alkenyldiarylmethane Series of Non-Nucleoside Reverse Anticriptase Inhibitors with Isoxazolone, Isoxazole, Oxazolone, or Cyano Substituents. J. Med. Chem. 2006, 49, 5316–5323. [Google Scholar] [CrossRef]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in Pharmaceuticals: Looking beyond Intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef]
- Leroux, F.; Jeschke, P.; Schlosser, M. α-Fluorinated Ethers, Thioethers, and Amines: Anomerically Biased Species. Chem. Rev. 2005, 105, 827–856. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, J.; Wang, S.; Gu, Z.; Aceña, J.L.; Izawa, K.; Liu, H.; Soloshonok, V.A. Recent Advances in the Trifluoromethylation Methodology and New CF3-Containing Drugs. J. Fluor. Chem. 2014, 167, 37–54. [Google Scholar] [CrossRef]
- Novás, M.; Matos, M.J. The Role of Trifluoromethyl and Trifluoromethoxy Groups in Medicinal Chemistry: Implications for Drug Design. Molecules 2025, 30, 3009. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, Y.; Zhang, X.; Yan, M. Regioselective Conjugate Addition of Isoxazol-5-ones to Ethenesulfonyl Fluoride. Org. Biomol. Chem. 2022, 20, 4714–4718. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-Q.; Li, T.-Z.; Liu, S.-J.; Shi, F. Advances in Organocatalytic Asymmetric [3 + 3] Cycloadditions: Synthesis of Chiral Six-Membered (Hetero)Cyclic Compounds. Org. Chem. Front. 2024, 11, 5573–5604. [Google Scholar] [CrossRef]
- Wang, Y.; Du, D.-M. Highly Diastereo- and Enantioselective Synthesis of Isoxazolone-spirooxindoles via Squaramide-Catalyzed Cascade Michael/Michael Addition Reactions. J. Org. Chem. 2020, 85, 15325–15336. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Bai, L.; Bai, Y.; Gan, Z.; Zhou, H.; Pan, T.; Yu, Y.; Zhou, J. High Diastereoselective Synthesis of Spiro-isoxazolonechromans via Domino Oxa-Michael/1,6-Addition Reactions of ortho-Hydroxyphenylsubstituted para-Quinone Methides with Unsaturated Isoxazolones. Tetrahedron 2019, 75, 682–687. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, C.; Xie, D.-H.; Du, D.-M. A Bifunctional Squaramide-Catalysed Enantioselective Vinylogous Michael Addition/Cyclization Cascade Reaction of 4-Unsaturated Isoxazol-5-ones and α,α-Dicyanoalkenes. Org. Biomol. Chem. 2021, 19, 8572–8577. [Google Scholar] [CrossRef]
- Kamlar, M.; Putatunda, S.; Císařová, I.; Veselý, J. Enantioselective Synthesis of Spirocyclic Isoxazolones Using a Conia-Ene Type Reaction. J. Org. Chem. 2025, 90, 3615–3627. [Google Scholar] [CrossRef]
- Foti, F.; Grassi, G.; Risitano, F.; Nicolò, F.; Rotondo, A. Regioselection in C-Bromo-N-phenylnitrilimine Cycloaddition to (Z)-4-(Arylmethylidene)azol-5-ones. Helv. Chim. Acta 2001, 84, 3313–3318. [Google Scholar] [CrossRef]
- CCDC 2500811 (for syn-3h) Contains the Supplementary Crystallographic Data for this Paper. These Data Can be Obtained Free of Charge Via. Available online: http://www.ccdc.cam.ac.uk/structures/ (accessed on 6 November 2025).
- Vekariya, R.H.; Patel, K.D.; Patel, H.D. Fruit Juice of Citrus Limon as a Biodegradable and Reusable Catalyst for Facile, Eco-friendly and Green Synthesis of 3,4-Disubstituted Isoxazol-5(4H)-ones and Dihydropyrano[2,3-c]-pyrazole Derivatives. Res. Chem. Intermed. 2016, 42, 7559–7579. [Google Scholar] [CrossRef]
- Laroum, R.; Debache, A. New Eco-friendly Procedure for the Synthesis of 4-Arylmethylene-isoxazol-5(4H)-ones Catalyzed by Pyridinium p-Toluenesulfonate (PPTS) in Aqueous Medium. Synth. Commun. 2018, 48, 1876–1882. [Google Scholar] [CrossRef]
- Rikani, A.B.; Setamdideh, D. One-Pot and Three-Component Synthesis of Isoxazol-5(4H)-one Derivatives in the Presence of Citric Acid. Orient. J. Chem. 2016, 32, 1433–1437. [Google Scholar] [CrossRef]
- Tanaka, K.; Maeno, S.; Mitsuhashi, K. Cycloadditions of N-Aryl- C-(trifluoromethyl)mitrilimines with Dimethyl Fumarate and Maleate. J. Heterocycl. Chem. 1985, 22, 565–568. [Google Scholar] [CrossRef]
- Mlostoń, G.; Urbaniak, K.; Utecht, G.; Lentz, D.; Jasiński, M. Trifluoromethylated 2,3-Dihydro-1,3,4-thiadiazoles via the Regioselective [3+2]-Cycloadditions of Fluorinated Nitrile Imines with Aryl, Hetaryl, and Ferrocenyl Thioketones. J. Fluor. Chem. 2016, 192, 147–154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.